
Sphincter of Oddi laxity alters bile duct microbiota and contributes to the recurrence of choledocholithiasis
Introduction
Choledocholithiasis is an intractable disease with an endemic distribution in East Asia and might lead to cholangiocarcinoma (1). In Asian countries, such as China, Japan, and South Korea, the incidence of choledocholithiasis is considerably higher than that of Western countries. Many risk factors, including bile duct anatomy, diet, and sanitary conditions, are believed to contribute to the occurrence of choledocholithiasis (2). Inflammation, infection, and bile duct stones are believed to be closely associated (3); however, few studies have presented direct evidence.
The sphincter of Oddi is the gate keeper of the common bile duct that controls bile duct release and avoids intestinal fluid reflux (4). The reflux of intestinal contents that are unsterile and corrosive, was hypothesized to be critical in the formation of bile duct stones by altering the microbial community and the inflammatory microenvironment of bile ducts (5). Previously, we reported that sphincter of Oddi laxity (SOL) occurred frequently in the Chinese population and might contribute to the high incidence of choledocholithiasis (6). The microbiota in the gut is closely related to many diseases, such as inflammatory bowel disease, autism, and arthritis (7). Using a combined analysis of microbiomes and metabolomics, we found that patients with SOL showed significantly different microbiomes and metabolomes compared to those with normal sphincter of Oddi function and proposed a mechanism for choledocholithiasis (8). However, whether SOL leads to bile duct stone recurrence and the role of the altered bile duct microbiome in disease recurrence warrant further investigation. In addition, although a special surgical algorithm (including fibrocholedochoscopic extraction of stones via T tube tract, endoscopic extraction of stones, choledocholithotomy and T tube drainage, choledochojejunostomy, percutaneous transhepatic cholangioscopy, etc.) to treat patients with recurrent choledocholithiasis and SOL has been proposed (9), no evidence obtained to date provides strong support for this approach. Understanding the true roles of SOL in altering the bile duct microbiota and bile duct stone recurrence is important to guide the treatment of patients with SOL and choledocholithiasis.
In the present study, we used a prospectively established cohort of patients with choledocholithiasis to evaluate the role of SOL in bile duct stone recurrence and explored the potential role of the bile duct microbiota in the recurrence of choledocholithiasis. We present the following article in accordance with the STORE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3295).
Methods
Patients and sample collection
The protocol of the current study was approved by the ethics committee of our hospital. The work has been reported in line with the STROCSS criteria and in accordance with the STORE reporting checklist (10). All patients with choledocholithiasis were prospectively recruited in our department between February 2014 and July 2018. Meanwhile, some of these patients were also recruited into a randomized controlled trial (NCT01459549) in accordance with the declaration of Helsinki (as revised in 2013). Informed consents to collect bile samples were obtained from all patients before surgery. In the past, SOL was diagnosed by experienced surgeons as previously described (8). In brief, SOL was intraoperatively diagnosed if the choledochoscope (Olympus CHF P20, with an external diameter of 4.9 mm) could reach the duodenum smoothly through the sphincter of Oddi without any dilation procedures. To provide objective parameters in the current study, we diagnosed SOL patients by a physician if the smallest diameter of the opening of the sphincter of Oddi was no less than 5 mm tested from each video filmed by a choledochoscope (Videos 1,2). Bile samples were collected from the common bile duct using a 5-mL sterile syringe before any invasive manipulation was performed on the bile duct. The samples were frozen in liquid nitrogen immediately and moved to −80 °C for storage until analysis.
Follow-up
All patients were followed up by nurses and physicians in our department. Residual bile duct stones were diagnosed based on the results of imaging at the first follow-up 1 month after surgery. Recurrence of choledocholithiasis was confirmed only in patients without residual bile duct stones at the first follow-up and who were found to have bile duct stones by imaging at a subsequent follow-up at least 3 months after surgery. Methods for detection of bile stone included T-tube radiography, B-mode ultrasound, computed tomography scan or magnetic resonance cholangiopancreatography. The diagnosis of residual bile duct stone or recurrence was made by two senior attending surgeons independently and was judged by a third surgeon in the case of disagreement.
DNA extraction and 16S ribosomal RNA (rRNA) gene analysis
DNA extraction from bile samples and MiSeq pyrosequencing of 16S rRNA gene amplicons were conducted as previously described (8) by ProMeGene (Shenzhen, China). In brief, extracted DNA was subjected to quality control and quantification using a Nanodrop Microvolume Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The 16S rRNA genes were amplified using primers with 8- to 12-bp barcodes, and polymerase chain reaction was performed. The qualified products were used for sequencing library generation using a TruSeq DNA Kit (Illumina, San Diego, CA, USA). Pyrosequencing was performed according to the protocol of the Illumina MiSeq system.
Data analysis and statistics
Sequencing data was analyzed as previously described (8). All data were presented as means and standard deviation. Student’s t-test or the Mann-Whitney U test were used for comparison, as appropriate. Recurrence-free survival was calculated using the Kaplan-Meier method and compared using the log rank test. P<0.05 was considered to be statistically significant.
Results
Demographic and clinical characteristics of patients
Overall, 202 patients were included in our study, of whom 113 were diagnosed with SOL, and 89 patients were diagnosed as non-SOL (Table 1; Figure 1A; Videos 1,2). No significant differences in demographic parameters were noted between patients with and without SOL. However, patients with SOL were more likely to have stones in both intra- and extra-hepatic locations, and had more positive bile bacterial cultures (P=0.004), although the proportions of patients with fever were comparable between the SOL and non-SOL groups (P=0.709). More patients in SOL group had received endoscopic retrograde cholangiopancreatography (ERCP; P=0.014). However, in the sub-cohort, there was no significant difference in the number of patients who had received ERCP between the two groups (P=0.116). The dilation of the common bile duct in SOL group is more obvious than non-SOL group (P=0.011). A similar difference was found in the sub-cohort (P=0.003). Intriguingly, patients with SOL had a higher incidence of previous cholecystectomy. Generally, similar results were observed in the sub-cohort of patients with bile microbiota analysis.
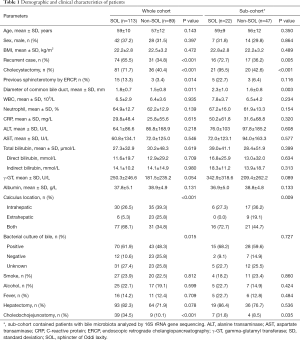
Full table
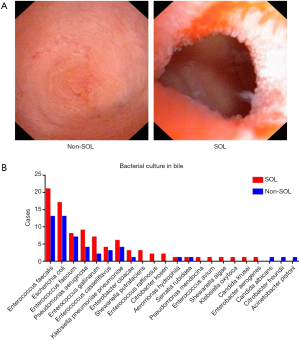
Differences of bile microbiota in patients with and without SOL
Bacterial culture of bile samples was conducted in 148 (73.27%) patients in the study. Although the species of the cultured bacteria were similar between the two groups (Figure 1B), patients with SOL were more likely to have positive bacterial cultures than non-SOL patients (85.37% vs. 65.15%, P=0.004). In particular, 23 out of 70 patients with SOL and 12 out of 43 patients with non-SOL suffered from complicated infections (i.e., with more than two types of bacteria successfully cultured).
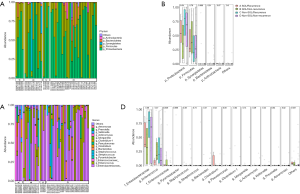
A total of 88 available bile samples (23 in the SOL group, 65 in the non-SOL group) with enough quantity were subjected to DNA extraction; library generation failed in 1 and 17 samples in the SOL and non-SOL groups (P=0.026), respectively, because of an inadequate abundance of bacterial DNA. As expected, the absolute abundance of the bile duct microbiota was significantly higher in patients with SOL patients (P<0.001). By searching the Greengenes database, we analyzed the operational taxonomic units (OTUs) at the phylum, class, order, and family levels. In the majority of samples, Firmicutes and Proteobacteria were predominant among the OTUs (Figure 2A,B), which were generally consistent with the result of bacterial cultures. In four cases (3/22 in the SOL group and 1/48 in the non-SOL group), Synergistetes accounted for a considerable proportion (>1/3) of the microbiota, which was very rare or even undetected in other cases. In fact, Synergistetes was the only phylum with a significantly different abundance between the two groups (P=0.012, Figure 2B). At the family level, Enterobacteriaceae and Enterococcus were the two most widespread families in the bile samples (Figure 2C,D). Pseudomonas, Streptococcus, Enterococcaceae, and Pyamidobacter also occupied a large part of the microbiota in some samples. Differences in abundance were observed for Enterococcaceae, Pyamidobacter, Clostridium, Morganella, and Shewanella, all of which had an average abundance of less than 2% (Figure 2D).
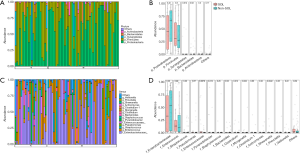
We then used alpha-diversity indexes and beta-diversity indexes to compare the bile microbial communities between the two groups. For alpha diversity assessment, although the evenness index and Shannon index did not show significant differences in the two groups, the observed OTUs and Faith’s phylogenetic diversity (PD) index suggested a trend of higher variation of microorganisms in the SOL samples (P=0.053 and 0.054, respectively; Figure 3A). For beta-diversity evaluation, both unweighted and weighted unifracs, together with the Bray-Curtis index, showed statistically significant differences between the two groups (P<0.001), indicating that the SOL samples had a higher between-habitat diversity (Figure 3B). These results suggested distinct bile microbial communities between patients with SOL and the non-SOL patients.
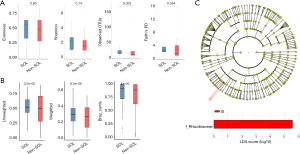
In addition, we used linear discriminant analysis effect size (LEfSe) analysis to identify potential biomarkers between the two groups using the relative abundance of microorganisms. Significant differences in the detection of Rhizobiaceae in patients with or without SOL (Figure 3C) indicated that this family was a possible biomarker of microbiota alteration caused by SOL.
SOL was associated with bile duct stone recurrence
We hypothesized that the SOL-related specific microbiota in bile duct induced the recurrence of choledocholithiasis. The median follow-up time was 21.9 (range, 0.1 to 55.2) and 14.9 (range, 1.0 to 55.6) months in the SOL and non-SOL groups, respectively. Fourteen patients in the SOL group and one patient in the non-SOL group were diagnosed as having recurrence after exclusion of residual bile duct stones, the rates of which were not significantly different between the two groups (15.93% vs. 20.22%; Table 2). The median time of recurrence was 20.3 months, ranging from 3.9 to 47.6 months. Compared with the non-SOL group, patients with SOL had a significantly higher rate of choledocholithiasis recurrence (16.67% vs. 1.82%, P=0.006). In addition, the recurrence plot showed a significantly shorter recurrence-free time in patients with SOL [P=0.0118, hazard ratio (HR) =8.631, 95% confidence interval (CI): 3.045 to 24.470; Figure 4A]. Diverse patterns of recurrence were noted (Figure 4B). The recurrence pattern of the only case from the non-SOL group was extrahepatic to intrahepatic. Taken together, these results suggested that SOL patients had a significantly higher risk of choledocholithiasis recurrence.

Full table
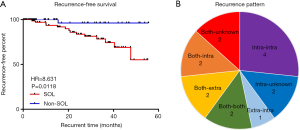
Species associated with recurrence of choledocholithiasis
We then explored which species in the bile could be associated with bile duct stone recurrence. We first compared the difference in the microbiota of patients with and without bile duct stone recurrence. No significant difference was noted between the patients with and without recurrence at the phylum and family levels (Figure S1), and at the general microbial community level (Figure S2). We hypothesized that the baseline characteristics, such as SOL, at the time of recruitment could influence the bile duct stone recurrence. Therefore, we divided all patients into four groups according to their sphincter of Oddi status and recurrence. Intriguingly, although no difference was found among the four groups at the order level (Figure 5A,B), at the genus level, we found diversity of the bile duct microbiota in the patients with SOL. The abundances of Clostridium and Enterococcaceae were significantly increased in SOL patients with and without bile duct stone recurrence, respectively (Figure 5C,D). Compared with patients with SOL and recurrence, patients with SOL patients who were free from recurrence had a higher abundance of Enterococcus (Figure 5D). Additionally, sphincterotomy by ERCP did not adequately change the bile microbiota (Figure S3). These results suggested that in patients with SOL patients, certain species, such as Clostridium, Enterococcaceae, and Enterococcus may be closely associated with bile duct stone recurrence after surgery.
Risk factors for recurrent choledocholithiasis
We further analyzed the risk factors that could affect choledocholithiasis recurrence in patients that underwent surgery for bile duct stones. Univariate analysis revealed that sphincter of Oddi status (P=0.006), previous cholecystectomy (P=0.048), ERCP (P=0.303), and bacterial culture positivity (P=0.111) were significantly or potentially associated with choledocholithiasis recurrence. Multivariate analysis further revealed that the sphincter of Oddi status (P=0.024, HR =10.800, 95% CI: 1.377–84.701) was an independent risk factor for choledocholithiasis recurrence after surgery.
Discussion
Choledocholithiasis is prevalent in East Asia probably because of the relatively poor hygiene conditions and possible race specificity. Notably few investigations of SOL have been reported. During the past decade, we have focused on the sphincter of Oddi dysfunction and regularly evaluate the sphincter of Oddi status during surgery. Our previous and current data suggested that approximately one half of patients with choledocholithiasis suffered from SOL (6). Thus, SOL is an important issue in this disease that warrants further research. Normally, the sphincter of Oddi opens upon increased pressure at the common bile duct and can prevent the reflux of duodenal content into the duct (5). SOL is a dysfunction of the sphincter of Oddi that can facilitate the reflux of intestinal content into the common bile duct. Microbiota implantation in the biliary ducts not only causes local inflammation but also influences bile metabolism to a great extent (11). However, whether an association exists among SOL, biliary microbiota, and choledocholithiasis is unknown.
The present study is a follow-up study based on our previous observations that SOL was associated with an increased recurrence of choledocholithiasis and that SOL led to a distinct microbiota in bile ducts (6,8). We used a prospective cohort of patients, expanded the sample size, and perfected the patients’ follow-up to confirm the previous results and to demonstrate an association between SOL-associated specific microbiota and bile duct stone recurrence. Previously, we used a recurrence index, which considered the time of recurrence and number of surgeries to assess the risk of bile duct stone recurrence (6). In the current study, we directly evaluated the recurrence time after surgery. Both methods demonstrated that SOL was associated with a higher risk of bile duct stone recurrence.
The patients without SOL had less abundant microbiota, which was supported by both the low number of positive bacterial cultures and the high failure rate of DNA amplification from bile samples. The components of the bile microbiota were consistent with our previous study and other reports (8,12). However, in this study, we focused on the diversity of the bile microbiota that might be associated with choledocholithiasis recurrence. We identified bacterial species in three families/genera (i.e., Clostridium, Enterococcaceae, and Enterococcus) that could serve as biomarkers for postoperative recurrence of choledocholithiasis in patients with SOL compared with non-SOL patients. This finding might reflect the fact that patients with SOL had both a higher abundance and higher diversity of the bile duct microbiota. In non-SOL patients, however, because of limited abundance and relatively lower diversity, the change in potential species in the bile duct microbiota might be nonsignificant and might not lead to choledocholithiasis recurrence. Among the three families, Clostridium is of particular interest because it is a known human pathogen that is increased in patients with recurrent SOL. Clostridium naturally exists in human intestines and plays a key role in antibiotic-induced enteritis. In chickens, inoculation of Clostridium perfringens induced cholangiohepatitis (13). Interestingly, the Clostridium genus is closely related to human bile salt metabolism because of their NADP-dependent 7α- and 7β-hydroxysteroid dehydrogenases (14). However, the exact role of this bacterial species in bile duct stone recurrence requires confirmation, and its potential pathogenic mechanism is unclear.
The present study had some limitations. First, not all bile samples were collected from the patients, which might introduce bias when their microbiome was analyzed. Second, more patients underwent choledochojejunostomy in the SOL group than in the non-SOL group. This procedure obviously eliminates the function of the sphincter of Oddi, which is functionally similar to severe SOL, leading to a reflux of the intestinal content and a change to the biliary microbiota. We believe the specific biliary microbiota in the liver caused by the long-term (several years or even longer) influence of SOL might be the main reason for bile duct stone recurrence. The specific alteration of the hepatic microbiota in patients with SOL is hypothesized to reflect biliary retrograde infection. The short-term influence of the removal of the sphincter of Oddi after surgery could change the common bile duct microbiota; however, it may not produce a profound alteration in the hepatic microbiota. Actually, more than 80% of recurrent calculi were found in the liver. Therefore, SOL-related long-term changes to the microbiota in the liver may be the cause of the increased recurrence rate of choledocholithiasis. Third, previous cholecystectomy and sphincterotomy by ERCP existed significant difference between patients with and without SOL. The loss of the gallbladder causes accumulation of bile and increases the pressure of common bile duct. ERCP may cause direct damage to the sphincter. Thus, both of them may lead to secondary SOL and can amplify the influence of SOL on the recurrence of the disease; however, these assumptions need further research to confirm. Forth, the actual recurrence-free time should be considerably shorter than that we have shown in this study, because most patients with choledocholithiasis were not followed up regularly with imaging examination, as was performed for tumor patients. Fourth, different imaging methods with different detection sensitivities were used to detect bile duct stones. Moreover, because of the complexity of the microbiota and the diversity of patients, it was difficult to identify the species responsible for bile duct stone recurrence. Fortunately, we determined that Clostridium is associated with an increased recurrence rate; however, this observation requires confirmation because of the relatively small number of patients with a significantly greater abundance of Clostridium in the current study. Additionally, the exact pathogenic mechanism by which Clostridium increased the risk of choledocholithiasis recurrence warrants further investigation. Last but not least, some patients received antibiotics before surgery, although it is balanced in both groups, it inevitably influenced the microbiota in the bile duct.
In conclusion, we confirmed that SOL led to significant alterations of the biliary microbiota and was associated with choledocholithiasis recurrence. We also found that Clostridium might serve as a prognostic biomarker of choledocholithiasis recurrence in patients with SOL. Therefore, in patients with SOL, close monitoring may be clinically valuable for the early detection of choledocholithiasis recurrence.
Acknowledgments
We thank Mr. Jianfeng Wang for his support in sample collection and storage.
Funding: This work was financially supported by the Project of Medical and Health Technology Platform of Zhejiang Province (No. 2017RC003), the National Natural Science Foundation of China (No. 81871320), and the Innovation Center for the Study of Pancreatic Diseases of Zhejiang Province, China.
Footnote
Reporting Checklist: The authors have completed the STORE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3295
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-3295
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3295). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol of the current study was approved by the ethics committee of our hospital (No. 2013-187). All patients with choledocholithiasis were prospectively recruited in our department between February 2014 and July 2018. Meanwhile, some of these patients were also recruited into a randomized controlled trial (NCT01459549) in accordance with the declaration of Helsinki (as revised in 2013). Informed consents to collect bile samples were obtained from all patients before surgery.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cai H, Kong WT, Chen CB, et al. Cholelithiasis and the risk of intrahepatic cholangiocarcinoma: a meta-analysis of observational studies. BMC Cancer 2015;15:831. [Crossref] [PubMed]
- Tazuma S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best Pract Res Clin Gastroenterol 2006;20:1075-83. [Crossref] [PubMed]
- Ochoa Villalabeitia Begoña T, González Serrano María C, Vazquez Melero A, et al. Hepatolithiasis as a Cause of Recurrent Cholangitis. Surgical Infections Case Reports 2017;2:113-5. [Crossref]
- Zhang ZH, Wu SD, Wang B, et al. Sphincter of Oddi hypomotility and its relationship with duodenal-biliary reflux, plasma motilin and serum gastrin. World J Gastroenterol 2008;14:4077-81. [Crossref] [PubMed]
- Toouli J. Sphincter of Oddi: Function, dysfunction, and its management. J Gastroenterol Hepatol 2009;24 Suppl 3:S57-62. [Crossref] [PubMed]
- Liang TB, Liu Y, Bai XL, et al. Sphincter of Oddi laxity: an important factor in hepatolithiasis. World J Gastroenterol 2010;16:1014-8. [Crossref] [PubMed]
- Gilbert JA, Blaser MJ, Caporaso JG, et al. Current understanding of the human microbiome. Nat Med 2018;24:392-400. [Crossref] [PubMed]
- Liang T, Su W, Zhang Q, et al. Roles of Sphincter of Oddi Laxity in Bile Duct Microenvironment in Patients with Cholangiolithiasis: From the Perspective of the Microbiome and Metabolome. J Am Coll Surg 2016;222:269-280.e10. [Crossref] [PubMed]
- Zhou B, Hu J, Zhong Y. Surgical treatments for patients with recurrent bile duct stones and Oddis sphincter laxity. Intractable Rare Dis Res 2017;6:172-6. [Crossref] [PubMed]
- Agha RA, Borrelli MR, Vella-Baldacchino M, et al. The STROCSS statement: Strengthening the Reporting of Cohort Studies in Surgery. Int J Surg 2017;46:198-202. [Crossref] [PubMed]
- Ma C, Han M, Heinrich B, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018;360:eaan5931.
- Pereira P, Aho V, Arola J, et al. Bile microbiota in primary sclerosing cholangitis: Impact on disease progression and development of biliary dysplasia. PLoS One 2017;12:e0182924. [Crossref] [PubMed]
- Sasaki J, Goryo M, Okada K. Cholangiohepatitis in chickens induced by bile duct ligations and inoculation of Clostridium perfringens. Avian Pathol 2000;29:405-10. [Crossref] [PubMed]
- Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 2006;47:241-59. [Crossref] [PubMed]


