
Citri Reticulatae Pericarpium protects against isoproterenol-induced chronic heart failure via activation of PPARγ
Introduction
Cardiovascular disease (CVD) is the leading cause of death which is responsible for more than one third of all deaths among adults and the elderly (1). Heart failure (HF) results from myocardial injury and is a common end phase of many kinds of CVD, including coronary heart disease, hypertension and diabetes. HF has become one of the fastest growing global health problems (2). Currently, novel therapy for HF is highly needed.
Heart is an organ with high metabolic demand and energy consumption. Abnormality of heart energy metabolism will eventually deteriorate cardiac function (3). Peroxisome proliferator-activated receptors (PPARs) are ligand-activated nuclear hormone receptor superfamily transcription factors, consisting of three members, PPARα, PPARγ and PPARδ. Previous studies showed that PPARγ participates in many biological processes, such as adipogenesis, energy metabolism and cellular proliferation (4,5). PPARγ coactivator-1α (PGC-1α) forms a complex with PPARγ, coactivates multiple transcription factors and coordinately governs transcriptional control of several metabolic processes (6). PPARγ downregulation has been found to be associated with pathogenesis of various CVDs, including HF (4,5). PPARγ activation increases glucose and free fatty acid uptake and glycerol lipid biosynthesis (7-9). Many studies found that PPARγ activation induced by PPARγ agonists has beneficial effects on heart, including inhibition of post-myocardial infarction (MI) cardiac remodeling and subsequent HF (10), alleviation of autoimmune myocarditis (11) and prevention of atherosclerosis in diabetic cardiopathy (12), suggesting that PPARγ could be potential therapeutic target for CVD.
Our previous studies showed that Qiliqiangxin (QLQX), a traditional Chinese medicine formula, has protective effects on inhibiting pathological cardiac remodeling, mechanistically via activation of PPARγ (13-16). In addition, QLQX was found to promote mitochondrial biosynthesis and improve myocardial energy metabolism in cardiomyocytes (17). Since QLQX includes 11 herbs, further studies are still needed to identify the authentic effective molecules in QLQX. Here, in the present study, we found that Citri Reticulatae Pericarpium (CRP), one of the herbal ingredients in QLQX, could protect against myocardial dysfunction both in vivo and in vitro. CRP is the dry and mature pericarp of Citrus reticulata Blanco and its cultivar (18). CRP contains numerous effective ingredients, such as flavonoids, alkaloids and volatile oils (19). CRP has been used in many prescriptions for improving digestive function, cardiovascular and respiratory system, as well as anti-tumor, anti-oxidant and anti-inflammation (20-23). Among hundreds of chemical compounds isolated and identified in CRP, hesperidin, nobiletin, naringin and naringenin were found to possess cardioprotective effects (24). Hesperidin, hesperetin and their derivatives were found to have beneficial effects against myocardial injury and cardiac remodeling, myocardial ischemia and infarction (25). Hesperidin has been found to inhibit cardiomyocyte apoptosis and reduce oxidative stress (OS) damage through up-regulating PPARγ expression (26,27). Nobiletin has been proven to be effective for alleviating myocardial dysfunction in diabetes rat model (28), attenuating myocardial ischemia and reperfusion injury and inhibiting endoplasmic reticulum stress-associated apoptosis via regulating PI3K/AKT signalling pathway (29). Naringenin was found to exert anti-ischemic effects by the activation of mitochondrial BK channels (30). Naringin was found to protect rats against LPS-induced myocardial dysfunction via regulation of PI3K/AKT/NF-κB Pathway (31). Many researchers have conducted extensive research on the chemical composition of CRP and the therapeutic effects of the bioactive ingredients. Few studies have explored in depth about the therapeutic effects of CRP on heart, Ou et al. showed that extracts from CRP and rhizoma zingiberis relieve myocardial ischemia (32), however, the underlying mechanisms remain to be further elucidated.
Here in this study, we used ISO to induce chronic HF, evaluated the therapeutic effect of CRP on cardiac remodeling and explored the protective mechanisms of CRP. We found that CRP could attenuate ISO-induced cardiac hypertrophy, fibrosis and apoptosis by upregulating PPARγ and PGC-1α. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-2200).
Methods
Animals
Male C57BL/6 mice (aged 7–8 weeks old, weighed 18–20 g) used in this study were obtained from Beijing Vital River Laboratory Animal Technology Corporation. All mice were maintained in a specific pathogen-free (SPF) conditions (temperature: 25±2 °C, humidity: 50%±5%) under a standard 12-hour dark/light cycle, supplied with the standard rodent diet and water in their cages during the entire experimental process.
All animal experiments in this study were approved by the ethical committees of the Nanjing Medical University and were in accordance with the regulations on laboratory animals care and use in scientific research published by National Institutes of Health (No. 85-23, revised 1996).
In vivo ISO model and CRP treatment
The animals were randomly separated into 4 experimental groups: Control; CRP treatment; ISO treatment; ISO + CRP treatment, eight mice in each group. ISO (Isoprenaline, Sigma-Aldrich, St Louis, USA) was dissolved in sterile saline and intraperitoneally injected once daily (30 mg/kg/day for 21 days consecutively) to induce mice chronic HF, while the control group was injected with sterile saline. CRP was obtained from Shijiazhuang Yiling pharmaceutical Co., Ltd (Shijiazhuang, Hebei, China). CRP contains approximately 140 chemical compounds, and normally the composition of Hesperidin (no less than 2.5%) is chosen as an indicator for the determination of the quantity of ingredients (18). In this study, CRP was dissolved in sterile saline and was given to mice according to the administration method of QLQX as previously described (14,16). The dosage of CRP used in this study was 0.5 g/kg/d and CRP was given intragastrically to ISO-infused and control mice once daily for 3 weeks.
To explore whether CRP exerts cardio-beneficial effects through upregulating PPARγ expression, mice were randomly divided into 4 groups: control; ISO treatment; ISO + CRP treatment, ISO + CRP +PPARγ inhibitor (T0070907, Selleck Chemical, St Louis, USA) treatment. ISO and CRP were given to mice the same methods as above. PPARγ inhibitor (T0070907) was intraperitoneally (1 mg/kg/d for 21 days) injected to ISO-infused and/or CRP-treated mice.
Left ventricular function was measured by echocardiography the day after the final administration. Then the mice were sacrificed and the heart tissues were harvested for further analysis.
Echocardiography
The mice were anesthetized with 2–4% isoflurane. Trans-thoracic echocardiography was performed by a Vevo 2100 (Visual Sonics Inc., Toronto, Ontario, Canada) equipped with a 35 MHz transducer. Two-dimensional M-mode images at the papillary muscle level were used for assessing cardiac parameters, including left ventricular fractional shortening (LVFS) and left ventricular ejection fraction (LVEF).
Hematoxylin-eosin (HE) staining
HE staining of paraffin-embedded mice heart sections was carried out as previously described (16) and was observed by the Nikon Eclipse microscope with NIS Elements software. At least 10 fields of view were analyzed for each section. Image J software was used to measure the myocardial cross-sectional area and to evaluate the morphological changes and cardiomyocyte size.
Masson’s trichrome staining
Masson’s Trichrome staining of paraffin-embedded mice heart sections was performed to examine the myocardial fibrosis as previously described (14). Images were captured by the Nikon Eclipse microscope with NIS Elements software. At least 20 fields of view were analyzed for each section. Image J software was used to calculate the degree of cardiac fibrosis (the ratio of fibrotic area to total myocardial area).
Neonatal rat ventricular cardiomyocytes (NRVMs) isolation, culture and treatment
The neonatal Sprague-Dawley rat pups (0–3 days old) were provided by Experimental Animal Center of Nanjing Medical University. NRVMs were obtained from heart ventricles by trypsin collagenase digestion and by Percoll gradient centrifugation as previously described (16,17). NRVMs were counted and seeded in the corresponding plastic dishes coated with 1% gelatin in advance.
NRVMs were cultured with high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Pasadena, CA, USA) with 5% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA), 10% horse serum (HS) (Gibco, Carlsbad, CA, USA), 1% penicillin-streptomycin for 24 hours. Then, NRVMs were cultured in serum starvation medium (DMEM containing 1% FBS) for another 24 hours to synchronize the cells. After that, NRVMs were treated with ISO and/or CRP for 48 hours with reagents in the serum starvation medium and harvested for further analysis.
To investigate the protective effects of CRP on cardiac pathological hypertrophy induced by ISO treatment in vitro, NRVMs were divide into four groups and were treated as follows: control group; CRP group (0.5 µg/mL CRP); ISO group (10 µM ISO); ISO + CRP group (10 µM ISO and 0.5 µg/mL CRP).
To explore whether PPARγ mediates the protective effects of CRP against ISO-induced cardiac pathological hypertrophy in vitro, NRVMs were divided into five groups, control, ISO group, ISO + CRP group, ISO + CRP +PPARγ agonist Rosiglitazone (10 µM ISO, 0.5 µg/mL CRP and 1 µM PPARγ agonist) group, ISO + CRP +PPARγ inhibitor T0070907 (10 µM ISO, 0.5 µg/mL CRP and 1 µM PPARγ inhibitor) group.
Immunofluorescence staining
The NRVMs were fixed with 4% paraformaldehyde for 30 min and washed 3 times, each time for 5 min with phosphate buffer saline (1×, PH 7.2–7.4). Next, the cells were permeabilized with 0.2% Triton X-100 for 20 min and blocked with 10% goat serum in PBS for 1h at room temperature. Subsequently, the cells were incubated with mouse α-actinin monoclonal antibody (1:200, Sigma-Aldrich, St Louis, MO, USA) overnight at 4 °C. The cells were washed three times with PBS buffer, then were incubated with FITC labelled secondary IgG antibody (1:200, Jackson, USA) at 37 °C in dark for 2 h. Finally, the cells were stained with DAPI (1:100, Sigma-Aldrich’s Louis, USA) to label the nuclei. To measure cell surface area, the cells were observed by a fluorescence microscope (Carl Zeiss, Oberkochen, Germany). At least 600 cardiomyocytes in 60 fields of view were examined in each group. The cell size was measured by Image J software.
Quantitative real-time PCR analysis (qRT-PCR)
Total RNA was extracted from mice heart samples or NRVMs by Trizol reagent according to manufacturer’s protocol (Takara, Japan). Then RNA was quantified using Nanodrop 2000 pectrophotometer. RNA (500 ng) was reverse transcribed using iScriptTM cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). QRT-PCR was performed using SYBR Green qPCR Master Mix (Bio-Rad, Hercules, CA, USA) kit and was detected with ABI-7900 Real-Time PCR Detection System (7900HT, Applied Biosystems, CA, USA). Relative mRNA expression was determined using the 2-△△Ct method. The forward and reverse primer sequences used in this study were listed as follows (5'-3' sequence): Rat-atrial natriuretic polypeptide (ANP): GAG CAA ATC CCG TAT ACA GTGC, ATC TTC TAC CGG CAT CTT CTCC; Rat-brain natriuretic polypeptide (BNP): GCT GCT GGA GCT GAT AAG AGAA, GTT CTT TTG TAG GGC CTT GGTC; Rat-Glyceraldehyde 3-phosphate dehydrogenase antibody (GAPDH): AAG CTC ACT GGC ATG GCCTT, CGG CAT GTC AGA TCC ACAAC; Mouse-ANP: AGG CAG TCG ATT CTG CTT, CGT GAT AGA TGA AGG CAG GAAG; Mouse-BNP: TAG CCA GTC TCC AGA GCA ATTC, TTG GTC CTT CAA GAG CTG TCTC; Mouse-GAPDH: CCT TCC GTG TTC CTA CCCC, GCC CAA GAT GCC CTT CAGT. GAPDH was used as an internal control.
Western blotting assay
Total protein was extracted from mice heart samples or NRVMs lysed by RIPA buffer (P0013C, Beyondtime) supplemented with 1 mM PMSF (ST505, Beyondtime). Equivalent protein was separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. Subsequently, PVDF membranes were blocked with 5% milk for 2 hours and then incubated with the primary antibodies overnight at 4 °C. PVDF membranes were washed 3 times with Tris Buffered Saline+Tween-20 (TBS-T) and incubated with HRP labeled secondary IgG antibody at room temperature for 2 hours. The intensity of protein bands was visualized by Lab Works software (Bio-Rad, USA) and analyzed by Image J software. The primary antibodies used in this study were listed as follows: Proliferator-activated receptor-gamma (PPARγ, 1:1,000, Proteintech Group, Wuhan, China), PPARγ coactivator 1-alpha (PGC-1α, 1:1,000, Novus Biologicals, Littleton, COLO, USA), B-cell lymphoma 2 (Bcl-2, 1:1,000, Cell Signaling Technology, Boston, Massachusetts, USA), Bcl-2-associated X protein (Bax, 1:1000, Cell Signaling Technology, Boston, Massachusetts, USA), caspase 3 (1:1,000, Proteintech Group, Wuhan, China), collagen type 1 (Collagen 1, 1:1,000, Proteintech Group, Wuhan, China), collagen type 3 (Collagen 3, 1:1,000, Proteintech Group, Wuhan, China), α-smooth muscle actin (α-SMA, 1:1,000, Proteintech Group, Wuhan, China). β-tubulin (1:1,000, Bioworld Technology, Minnesota, USA) and Glyceraldehyde 3-phosphate dehydrogenase antibody (GAPDH, 1:1,000, Kangchen, Shanghai, China) were used as loading control.
Statistical analysis
All data were presented as mean ± SD. Comparisons between two groups were performed by an independent-sample t-test. Comparisons among more than two groups were assessed by one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test. All statistical analyses were performed with GraphPad Prism 6.0 Software. P<0.05 was considered statistically significant.
Results
CRP attenuates ISO-induced cardiac dysfunction and pathological hypertrophy
The C57BL/6 male mice were intraperitoneally injected with ISO once daily for 21 days consecutively. After the final injection, cardiac systolic function was detected by echocardiography (Figure 1A). Compared to the control group, left ventricular ejection fraction (LVEF%) and fraction shortening (LVFS%) were decreased in ISO-infused mice, indicating that long-term ISO infusion deteriorated the myocardial function. The significantly improvement of ISO-induced cardiac dysfunction was found in CRP-treated mice. HE staining analysis of heart sections showed that cardiomyocyte cross-sectional area in ISO-induced mice was increased, and CRP treatment could significantly relieve ISO-induced cardiac pathological hypertrophy (Figure 1B). Furthermore, qRT-PCR analysis showed that upregulated expression of hypertrophic marker ANP and BNP in ISO-infused mice were reversed in CRP-treated mice (Figure 1C). The protective effects of CRP were further examined by in ISO and/or CRP-treated NRVMs in vitro. Comparation of the size of NRVMs 48 hours after ISO and/or CRP treatment measured by immunostaining analysis, showed that CRP could alleviate ISO-induced pathological hypertrophy of NRVMs (Figure 1D). Similarly, the ANP and BNP expression were found upregulated in ISO-treated NRVMs and were downregulated by CRP administration (Figure 1E). All these in vivo and in vitro data above demonstrate that CRP has cardio-beneficial effects.
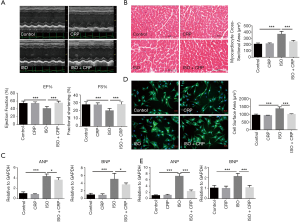
CRP reduces ISO-induced cardiac fibrosis and apoptosis
Long-term ISO infusion was found to impair heart function and cause abnormal apoptosis of cardiomyocyte, excessive proliferation of cardiac fibroblast and cardiac deposition of excessive extracellular matrix protein like collagen type I and collagen type III. Masson’s trichrome staining analysis showed that the elevated cardiac fibrosis in ISO-treated mice was decreased by the application of CRP (Figure 2A). Compared to ISO-infused mice, significantly decreased cardiac deposition of collagen type I, collagen type III and α-SMA was found in ISO and CPR co-treated mice, further demonstrating that CRP could prevent the progression of cardiac fibrosis (Figure 2B). Moreover, western blotting analysis revealed the increased ratio of pro-apoptotic molecule (Bax) to anti-apoptotic molecule (Bcl-2) and activated cleaved-caspase-3 to caspase-3 in ISO-treated mice, was obviously decreased by CRP administration (Figure 2C), suggesting that CRP could prevent cardiac apoptosis induced by ISO infusion.
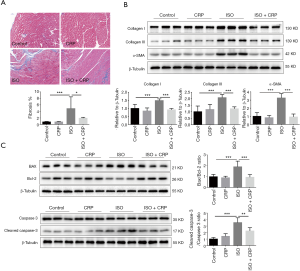
CRP protects against ISO-induced cardiac remodeling via activation of PPARγ
PPARs and its coactivator PGC-1α have been found to play critical roles in the regulation of energy metabolic processes. Our previous studies showed that PPARγ and PGC-1α were downregulated during ISO-induced cardiac remodeling. Western blotting analysis of extracts from ISO-infused mice hearts (Figure 3A) or ISO-treated NRVMs (Figure 3B), showed that the expression of PPARγ and PGC-1α was downregulated and CRP treatment could significantly increase PPARγ and PGC-1α expression, indicating that PPARγ and PGC-1α maybe participated in CRP-mediated improvement of ISO-induced cardiac dysfunction.
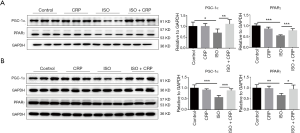
The protective efficacy of CRP on ISO-induced myocardial dysfunction and pathological hypertrophy was abolished by PPARγ inhibitor
To investigate whether the PPARγ signaling pathway was essential for CRP in rescue of ISO-induced cardiac dysfunction, the mice and the NRVMs were both treated with PPARγ inhibitor (T0070907). Western blot analysis of extracts of heart samples from mice treated with ISO, CRP combined with PPARγ inhibitor showed that PPARγ inhibitor could effectively downregulate the PPARγ expression (Figure 4A). Left ventricular function of PPARγ inhibitor-treated mice detected by echocardiography (Figure 4B) showed that cardio-protective effects of CRP to ISO-infused mice were blocked. Both HE staining analysis of heart sections (Figure 4C) and immunofluorescent staining of NRVMs (Figure 4D) showed that CRP mediated improvement of cardiomyocyte pathological hypertrophy caused by ISO stimulation, was inhibited by the application of PPARγ inhibitor. The CRP mediated downregulation of hypertrophic maker ANP and BNP in ISO-infused mice (Figure 4E) or ISO-treated NRVMs (Figure 4F) was reversed by PPARγ inhibitor examined by qRT-PCR analysis. Moreover, compared cell size (Figure 4D) and hypertrophic marker ANP, BNP expression levels of NRVMs (Figure 4F) treated with or without PPARγ agonist (rosiglitazone), similar protective effects were found in these two groups. PPARγ agonist treatment did not further enhance the beneficial effects of CRP on ISO-induced pathological hypertrophy, indicating that CRP is participated in cardiac protection mainly through PPARγ upregulation.

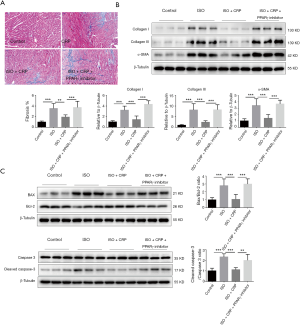
The protective efficacy of CRP on ISO-induced cardiac fibrosis and apoptosis was abrogated by PPARγ inhibitor
Comparation of the ratio of fibrotic area to total myocardial area determined by Masson’s trichrome staining analysis (Figure 5A) and the deposition of fibrosis-related proteins including collagen type I, collagen type III and α-SMA evaluated by western blot analysis (Figure 5B) of heart samples treated with ISO, CRP combined with PPARγ inhibitor, showed that blocking CRP-mediated PPARγ activation impaired its beneficial effects on ISO-induced cardiac fibrosis. Western blot analysis of the ratio of Bax to Bcl-2, the activated cleaved-caspase-3 to caspase-3, showed that the protective effects on cardiac apoptosis in ISO-infused mice mediated by CRP were suppressed when cotreated with PPARγ inhibitor (Figure 5C).
Consequently, all above results demonstrated that CRP could effectively upregulate the expression of PPARγ and its coactivator PGC-1α, thus exerts beneficial effects on myocardial pathological hypertrophy, cardiac fibrosis and apoptosis caused by sustained ISO activation.
Discussion
The long-term infusion of β-adrenergic receptor agonist, such as isoproterenol (ISO), is widely applied in the establishment of myocardial injury models in animal studies (33). There are two main methods to administer exogenous ISO to mice, either through osmotic pump continuous infusion or by repeated daily injection, both will eventually induce comparable cardiac hypertrophy (34). Repeated intraperitoneal injection or subcutaneous injection functions in a pulsatile fashion and results in greater peak concentrations, while osmotic pump infusion will maintain a stable dose and produce continuous stimulation to the mice, which may lead to receptor desensitization and downregulation and thus reduced effectiveness. Since many hormones are produced in a pulsatile fashion, compared two methods, daily injection might be the more effective means to induce HF. ISO, a synthetic catecholamine, short-termly given to mice, causes the activation of adrenergic system and neurohumoral system, results in increased beating rate of heart, hemodynamic stresses and contractile force and enhances cardiac output and oxygen consumption. However, when ISO infusion is sustained, myocardial ischemia and injury occurred. Excessive adrenaline activity destroys the homeostasis of the heart, changes its morphology and gene expression pattern and induces cardiomyocyte apoptosis and interstitial fibrosis (1,35,36). Cardiac apoptosis and fibrosis resulted from cardiac remodeling eventually impair heart function and induce HF (37,38). To investigate the potential new drugs to treat ISO-induced cardiac pathological hypertrophy and remodeling could shed light on dealing with chronic HF.
Previously, we studied the therapeutic effect of QLQX, a traditional Chinese medicine formula on multiple CVDs, including HF, myocardial infarction, as well as cardiac pathological hypertrophy and myocardial ischemia/ reperfusion (I/R) injury (13-16). Mechanistically, our previous studies showed that the beneficial effects of QLQX were closely related to the activation of PPARγ and its coactivator PGC-1α. When PPARγ upregulation mediated by QLQX was inhibited by PPARγ inhibitor, the protective efficacy of QLQX was abrogated, indicating that effective ingredients in QLQX may play a therapeutic role in heart protection similar as PPARγ agonist. QLQX combined with PPARγ agonist (rosiglitazone) treatment did not further enhance the beneficial effects of QLQX on cardiac remodeling, further confirming that QLQX might regulate cardiac remodeling in the same way as PPARγ agonist. Since QLQX is extracts of 11 herbal medicines, which consists numerous effective ingredients, this study focuses on investigating if a single herb could provide similar protective effects on HF as QLQX. We found CRP administration could protect against ISO-induced HF. Analysis of ISO-treated NRVMs and ISO-infused mice, showed that CRP could decrease cardiac hypertrophy, alleviate cardiac fibrosis and apoptosis and reduce the deposition of extracellular proteins. Similar as the therapeutic function of QLQX, CRP provided cardio-beneficial effects via PPARγ activation and could be blocked by the application of PPARγ inhibitor.
Pathological cardiac hypertrophy, a major and independent risk factor for HF, is characterized by cardiomyocyte hypertrophy, disarray and interstitial fibrosis, myocytes apoptosis and releasing of ANP and BNP (39). Compared with the saline-treated control group, CRP obviously improved the ISO-induced cardiac dysfunction, decreased the cardiomyocytes size, preserved the morphology of the myocardium and reduced the mRNA level of ANP and BNP, indicating its effective role in reversing cardiac hypertrophy and pathological reconstruction. Until now, up to 140 chemical components from CRP have been isolated and identified, including alkaloids, flavonoids, and essential oils (19). Flavonoids, such as hesperidin, nobiletin were considered as the primary bioactive constituents in CRP. Hesperidin has been found to be effective in inhibiting cardiac remodeling via blocking PKCα/βII-AKT, JNK and TGFβ1-Smad signaling pathways (40). Nobiletin was reported to inhibit cardiomyocyte hypertrophy and HF through regulation of NBP1 activity (41). However, further studies are still needed to verify which chemical component in CRP is responsible for its protective effects on cardiac pathological hypertrophy.
Pathological myocardial fibrosis caused by the abnormal deposition of extracellular protein and excessive proliferation of cardiac fibroblasts, is the key contributor to cardiac dysfunction, arrhythmia and HF (42,43). ISO-induced cardiac fibrosis marked by increased deposition of collagen type I, collagen type III, α-SMA and histopathology accumulation of collagen, was significantly suppressed by the application of CRP in our study. Previous studies showed that nobiletin could decrease cardiac dysfunction and interstitial fibrosis via suppressing JNK, P38, and NF-κB signaling pathways (44). Whether the function of CRP in regulation of cardiac fibrosis are related to nobiletin or the other effective component needed to be further studied.
Oxidative stress (OS) and excessive free radicals produced by mitochondria due to sustained ISO infusion result in DNA damage and membrane peroxidation, enhance membrane permeability and release of Bax and cytochrome C into cytoplasm from mitochondria, activate caspase and eventually increase cell apoptosis (45,46). The administration of CRP in ISO-infused mice decreased the ratio of Bax to Bcl-2 and activated Cleaved-caspase3 to Caspase3, suggesting that CRP reduced cardiac apoptosis. Hesperidin was found played an anti-apoptotic role in regressing cardiac hypertrophy by activating PPARγ (27). Among approximately 140 components in CRP, hesperidin is the main effective ingredient and was well characterized. Many studies show that hesperidin could protect and preserve cardiac function, however whether the anti-apoptosis effects of CRP is dependent on hesperidin are still unclear.
CVDs have become a major problem affecting the health of all ages (47). There is a critical need to identify new molecular therapeutic targets through laboratory researches (48). In this study, we found similar as QLQX, CRP-mediated PPARγ activation is responsible for its therapeutic effects on cardiac remodeling. The new findings in this study further demonstrate that PPARγ and PGC-1α could be potential therapeutic targets for the treatment of cardiac dysfunction and HF.
Conclusions
In conclusion, the present study reveals for the first time that CRP was effective in preventing cardiac hypertrophy, fibrosis and apoptosis and preserving heart function in ISO-induced mice through meditating the activation of PPARγ and PGC-1α. More importantly, the new findings in this study provide experimental evidence for the application of CRP to treat cardiac dysfunction and HF. Clinical trials are still required to evaluate the potential clinical use of CRP in the future.
Acknowledgments
Funding: This study was supported by the grants from National Natural Science Foundation of China (81730106 and 81670347 to X Li and 81770396 to H Zhang). National Key Research and Development Program (2017YFC1700505 and 2017YFC1700401) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD20102013 to X Li).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-2200
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-2200
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-2200
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2200). Dr. XL received research grants from Shijiazhuang Yiling Pharmaceutical Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments in this study were approved by the ethical committees of the Nanjing Medical University and were in accordance with the regulations on laboratory animals care and use in scientific research published by National Institutes of Health (No. 85-23, revised 1996).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol 2012;21:365-71. [Crossref] [PubMed]
- Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016;13:368-78. [Crossref] [PubMed]
- Lopaschuk GD, Ussher JR. Evolving Concepts of Myocardial Energy Metabolism: More Than Just Fats and Carbohydrates. Circ Res 2016;119:1173-6. [Crossref] [PubMed]
- Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med 2002;53:409-35. [Crossref] [PubMed]
- Singh A, Borah AK, Deka K, et al. Arginylation regulates adipogenesis by regulating expression of PPARgamma at transcript and protein level. Biochim Biophys Acta Mol Cell Biol Lipids 2019;1864:596-607. [Crossref] [PubMed]
- Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 2011;1813:1269-78. [Crossref] [PubMed]
- Krishnan J, Suter M, Windak R, et al. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab 2009;9:512-24. [Crossref] [PubMed]
- Aubert G, Martin OJ, Horton JL, et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016;133:698-705. [Crossref] [PubMed]
- Pascual F, Coleman RA. Fuel availability and fate in cardiac metabolism: A tale of two substrates. Biochim Biophys Acta 2016;1861:1425-33. [Crossref] [PubMed]
- Shiomi T, Tsutsui H, Hayashidani S, et al. Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 2002;106:3126-32. [Crossref] [PubMed]
- Yuan Z, Liu Y, Liu Y, et al. Cardioprotective effects of peroxisome proliferator activated receptor gamma activators on acute myocarditis: anti-inflammatory actions associated with nuclear factor kappaB blockade. Heart 2005;91:1203-8. [Crossref] [PubMed]
- Tikellis C, Jandeleit-Dahm KA, Sheehy K, et al. Reduced plaque formation induced by rosiglitazone in an STZ-diabetes mouse model of atherosclerosis is associated with downregulation of adhesion molecules. Atherosclerosis 2008;199:55-64. [Crossref] [PubMed]
- Tao L, Shen S, Fu S, et al. Traditional Chinese Medication Qiliqiangxin attenuates cardiac remodeling after acute myocardial infarction in mice. Sci Rep 2015;5:8374. [Crossref] [PubMed]
- Shen S, Jiang H, Bei Y, et al. Qiliqiangxin Attenuates Adverse Cardiac Remodeling after Myocardial Infarction in Ovariectomized Mice via Activation of PPARgamma. Cell Physiol Biochem 2017;42:876-88. [Crossref] [PubMed]
- Zhang J, Huang M, Shen S, et al. Qiliqiangxin attenuates isoproterenol-induced cardiac remodeling in mice. Am J Transl Res 2017;9:5585-93. [PubMed]
- Gao RR, Wu XD, Jiang HM, et al. Traditional Chinese medicine Qiliqiangxin attenuates phenylephrine-induced cardiac hypertrophy via upregulating PPARgamma and PGC-1alpha. Ann Transl Med 2018;6:153. [Crossref] [PubMed]
- Zhang H, Li S, Zhou Q, et al. Qiliqiangxin Attenuates Phenylephrine-Induced Cardiac Hypertrophy through Downregulation of MiR-199a-5p. Cell Physiol Biochem 2016;38:1743-51. [Crossref] [PubMed]
- China SPCoPsRo. Pharmacopoeia of the People's Republic of China. 10th ed. Beijing, China: China Medical Science and Technology Press; 2015.
- Yu X, Sun S, Guo Y, et al. Citri Reticulatae Pericarpium (Chenpi): Botany, ethnopharmacology, phytochemistry, and pharmacology of a frequently used traditional Chinese medicine. J Ethnopharmacol 2018;220:265-82. [Crossref] [PubMed]
- Guo J, Tao H, Cao Y, et al. Prevention of Obesity and Type 2 Diabetes with Aged Citrus Peel (Chenpi) Extract. J Agric Food Chem 2016;64:2053-61. [Crossref] [PubMed]
- Bhargava P, Verma VK, Malik S, et al. Hesperidin regresses cardiac hypertrophy by virtue of PPAR-gamma agonistic, anti-inflammatory, antiapoptotic, and antioxidant properties. J Biochem Mol Toxicol 2019;33:e22283. [Crossref] [PubMed]
- Seyedrezazadeh E, Kolahian S, Shahbazfar AA, et al. Effects of the flavanone combination hesperetin-naringenin, and orange and grapefruit juices, on airway inflammation and remodeling in a murine asthma model. Phytother Res 2015;29:591-8. [Crossref] [PubMed]
- Byun EB, Kim HM, Song HY, et al. Hesperidin structurally modified by gamma irradiation induces apoptosis in murine melanoma B16BL6 cells and inhibits both subcutaneous tumor growth and metastasis in C57BL/6 mice. Food Chem Toxicol 2019;127:19-30. [Crossref] [PubMed]
- Yu JJ, Su J, Lv GY. Research progress in anti-cardiovascular and cerebrovascular disease activity of Cirri Reticulatae Pericarpium. Chinese Traditional and Herbal Drugs 2016;47:3127-32.
- Roohbakhsh A, Parhiz H, Soltani F, et al. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci 2015;124:64-74. [Crossref] [PubMed]
- Selvaraj P, Pugalendi KV. Hesperidin, a flavanone glycoside, on lipid peroxidation and antioxidant status in experimental myocardial ischemic rats. Redox Rep 2010;15:217-23. [Crossref] [PubMed]
- Agrawal YO, Sharma PK, Shrivastava B, et al. Hesperidin blunts streptozotocin-isoproternol induced myocardial toxicity in rats by altering of PPAR-γ receptor. Chem Biol Interact 2014;219:211-20. [Crossref] [PubMed]
- Parkar NA, Bhatt LK, Addepalli V. Efficacy of nobiletin, a citrus flavonoid, in the treatment of the cardiovascular dysfunction of diabetes in rats. Food Funct 2016;7:3121-9. [Crossref] [PubMed]
- Zhang BF, Jiang H, Chen J, et al. Nobiletin ameliorates myocardial ischemia and reperfusion injury by attenuating endoplasmic reticulum stress-associated apoptosis through regulation of the PI3K/AKT signal pathway. Int Immunopharmacol 2019;73:98-107. [Crossref] [PubMed]
- Testai L, Martelli A, Marino A, et al. The activation of mitochondrial BK potassium channels contributes to the protective effects of naringenin against myocardial ischemia/reperfusion injury. Biochem Pharmacol 2013;85:1634-43. [Crossref] [PubMed]
- Sun LJ, Qiao W, Xiao YJ, et al. Naringin mitigates myocardial strain and the inflammatory response in sepsis-induced myocardial dysfunction through regulation of PI3K/AKT/NF-κB pathway. Int Immunopharmacol 2019;75:105782. [Crossref] [PubMed]
- Ou LJ, Sun XP, Liu QD, et al. Effects of Rhizoma zingiberis and Pericarpium citri reticulatae extracts on myocardial ischemia in rats. Zhong Yao Cai 2009;32:1723-6. [PubMed]
- Nichtova Z, Novotova M, Kralova E, et al. Morphological and functional characteristics of models of experimental myocardial injury induced by isoproterenol. Gen Physiol Biophys 2012;31:141-51. [Crossref] [PubMed]
- Hohimer AR, Davis LE, Hatton DC. Repeated daily injections and osmotic pump infusion of isoproterenol cause similar increases in cardiac mass but have different effects on blood pressure. Can J Physiol Pharmacol 2005;83:191-7. [Crossref] [PubMed]
- Branco AF, Moreira AC, Cunha-Oliveira T, et al. beta-adrenergic over-stimulation and cardio-myocyte apoptosis: two receptors, one organelle, two fates? Curr Drug Targets 2014;15:956-64. [Crossref] [PubMed]
- Piek A, de Boer RA, Sillje HH. The fibrosis-cell death axis in heart failure. Heart Fail Rev 2016;21:199-211. [Crossref] [PubMed]
- Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res 2013;113:739-53. [Crossref] [PubMed]
- Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol 2017;14:30-8. [Crossref] [PubMed]
- Bernardo BC, Weeks KL, Pretorius L, et al. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther 2010;128:191-227. [Crossref] [PubMed]
- Deng W, Jiang D, Fang Y, et al. Hesperetin protects against cardiac remodelling induced by pressure overload in mice. J Mol Histol 2013;44:575-85. [Crossref] [PubMed]
- Sunagawa Y, Funamoto M, Suzuki A, et al. A Novel Target Molecule of Nobiletin Derived from Citrus Peels has a Therapeutic Potency Against the Development of Heart Failure. Eur Cardiol 2017;12:105. [Crossref] [PubMed]
- de Boer RA, De Keulenaer G, Bauersachs J, et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur J Heart Fail 2019;21:272-85. [Crossref] [PubMed]
- Frangogiannis NG. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med 2019;65:70-99. [Crossref] [PubMed]
- Zhang N, Yang Z, Xiang SZ, et al. Nobiletin attenuates cardiac dysfunction, oxidative stress, and inflammatory in streptozotocin: induced diabetic cardiomyopathy. Mol Cell Biochem 2016;417:87-96. [Crossref] [PubMed]
- Prabhu SD, Wang G, Luo J, et al. Beta-adrenergic receptor blockade modulates Bcl-X(S) expression and reduces apoptosis in failing myocardium. J Mol Cell Cardiol 2003;35:483-93. [Crossref] [PubMed]
- Sahu BD, Putcha UK, Kuncha M, et al. Carnosic acid promotes myocardial antioxidant response and prevents isoproterenol-induced myocardial oxidative stress and apoptosis in mice. Mol Cell Biochem 2014;394:163-76. [Crossref] [PubMed]
- Cristi-Montero C, Chillón P, Labayen I, et al. Cardiometabolic risk through an integrative classification combining physical activity and sedentary behavior in European adolescents: HELENA study. J Sport Health Sci 2019;8:55-62. [Crossref] [PubMed]
- Wang L, Lv Y, Li G, et al. MicroRNAs in heart and circulation during physical exercise. J Sport Health Sci 2018;7:433-41. [Crossref] [PubMed]


