Osteonecrosis of the knee: review
Introduction
First described by Ahlbäck et al. (1) in 1968, osteonecrosis of the knee can be a devastating disease that leads to end-stage arthritis of the knee. Osteonecrosis of the knee is the second most common affected location, following the hip (2). It remains a rare cause of knee pain for which treatment options and recommendations continue to evolve as we learn more about the etiology and pathophysiology of this disease (1).
Knee osteonecrosis has been delineated into three categories: spontaneous [also called primary osteonecrosis or osteonecrosis of the knee (SONK)], secondary (also called atraumatic, ischemic, or idiopathic osteonecrosis), and post-arthroscopic (2). SONK is considered to be the most common form of osteonecrosis of the knee, with a higher prevalence observed in patients over 50 years of age (3). Recent studies have reported a 3.4% and 9.4% incidence of SONK in persons older than 50 and 65 years of age, respectively (3). Conversely, secondary osteonecrosis is more commonly seen in younger patients, and is associated with a number of medical conditions and risk factors such as sickle cell disease, myeloproliferative disorders, alcohol, corticosteroids, and tobacco consumption. Post-arthroscopic osteonecrosis is the rarest form of osteonecrosis that affects the knee, however Cetik et al. (4) reported that it affected 4% of patients (n=50) following arthroscopic knee surgery, most commonly following meniscectomy.
Due to the paucity of studies describing the different alternatives in patients with knee osteonecrosis sub-stratified by spontaneous, secondary, and post-arthroscopic, we attempted to provide a broad overview of the etiologies, clinical presentation, radiographic findings, and most current treatment recommendations for each of these three entities.
SONK: etiology and pathophysiology
SONK is most commonly seen in patients who are 60 years of age or older. This disease more often affects women than men, and is typically unilateral (5). Although the true incidence is not known, it may be more prevalent than secondary osteonecrosis. However, the real prevalence may be underestimated because many patients who present with end-stage osteoarthritis may have actually had occult undiagnosed SONK (6). In addition, SONK is classically described as a focal, superficial subchondral lesion, mainly affecting the medial femoral condyle (7). This is supported by al-Rowaih et al., who demonstrated that in 109 patients who were diagnosed with SONK, the medial femoral condyle was affected 94% of the time (102 out of 109 knees) (8). Furthermore, the medial femoral condyle predominance has been purported to be as a result of varying blood blood supply between the medial and lateral condyles. A cadaveric study by Reddy and Fredericks demonstrated that the medial femoral condyle has limited intraosseous blood supply with apparent watershed areas, whereas, the lateral femoral condyle has both a rich intra- and extra-osseous vascular supply (9).
Historically, SONK was thought to occur secondary to ischemia, which would result in necrosis. However, recent evidence has demonstrated that it may be due to subchondral insufficiency fractures in osteopenic bone, with no evidence of necrosis (7). These insufficiency fractures may lead to fluid accumulation in the bone marrow, which results in subsequent edema with focal ischemia, and eventual necrosis (7). This is supported by a recent study demonstrating that there was a positive association between low bone mineral density and the incidence of SONK in women over 60 years of age (10). Akamatsu et al. reported that the mean lateral femoral and tibial condyle BMD was significantly lower in SONK group than in the OA group (P<0.001), however, they did not give an indication of a BMD level that should raise concern for SONK. Therefore, the term “SONK” may in fact be a misnomer, and authors have suggested it be redefined as an “unstable fracture resulting in bone death of the displaced fracture fragment” (6).
SONK: clinical presentation and diagnosis
Patients who have SONK usually present with acute onset of medial-sided knee pain not precipitated by trauma. Focal tenderness over the medial femoral condyle is the most common finding on physical examination (11). Patients often complain that pain is worse at night and on weight-bearing, frequently mimicking the pain experienced following a medial meniscus tear (6). At initial evaluation, plain anteroposterior (AP), lateral, and oblique radiographs should be obtained, although in the early course of the disease they are often negative, and in some cases remain negative for the full duration of clinical symptoms (12). Typical radiographic findings of late stage disease include radiolucencies or flattening of the affected condyle. Magnetic resonance imaging (MRI) is recommended for detection of earlier stages of the disease, due to its high sensitivity in detecting bone edema (13). Initial MRI characteristics of early stage osteonecrosis include a subchondral area of low signal intensity on T2-weighted images, focal epiphyseal contour depressions, and lines of low signal intensity deep in the affected condyle (14). Bone scintigraphy may show increased uptake in the affected condyle, however, this method is less sensitive in identifying disease than MRI (15). Therefore, bone scans are not indicated for the diagnosis of osteonecrosis, because of their poor sensitivity and specificity, with one study demonstrating that bone scanning detected only 56% of the lesions previously detected by MRI in the setting of multifocal disease, which was histopathologically confirmed (16).
The Koshino classification was the first classification for SONK described in 1979. There are four stages in the Koshino staging system, which are based on clinical and radiographic findings (17). A patient who has knee symptoms, but normal radiographic findings are classified as stage I. Stage II demonstrates the weight-bearing area with flattening and subchondral radiolucencies surrounded by osteosclerosis. Stage III demonstrates extension of the radiolucencies around the affected area and subchondral collapse. Stage IV is the degenerative phase with osteosclerosis and osteophyte formation around the condyles.
SONK: treatment
Patients who have SONK may be managed non-operatively or operatively based on symptoms and disease staging. The decision to treat epiphyseal lesions is based largely on the size of the osteonecrotic area, which is taken by measuring the greatest width in the anterior-posterior radiograph and the greatest length in the lateral radiograph. Small lesions (typically <3.5 centimeters squared) usually regress with non-surgical management, medium lesions (3.5 to 5.0 centimeters squared) may or may not regress, and large lesions (>5 centimeters squared) usually lead to condyle collapse (6,18). A study by Lotke et al. utilized another method of determining the size of the osteonecrotic lesion. Their method was to calculate the width of the lesion in the AP radiograph as a percentage of the affect femoral condyle. They found that in patients with lesions involving 32% of the medial femoral condyle, only 6 of 23 knees required surgical management, whereas, the patients with lesions involving more than 50% of the medial femoral condyle, all required prosthetic arthroplasty (11). A recent study by Juréus et al. also evaluated the long term outcomes in relation to need for major knee surgery in a cohort of 40 patients with osteonecrosis of the knee. After a 27 follow-up, of the 40 patients, 15 required arthroplasty, and 2 individuals required osteotomy. The majority of patients (6 out of 7) who had a lesion of greater than 40% of the affected femoral condyle required a knee prosthesis, whereas none of the ten patients with less than 20% of the condyle affected required surgery (19) (refer below and to Figure 1 for treatment algorithm).
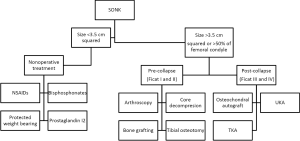
Nonsurgical management
Non-operative management is usually reserved for smaller lesions (<3.5 centimeters squared), which includes treatment with non-steroidal anti-inflammatory drugs (NSAIDs), protected weight bearing, and analgesics as needed (20). This was observed in a case series of patients who had early stage SONK diagnosed by MRI (n=20 knees), in which all patient’s symptoms resolved and their MRIs returned to normal within three to eight months with non-operative management (21).
Some authors have reported the potential efficacy of bisphosphonate drugs in preventing or postponing the need for surgery in patients who have SONK (22). Bisphosphonates are a group of drugs that are typically utilized in osteoporosis and bone malignancies. They exert their action by binding to bone, which subsequently is resorbed by osteoclasts. Once internalized by the osteoclast, the bisphosphonate then interferes with the cellular metabolism, leading to apoptosis and ultimately inhibition of bone resorption. Bisphosphonates have been shown to prevent subchondral collapse in osteonecrosis of the femoral head, by preventing resorption of the necrotic region (23). Jureus et al. evaluated the use of 70 mg of alendronate once a week for a minimum of 6 months in 17 patients who had SONK. The authors demonstrated that only 18% (3 out of 17) of the patients went on to eventual subchondral collapse, moreover, they noted that 2 of the 3 patients who failed treatment ended their medication regimen prematurely (22). However, a randomized, placebo-controlled trial by Meier et al. found that there was no benefit of bisphosphonates over anti-inflammatory medications for the treatment of early stage SONK (24). The authors reported that after 12 weeks, the mean pain score was reduced in both ibandronate and placebo cohorts (mean change, −2.98 versus −3.59), and groups after 24 and 48 weeks radiological outcome measures were comparable between cohorts (24).
Another area of non-operative management currently being studied is pulsed electromagnetic fields therapy. A recent case series by Marcheggiani Muccioli et al. evaluated whether pulsed electromagnetic fields treatments improved symptoms in the early stage of SONK. The authors found that at 6 months, pulsed electromagnetic fields significantly reduced pain measured with the Visual Analog Pain Scale (73.2 to 29.6, P<0.0001) and size of the necrotic lesion defined as a reduction of the total whole-organ MRI (WORMS) mean score (P<0.0001) and a reduction in the mean femoral bone marrow lesion’s area (P<0.05) (25).
Surgical management
Surgical management should be considered when patients do not improve clinically or radiographically after three months of non-operative treatment, as well as in patients who present with osteonecrotic lesions larger than 5 centimeters (6,20). Lotke et al. demonstrated that patients who had SONK with large lesions (n=79 patients), defined as lesions involving more than 50% of the medial femoral condylar surface, all progressed to collapse quickly without surgical intervention (11).
Joint-preserving surgical techniques such as arthroscopic meniscal tear repairs or other associated pathology, core decompression, and osteochondral autograft may successfully postpone the need for joint arthroplasty (26). Miller et al. suggested performing arthroscopic debridement for early treatment of SONK (27). Akgun et al. performed arthroscopic microfracture in 26 patients with SONK who failed non-operative treatment measures. The authors demonstrated that there were clinical improvement in 96% of patients at a mean follow-up of 27 months (mean Cincinnati Knee Rating System improved from six preoperatively and approximately 14 points post-operatively) (28).
Core decompression may be used to treat refractory SONK. Forst et al. reported a series of 16 patients who had SONK (15 stage 1 and 2, and one with flattening of the affected femoral condyle), treated with core decompression. The authors demonstrated that all patients reported improvement in knee pain immediately after core decompression. Additionally, the authors noted that of the 15 patients who had early stage disease demonstrated normalization of bone marrow signal on MRI at latest follow-up (29). Similarly, Deie et al. analyzed 12 patients who had SONK treated with core decompression and calcium hydroxyapatite artificial bone graft. All patients in this series reported improved knee pain, and avoided total knee arthroplasty (TKA) at a mean follow-up of 25 months (30).
However, patients who have progressed to subchondral collapse may benefit more from osteochondral autograft due to restoration of the cartilage surface. Osteochondral autografting has two main advantages: (I) reconstructing the mechanically durable articular bone plate and the articular cartilage; and (II) removal of collapsed tissue. Duany et al. reported excellent outcomes with a mean Knee Society Score of 85 points in 9 patients who underwent osteochondral autografts for the treatment of SONK (26). Similarly, Tanaka et al. reported on 6 patients who had SONK undergoing osteochondral autograft, in which all patients reported favorable pain relief after a mean follow-up of 28 months (31).
High tibial osteotomy is also known to be a reliable procedure with excellent patient outcomes (20). This procedure is indicated in younger or active patients who wish to avoid prosthetic replacement. The osteotomy is preformed to offload the proximal tibial metaphysis, therefore unloading the affected femoral condyle and potentially promoting healing of the lesion (20). A valgus producing medial opening wedge osteotomy may be utilized in SONK, as the disease typically affects the medial compartment of the knee. Saito et al. reported a procedure that combined opening-wedge high tibial osteotomy using a stable plate fixation system with a bone substitute. They reported significantly improved mean Knee Society Scores objective and functional scores at 5 to 10 years follow-up, from 50 to 88 points, and from 57 to 89 points, respectively (P<0.05) (32). Similarly, Takeuchi et al. evaluated the use of opening wedge high tibial osteotomy in combination with drilling of the necrotic lesion to treat patients who had spontaneous osteonecrosis of the medial femoral condyle of the knee at mean follow-up of 40 months (n=30 patients, 30 knees). They demonstrated the mean objective and functional Knee Society Score, with significant improvements from 51 to 93 points, and from 58 to 93 points, respectively (P<0.05) at final follow-up.
Unicompartmental knee arthroplasty (UKA) is a well known treatment for unicompartmental osteoarthritis. However, recent studies have shown that it can also be a successful treatment alternative for SONK, because there is frequent involvement of a single condyle (33). A study by Heyse et al. evaluated the functional outcomes in 52 cases of SONK of the femoral condyle treated with UKA. The authors described that a mean follow-up of approximately 11 years the KSS and WOMAC scores were 173 and approximately 8 points, respectively (33). Similarly, Langdown et al. compared the outcomes of medial UKA in 29 knees (27 patients) using the Oxford prosthesis for end-stage focal SONK (26 and 3 knees with medial femoral condyle and tibial plateau involvement, respectively) to 28 knees (26 patients) undergoing the same procedure for osteoarthritis. The authors demonstrated that at 1 year follow-up, no implants failed in either cohort. However, for patients who have extensive disease affecting multiple compartments, or in those whom more conservative measures have failed, TKA may be the only successful alternative. Myers et al. evaluated patients who had SONK and underwent TKA (n=148 patients). The authors described a significant increase in knee scores from a pre-operatively score of 57 points to a post-operatively score of 85 points (P<0.05) (34).
In summary, the decision to treat is based on the size of the osteonecrotic lesion with small lesions may regress with non-surgical management, whereas medium-to-large lesions may or may not regress and will likely require surgical intervention. When undergoing surgical intervention if the patient is in the pre-collapse state, joint preserving procedures should be attempted, however if post-collapse has occurred surgeons should consider total joint arthroplasty.
Secondary osteonecrosis: etiology and pathophysiology
The incidence of secondary or atraumatic osteonecrosis of the knee is approximately 90% less than the incidence of hip osteonecrosis. It usually affects patients younger than 45 years of age and frequently involves multiple lesions affecting numerous joints. Dissimilar to SONK, secondary osteonecrosis may involve both femoral condyles, as well as the epiphysis, diaphysis, and metaphysis of the involved femur and/or tibia. Most of the patients who have this pathology have bilateral involvement (>80%). However, like spontaneous osteonecrosis, it has a female predominance (2). Secondary osteonecrosis has been associated with numerous conditions and risk factors that can be separated into direct causes (sickle cell disease, caisson disease, Gaucher’s disease, myeloproliferative disorders) and indirect associations (alcohol, corticosteroids, tobacco, obesity).
The two most common risk factors for secondary osteonecrosis are corticosteroid use and alcohol abuse (>90%) (2). Recent evidence suggests that corticosteroids and alcohol cause bone marrow adipose cell enlargement, which can contribute to an increased intra-osseous pressure that may lead to bone ischemia (35). This theory of bone marrow crowding may be reasonably extended to other conditions associated with osteonecrosis, such as myeloproliferative disorders and glycogen storage diseases such as Gaucher’s. Other risk factors such as tobacco use, sickle cell disease, and other coagulopathic disorders have mainly vaso-occlusive effects. Cigarette smoking is known to cause vasoconstriction, in addition to an oxidative damage to the vasculature, thus compromising the endothelium and predisposing to atherosclerosis (36). The irregularly shaped, deformable red blood cells found in sickle cell disease are more likely to adhere to vascular walls and clump together (cell sickling), leading to vascular occlusion (37). Similarly, nitrogen gas emboli seen in decompression sickness (Caisson disease) may cause direct vascular occlusion. In the same way that these conditions cause vaso-occlusion elsewhere in the body, they can cause vaso-occlusion in the bone and lead to ischemia.
Secondary: clinical presentation, classification, and diagnosis
In contrast to the sudden onset of pain in spontaneous osteonecrosis, patients who have secondary osteonecrosis will usually describe a gradual onset of pain over the affected area. The pain is most often located over a femoral condyle, but approximately 20% of the time, the tibial condyle may also be involved. Patients may also complain of pain in other joints, because secondary osteonecrosis frequently involves multiple joints (20).
Similar to SONK, AP and lateral plain radiographs and MRI are the mainstays of diagnosing secondary osteonecrosis. Instead of a single lesion, multiple lesions may be observed, and 80% of cases will have bilateral knee involvement. Additionally, while SONK lesions are isolated to the epiphysis, lesions of atraumatic osteonecrosis of the knee may be seen in the epiphysis, metaphysis, and/or diaphysis of the femur. Bone scans are not recommended for secondary osteonecrosis, as they have been shown to be less effective than MRI when identifying osteonecrotic lesions (15).
Two staging classification systems are utilized for secondary osteonecrosis of the knee: the Koshino staging system, which was originally developed for SONK, and the Modified Ficat and Arlet staging system. The latter has been adapted for the knee from the original version which described femoral head osteonecrosis (38). It is based on plain radiographs and includes four stages based on joint-space narrowing, subchondral collapse, and trabecular pattern. Stages I and II have a normal joint-space and no subchondral collapse, though stage II will have some evidence of sclerosis in the trabeculae. Stage III may have a normal or slightly narrowed joint space with subchondral collapse and a sequestered appearance of the trabeculae, the so-called “crescent sign”. Stage IV has both joint-space narrowing and subchondral collapse (20).
Secondary: treatment
Nonsurgical management
Symptomatic patients who have secondary osteonecrosis of the knee will almost uniformly need surgery. Therefore, nonsurgical management is recommended only for those patients who are asymptomatic (2). This is supported by Mont et al., who found that only 19% (8 of 41) of the patients who had symptomatic lesions and were managed with observation had satisfactory outcomes (Knee Society Score >80) at a mean follow-up of 8 years. The authors also described that 70% (29 of 41) of the patients with symptomatic ON went on to eventually require TKA (2). Of the subgroup of asymptomatic lesions that were treated with observation, 80% (8 of 10) were successful in avoiding arthroplasty and had no signs of radiographic progression. The group also noted that large epiphyseal lesions had a significantly worse prognosis than metaphyseal or diaphyseal lesions (P<0.05).
In addition to observation, recently, studies have shown that bisphosphonates and prostaglandin I-2 may have a potential option in managing secondary osteonecrosis of the knee. Jäger et al. evaluated 28 patients who had knee osteonecrosis and were treated with bisphosphonates (initially given pamidronate 120 mg i.v. divided in 3 to 4 perfusions over 2 weeks, followed by oral 70 mg of alendronate weekly for 4 to 6 months) (39). The authors demonstrated that there was a significant reduction of the VAS from 8 point to 5 points at 4 to 6 weeks (P<0.0001), and by 6 months there was an 80% decrease in the mean VAS. The authors noted that at a 6-month follow-up there was complete symptom resolution in 15 individuals, while only 6 patients had minimal residual pain, and 18 patients had complete resolution of bone marrow edema on MRI with a substantial reduction in the remaining cohort. Similarly, Jäger et al. found that patients had significant improvement in pain, function and radiologic outcomes in bone marrow edema and early avascular necrosis stages after prostaglandin I-2 application. However, patients who had advanced stage osteonecrosis did not benefit from there infusions (39).
These non-operative measures have resulted in promising outcomes whenever used in the pre-collapse stages of ON, but larger prospective studies are needed to confirm these findings (refer to Figure 2 for treatment algorithm).
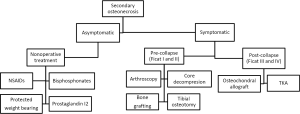
Surgical management
In the pre-collapse stages of secondary osteonecrosis, joint preserving surgery such as arthroscopy, core decompression, osteochondral autograft, and bone grafting may be performed in an attempt to avoid or postpone joint arthroplasty (6).
Arthroscopy is primarily recommended if other knee pathology, such as a meniscal tear or an osteochondral defect is present. Arthroscopy will allow addressing these defects, as well as direct visualization of the condition of the bone in the joint. Core decompression, as well as other therapeutic procedures such as osteochondral allografting, may be performed during arthroscopy as well (20).
Core decompression, as in SONK, may be used successfully in secondary osteonecrosis without subchondral collapse. Woehnl et al. and Lee et al. described techniques in which small diameter drills have been used to enter the affected condyle from the metaphysis under fluoroscopy, with subsequent partial weight bearing (20,40). Marulanda et al. reported a 92% success rate with percutaneous techniques combined with limited weight bearing for 4 to 6 weeks (41). The authors reported that in 56 out of 61 knees the KSS was greater than 80 points (41).
Bone impaction grafting has also been used in patients who have early stage osteonecrosis (42). In this procedure, the osteonecrotic lesion is excised, and then the bone defect is reconstructed with impacted bone autograft or fresh frozen allograft to prevent collapse and recreate the natural sphericity of the femoral condyle. Lee and Goodman, in their study of three patients, and Rijnen et al. in their study of nine patients demonstrated that bone grafting can be successful in knees with femoral collapse and may delay the need for TKA (40). Lee and Goodman demonstrated that there were improvements in the mean KSS from 63 to 89 points, and in functional KSS from 19 to 81 points. However, in patients with multiple osteonecrotic lesions affecting multiple condyles this alternative may not be a viable option (20).
Osteochondral allograft transplantation is another procedure which has demonstrated efficacy even in large or complex lesions, and has the advantage of restoring mature hyaline cartilage to the affected area (43). Flynn et al. treated eight patients with fresh frozen osteochondral allografts, and reported that 75% of patients had occasional to no pain, unlimited walking without aids, and greater than 95 degrees of flexion at latest follow-up (44). Similarly, Görtz et al., found that there was an 89% success rate in 28 knees that underwent osteochondral allografting for post-collapse disease, with an increase in mean Knee Society function scores from 60 to approximately 86 points at a mean follow-up of 2 years (45). However, a study by Bayne et al. demonstrated worse outcomes in patients with corticosteroid associated osteonecrosis of the knee, who underwent osteochondral allografting. The authors attributed this to the continued use of corticosteroids, causing poor revascularization of the allograft, resulting in graft subsidence (46). Currently, there are potential concerns with the continued use of corticosteroids and placing a graft in a necrotic bone bed in these patients. Thus, we believe that larger, long-term studies are needed to determine this treatments effectiveness.
Similar to patients who have SONK, TKA is the most appropriate surgical option in patients who have failed more conservative measures and present with subchondral bone collapse (Ficat stage III and IV disease). Conversely, UKA is not recommended for secondary osteonecrosis due to the frequent involvement of multiple condyles (6). Several studies have demonstrated excellent results of TKA (29,47). A study by Mont et al. (48), on 30 patient reported a 97% survival rate at mean follow-up of 108 months. Similarly, Parratte et al (49). found that there was a 12-year Kaplan-Meier survivorship of 96.7% in 10 patients, with a mean Knee Society score of 95 points (from 56 points pre-operatively) at 7 years follow-up. Due to the recent advances surgical techniques, modern implants, and better peri-operative medical management have likely led to these improved and predictable outcomes with low complication rates in patients undergoing TKA (refer to Figure 2 for treatment algorithm).
Post-arthroscopic: etiology and pathophysiology
Post-arthroscopic osteonecrosis of the knee is the rarest type of osteonecrosis, however, it has been reported that its incidence can be as high as 4% after these procedures (4). Post-arthroscopic osteonecrosis is usually seen following routine chondroplasty and meniscectomy, with less cases being seen with laser assisted meniscectomy and anterior cruciate ligament reconstruction (47). There are varying theories on the etiology. Pape et al. postulated that altered biomechanics of the knee following meniscectomy, which cause and increased contact pressures, and may lead to insufficiency fractures and intraosseous leak of synovial fluid, and eventually the development of osteonecrosis. Other authors have described the lesion as being in fact a subchondral fracture, and not pure osteonecrosis as traditionally described (47). Additional theories include thermal energy or photoacoustic shock to be the causing factor of knee ON (48).
Post-arthroscopic on: clinical presentation and diagnosis
Both men and women appear equally affected, with the occurrence most commonly in the fifth decade of life. The medial femoral condyle is reported to be most commonly affected, followed by the lateral femoral condyle, lateral tibial plateau, and medial tibial plateau. The affected area coincides with the area of pre-existing pathology and arthroscopic procedure (50). These patients typically present with sudden onset knee pain in contrast to the more gradual onset seen in secondary osteonecrosis. Usually symptoms start about six to eight weeks after the arthroscopic procedure (20).
Plain radiographs of the knee, as well as MRI evaluation are recommended in patients with suspected post-arthroscopic osteonecrosis. Bone marrow edema is typically seen in the compartment where the arthroscopic procedure was performed, with no evidence of edema seen prior to the arthroscopy (6). These lesions can be graded using the modified Ficat and Arlet staging system, similar to that of secondary osteonecrosis.
Post-arthroscopic: treatment
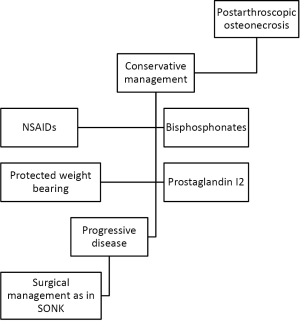
Nonsurgical management
Initial management of post-arthroscopic osteonecrosis of the knee should consist of protected weight bearing, non-steroidal anti-inflammatories, and analgesia. Bisphosphonates have also been shown to be beneficial in the nonsurgical management of post-arthroscopic osteonecrosis following meniscectomy (51). Kraenzlin et al. treated 22 patients who were diagnosed with knee osteonecrosis following arthroscopy with intravenous pamidronate 120 mg divided into 3 or 4 perfusions over 2 weeks, followed by oral alendronate (70 mg) weekly for 4 to 6 months. The authors demonstrated that treatment with bisphosphonate in a decrease in VAS from 8 to 5, and after 4 to 6 weeks (P<0.001). After 6 months, the VAS decreased by 80% (P<0.001). Non-operative management is best for patients with early stage, pre-collapse lesions. If lesions progress beyond this point, and are associated with degenerative articular surface changes, operative management is often necessary (6).
Surgical management
Joint preserving techniques can be employed such as core decompression, or osteochondral allograft repair. High tibial osteotomy may also be considered in young, active individuals who have failed core decompression and still have early stage disease (20). There is a paucity of reports detailing outcomes of joint preserving procedures for the management of post-arthroscopic osteonecrosis. Garino et al. reported on six cases in which post-operative pain persisted after arthroscopic surgery of the knee utilizing lasers (52). One patient had post-operative pain that persisted for 9 months, with MRI evidence of osteonecrosis in the medial femoral condyle. This patient subsequently went on to repeat arthroscopy with core decompression which failed, ultimately requiring UKA one year post-operatively. Another patient underwent arthroscopic chondroplasty of the medial femoral condyle and patella with an increase in pain post-operatively. This patient underwent repeat arthroscopy one week later with core decompression of the patella. One year following this procedure, the patient ultimately required patellectomy for resolution of her pain.
For patients with end stage arthritis, advanced stage disease, or failure of joint preserving treatment, UKA or TKA are recommended. Bonutti et al. reported a series of 19 knees with post-arthroscopic osteonecrosis, 4 of which were treated with UKA and 15 of which were treated with TKA (53). The authors demonstrated that 95% of patients had good to excellent clinical results with a Knee Society Score of greater than 80 points at a mean follow-up of 62 months. Unicompartmental knee arthroplasty may be a good option for patients who present with unicondylar disease, but for those who have multi-compartmental disease, TKA may be the better choice (20). However, there is limited data on the topic of post-arthroscopic osteonecrosis, as such the result of these studies used cautiously, until further registry, or prospective, multi-center studies are available (refer to Figure 3 for treatment algorithm).
Summary
There are three distinct types of osteonecrosis of the knee, including spontaneous, secondary, and post-arthroscopic. Secondary osteonecrosis, similar to osteonecrosis seen in the hip, is related to corticosteroid and alcohol use, and may be due to vascular occlusion and marrow crowding. Spontaneous and post-traumatic osteonecrosis may be due to subchondral insufficiency fractures. Plain radiographs and MRI are primarily used in the diagnosis and staging of these diseases, with MRI being the most sensitive and specific diagnostic modality. Spontaneous and post-arthroscopic osteonecrosis of the knee can initially be managed non-operatively, while secondary osteonecrosis is best treated surgically. Initial surgical treatment consists of joint preserving procedures in those patients in pre-collapse stages, and arthroplasty as the end stage treatment for those symptomatic patients who have progressed to advanced collapse and osteoarthritis.
Acknowledgements
Michael A. Mont receives royalties from Stryker; Wright Medical Technology, Inc.; is a paid consultant for Biocomposites; DJ Orthopaedics; Janssen; Joint Active Systems; Medtronic; Sage Products, Inc.; Stryker; TissueGene; Wright Medical Technology, Inc.; has received research support from DJ Orthopaedics; Joint Active Systems; National Institutes of Health (NIAMS & NICHD); Sage Products, Inc.; Stryker; Tissue Gene; Wright Medical Technology, Inc.; is in editorial/governing board of American Journal of Orthopedics; Journal of Arthroplasty; Journal of Bone and Joint Surgery-American; Journal of Knee Surgery; Surgical Techniques International; and is a board member for AAOS.
Disclosure: The authors declare no conflict of interest.
References
- Ahlbäck S, Bauer GC, Bohne WH. Spontaneous osteonecrosis of the knee. Arthritis Rheum 1968;11:705-33. [PubMed]
- Mont MA, Baumgarten KM, Rifai A, et al. Atraumatic osteonecrosis of the knee. J Bone Joint Surg Am 2000;82:1279-90. [PubMed]
- Pape D, Seil R, Fritsch E, et al. Prevalence of spontaneous osteonecrosis of the medial femoral condyle in elderly patients. Knee Surg Sports Traumatol Arthrosc 2002;10:233-40. [PubMed]
- Cetik O, Cift H, Comert B, et al. Risk of osteonecrosis of the femoral condyle after arthroscopic chondroplasty using radiofrequency: a prospective clinical series. Knee Surg Sports Traumatol Arthrosc 2009;17:24-9. [PubMed]
- Mears SC, McCarthy EF, Jones LC, et al. Characterization and pathological characteristics of spontaneous osteonecrosis of the knee. Iowa Orthop J 2009;29:38-42. [PubMed]
- Mont MA, Marker DR, Zywiel MG, et al. Osteonecrosis of the knee and related conditions. J Am Acad Orthop Surg 2011;19:482-94. [PubMed]
- Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am 2000;82:858-66. [PubMed]
- al-Rowaih A, Björkengren A, Egund N, et al. Size of osteonecrosis of the knee. Clin Orthop Relat Res 1993;68-75. [PubMed]
- Reddy AS, Frederick RW. Evaluation of the intraosseous and extraosseous blood supply to the distal femoral condyles. Am J Sports Med 1998;26:415-9. [PubMed]
- Akamatsu Y, Mitsugi N, Hayashi T, et al. Low bone mineral density is associated with the onset of spontaneous osteonecrosis of the knee. Acta Orthop 2012;83:249-55. [PubMed]
- Lotke PA, Abend JA, Ecker ML. The treatment of osteonecrosis of the medial femoral condyle. Clin Orthop Relat Res 1982;109-16. [PubMed]
- Houpt JB, Pritzker KP, Alpert B, et al. Natural history of spontaneous osteonecrosis of the knee (SONK): a review. Semin Arthritis Rheum 1983;13:212-27. [PubMed]
- Fotiadou A, Karantanas A. Acute nontraumatic adult knee pain: the role of MR imaging. Radiol Med 2009;114:437-47. [PubMed]
- Lecouvet FE, van de Berg BC, Maldague BE, et al. Early irreversible osteonecrosis versus transient lesions of the femoral condyles: prognostic value of subchondral bone and marrow changes on MR imaging. AJR Am J Roentgenol 1998;170:71-7. [PubMed]
- Mont MA, Ulrich SD, Seyler TM, et al. Bone scanning of limited value for diagnosis of symptomatic oligofocal and multifocal osteonecrosis. J Rheumatol 2008;35:1629-34. [PubMed]
- Pivec R, Johnson AJ, Harwin SF, et al. Differentiation, diagnosis, and treatment of osteoarthritis, osteonecrosis, and rapidly progressive osteoarthritis. Orthopedics 2013;36:118-25. [PubMed]
- Koshino T, Okamoto R, Takamura K, et al. Arthroscopy in spontaneous osteonecrosis of the knee. Orthop Clin North Am 1979;10:609-18. [PubMed]
- Aglietti P, Insall JN, Buzzi R, et al. Idiopathic osteonecrosis of the knee. Aetiology, prognosis and treatment. J Bone Joint Surg Br 1983;65:588-97. [PubMed]
- Juréus J, Lindstrand A, Geijer M, et al. The natural course of spontaneous osteonecrosis of the knee (SPONK): a 1- to 27-year follow-up of 40 patients. Acta Orthop 2013;84:410-4. [PubMed]
- Woehnl A, Naziri Q, Costa C, et al. Osteonecrosis of the knee. Orthopaedic Knowledge Online Journal 2012;10.
- Yates PJ, Calder JD, Stranks GJ, et al. Early MRI diagnosis and non-surgical management of spontaneous osteonecrosis of the knee. Knee 2007;14:112-6. [PubMed]
- Jureus J, Lindstrand A, Geijer M, et al. Treatment of spontaneous osteonecrosis of the knee (SPONK) by a bisphosphonate. Acta Orthop 2012;83:511-4. [PubMed]
- Nishii T, Sugano N, Miki H, et al. Does alendronate prevent collapse in osteonecrosis of the femoral head? Clin Orthop Relat Res 2006;273-9. [PubMed]
- Meier C, Kraenzlin C, Friederich NF, et al. Effect of ibandronate on spontaneous osteonecrosis of the knee: a randomized, double-blind, placebo-controlled trial. Osteoporos Int 2014;25:359-66. [PubMed]
- Marcheggiani Muccioli GM, Grassi A, Setti S, et al. Conservative treatment of spontaneous osteonecrosis of the knee in the early stage: pulsed electromagnetic fields therapy. Eur J Radiol 2013;82:530-7. [PubMed]
- Duany NG, Zywiel MG, McGrath MS, et al. Joint-preserving surgical treatment of spontaneous osteonecrosis of the knee. Arch Orthop Trauma Surg 2010;130:11-6. [PubMed]
- Miller GK, Maylahn DJ, Drennan DB. The treatment of idiopathic osteonecrosis of the medial femoral condyle with arthroscopic debridement. Arthroscopy 1986;2:21-9. [PubMed]
- Akgun I, Kesmezacar H, Ogut T, et al. Arthroscopic microfracture treatment for osteonecrosis of the knee. Arthroscopy 2005;21:834-43. [PubMed]
- Forst J, Forst R, Heller KD, et al. Spontaneous osteonecrosis of the femoral condyle: causal treatment by early core decompression. Arch Orthop Trauma Surg 1998;117:18-22. [PubMed]
- Deie M, Ochi M, Adachi N, et al. Artificial bone grafting [calcium hydroxyapatite ceramic with an interconnected porous structure (IP-CHA)] and core decompression for spontaneous osteonecrosis of the femoral condyle in the knee. Knee Surg Sports Traumatol Arthrosc 2008;16:753-8. [PubMed]
- Tanaka Y, Mima H, Yonetani Y, et al. Histological evaluation of spontaneous osteonecrosis of the medial femoral condyle and short-term clinical results of osteochondral autografting: a case series. Knee 2009;16:130-5. [PubMed]
- Saito T, Kumagai K, Akamatsu Y, et al. Five- to ten-year outcome following medial opening-wedge high tibial osteotomy with rigid plate fixation in combination with an artificial bone substitute. Bone Joint J 2014;96-B:339-44. [PubMed]
- Heyse TJ, Khefacha A, Fuchs-Winkelmann S, et al. UKA after spontaneous osteonecrosis of the knee: a retrospective analysis. Arch Orthop Trauma Surg 2011;131:613-7. [PubMed]
- Myers TG, Cui Q, Kuskowski M, et al. Outcomes of total and unicompartmental knee arthroplasty for secondary and spontaneous osteonecrosis of the knee. J Bone Joint Surg Am 2006;88:76-82. [PubMed]
- Motomura G, Yamamoto T, Miyanishi K, et al. Bone marrow fat-cell enlargement in early steroid-induced osteonecrosis--a histomorphometric study of autopsy cases. Pathol Res Pract 2005;200:807-11. [PubMed]
- Wright JL, Zhou S, Churg A. Pulmonary hypertension and vascular oxidative damage in cigarette smoke exposed eNOS(-/-) mice and human smokers. Inhal Toxicol 2012;24:732-40. [PubMed]
- Lemonne N, Lamarre Y, Romana M, et al. Does increased red blood cell deformability raise the risk for osteonecrosis in sickle cell anemia? Blood 2013;121:3054-6. [PubMed]
- Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br 1985;67:3-9. [PubMed]
- Jäger M, Tillmann FP, Thornhill TS, et al. Rationale for prostaglandin I2 in bone marrow oedema--from theory to application. Arthritis Res Ther 2008;10:R120. [PubMed]
- Lee K, Goodman SB. Cell therapy for secondary osteonecrosis of the femoral condyles using the Cellect DBM System: a preliminary report. J Arthroplasty 2009;24:43-8. [PubMed]
- Marulanda G, Seyler TM, Sheikh NH, et al. Percutaneous drilling for the treatment of secondary osteonecrosis of the knee. J Bone Joint Surg Br 2006;88:740-6. [PubMed]
- Rijnen WH, Luttjeboer JS, Schreurs BW, et al. Bone impaction grafting for corticosteroid-associated osteonecrosis of the knee. J Bone Joint Surg Am 2006;88:62-8. [PubMed]
- Bugbee W, Cavallo M, Giannini S. Osteochondral allograft transplantation in the knee. J Knee Surg 2012;25:109-16. [PubMed]
- Flynn JM, Springfield DS, Mankin HJ. Osteoarticular allografts to treat distal femoral osteonecrosis. Clin Orthop Relat Res 1994;38-43. [PubMed]
- Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for steroid-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res 2010;468:1269-78. [PubMed]
- Bayne O, Langer F, Pritzker KP, et al. Osteochondral allografts in the treatment of osteonecrosis of the knee. Orthop Clin North Am 1985;16:727-40. [PubMed]
- MacDessi SJ, Brophy RH, Bullough PG, et al. Subchondral fracture following arthroscopic knee surgery. A series of eight cases. J Bone Joint Surg Am 2008;90:1007-12. [PubMed]
- Mont MA, Rifai A, Baumgarten KM, et al. Total knee arthroplasty for osteonecrosis. J Bone Joint Surg Am 2002;84-A:599-603. [PubMed]
- Parratte S, Argenson JN, Dumas J, et al. Unicompartmental knee arthroplasty for avascular osteonecrosis. Clin Orthop Relat Res 2007;37-42. [PubMed]
- Pape D, Lorbach O, Anagnostakos K, et al. Osteonecrosis in the postarthroscopic knee. Orthopade 2008;37:1099-100, 1102-7. [PubMed]
- Kraenzlin ME, Graf C, Meier C, et al. Possible beneficial effect of bisphosphonates in osteonecrosis of the knee. Knee Surg Sports Traumatol Arthrosc 2010;18:1638-44. [PubMed]
- Garino JP, Lotke PA, Sapega AA, et al. Osteonecrosis of the knee following laser-assisted arthroscopic surgery: a report of six cases. Arthroscopy 1995;11:467-74. [PubMed]
- Bonutti PM, Seyler TM, Delanois RE, et al. Osteonecrosis of the knee after laser or radiofrequency-assisted arthroscopy: treatment with minimally invasive knee arthroplasty. J Bone Joint Surg Am 2006;88:69-75. [PubMed]


