
Delay in articular cartilage degeneration of the knee joint by the conditional removal of discoidin domain receptor 2 in a spontaneous mouse model of osteoarthritis
Introduction
The preservation of articular cartilage is one of the effective ways to prevent joints from being destroyed. Understanding the molecular basis underlying the progressive process of articular cartilage degeneration can provide valuable insights into the potential therapeutic targets for the preservation of articular cartilage. On the basis of results from previous investigations, it was concluded that a cell surface receptor tyrosine kinase, discoidin domain receptor 2 (Ddr2) for native collagen type II, may be a rate-limiting factor in articular cartilage degeneration (1-4). In related studies, the expression of Ddr2 was found to be increased in human osteoarthritic tissues and degenerative articular cartilages of osteoarthritis (OA) mouse models (5-8), and co-localized with the elevated expression of matrix metalloproteinase 13 (MMP-13) (9). At an early stage, activation of the collagen receptor DDR2 leads to upregulated expression of MMP13. This results in generation of fragments of collagen II and fibronectin in the territorial matrix. These fragments, as well as cytokines such as IL-1, further stimulate signaling pathways that induce MMP-13 expression. The result is a time-dependent increase in the degradation of the cartilage extracellular matrix. Based on previous studies of other independent research groups and our group, MMP-13 can degrade both proteoglycans and type II collagen effectively, and the expression of MMP13 is hardly detected in normal mature articular cartilage, while the activity and expression of this enzyme are increased in human osteoarthritic cartilage and in joints of OA mouse models (1,5,6,8). In addition, in vitro experiments demonstrated that the interaction of Ddr2 with collagen type II can induce the expression of MMP-13 in chondrocytes through the activation of Ras/Raf/MEK/ERK signaling pathways and p38 signaling pathways: the activation of DDR2 by type II collagen will lead to the phosphorylation of MEK, which subsequently activate the downstream molecule ERK via the Ras/Raf/MEK/ERK pathway, and p38 via p38 signaling pathways in chondrocytes. Then the phosphorylated ERK and p38 translocate into nucleus, result in elevated levels of MMP-13 mRNA, and therefore increase the expression of MMP-13 (5). More importantly, the conditional deletion of Ddr2 was revealed to significantly delay the progression of articular cartilage degeneration in a post-traumatic OA mouse model, with destabilization of the medial meniscus (DMM) (1). However, a question remains: what is the biological effect of the conditional removal of Ddr2 in other types of OA, such as in the case of aging-related OA? Collagen type XI-haploinsufficiency (Col11a1+/−) mouse strain provides us with an excellent opportunity to determine whether the conditional deletion of Ddr2 can significantly attenuate the pathogenesis of OA in an aging-related mouse model.
Chondrodysplasia (cho) in mice is a spontaneous mutant mouse strain (10). The genetic defect of cho is identified with a single nucleotide deletion in Col11a1, which leads to the absence of the normal collagen type XI in homozygous mutant mice (cho/cho) (11). Thus, the condition in cho/cho mice is equal to that in functional knockout collagen type XI in mice (Col11a1−/−). Despite having normal life span (about 30 months on average) without any overt abnormalities, heterozygous cho/+ (Col11a1+/−) mice may develop OA-like changes in knee joints starting at the age of 3 months old and form a typical osteoarthritic joint at the age of 15 months old (12). They may further present the pathological appearance of chondrocyte clusters and undergo an increase in the production of proteoglycans in the extracellular matrix, particularly in the pericellular matrix of chondrocytes. The degradation of proteoglycans and collagen type II is seen in Col11a1+/− mice with aging. Finally, fibrillation, missing cartilage, synovial inflammation, and osteophyte formation are evident in the mutant mice. This pathological progression is consistent with what is seen in the majority of human aging-related OA cases (13,14). Thus, Col11a1+/− mouse strain is a good aging-related OA mouse model.
In this study, for the first time, we explored the biological effect of the conditional removal of Ddr2 in aging-related OA, and used a mouse genetic approach to specifically remove Ddr2 in the articular cartilage of knee joints in Col11a1+/− mice at the age of the 10 weeks, an age at which Col11a1+/− mice do not demonstrate articular cartilage degeneration. Mice were then euthanized at the ages of 3, 9, and 15 months so that knee joints could be collected, and the joints were then examined for evidence of articular cartilage degeneration.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5786).
Methods
The conditional deletion of Ddr2 in the articular cartilage of knee joints of Col11a1+/− mice
All animal work described in this study was approved by and was conducted in accordance with the Institutional Animal Care and Use Committee of Harvard Medical School. Three mouse strains were used in this experiment. The first mouse strain, AcanCreERT2, was made available by the Jackson Laboratory (B6.Cg-Acantm1(cre/ERT2)Crm/J, stock# 019148), and expresses a recombinant protein consisting of Cre-recombinase and a modified estrogen receptor (CreERT2), driven by the endogenous aggrecan promoter (14). The second strain was the floxed Ddr2 (Ddr2flox/flox) mouse strain (1), with the exon 9 of Ddr2 flanked by loxP sites. The third strain was the Col11a1+/− mice. For the generation of Acan+/CreERT2;Ddr2flox/flox;Col11a1+/− mice, Acan+/CreERT2 mice were bred with Ddr2flox/flox mice to produce Acan+/CreERT2;Ddr2+/flox mice. Acan+/CreERT2;Ddr2+/flox mice were then crossed with Ddr2flox/flox mice to produce Acan+/CreERT2;Ddr2flox/flox mice. A similar breeding procedure was used to generate Col11a1+/−;Ddr2flox/flox mice. Finally, Acan+/CreERT2;Ddr2flox/flox mice were bred with Col11a1+/-;Ddr2flox/flox mice to produce Acan+/CreERT2;Ddr2flox/flox;Col11a1+/− mice (Figure 1A). The Acan+/CreERT2;Ddr2flox/flox;Col11a1+/− mice were identified by polymerase chain reaction (PCR). The detailed experimental procedures of PCR for the identification of Acan+/CreERT2, Ddr2flox/flox, and Col11a1+/− are described in our previous publications (1,12).
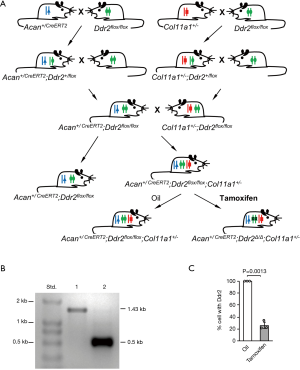
To specifically delete Ddr2 from the articular cartilage of knee joint in Col11a1+/− mice, 10-week-old Acan+/CreERT2;Ddr2flox/flox;Col11a1+/− mice were intraperitoneally injected with tamoxifen at 2 mg/10 g body weight daily with the concentration of 1mg/100ul for 10 consecutive days. Acan+/CreERT2;Ddr2flox/flox; Col11a1+/− mice were treated with tamoxifen. Meanwhile, Acan+/CreERT2;Ddr2flox/flox;Col11a1+/− mice treated with sunflower seed oil and their littermates, Acan+/CreERT2;Ddr2flox/flox mice (as wild-type control), were used as the two control groups. They were maintained in a mouse facility under a 12-hour light and dark cycle, and euthanized at the ages of 3, 9, and 15 months for knee joint collection.
Measurement of the removal efficiency of Ddr2 from the articular cartilage of knee joints
First, three 10-week-old Acan+/CreERT2;Ddr2flox/flox;Col11a1+/− mice were intraperitoneally injected with tamoxifen at 2 mg/10 g body weight each day for 10 consecutive days. The other three Acan+/CreERT2;Ddr2flox/flox;Col11a1+/− mice were injected with oil as control.
Second, the mice were euthanized at the age of 3 months old, and articular cartilages of knee joints were carefully sliced off from the tibial and femoral condyles, and collected individually from each mouse. For genomic DNA isolation, the articular cartilages were digested in a 1.7 mL Eppendorf tube containing 200 mL of the tail buffer and 2 mL of protease K (10 mg/mL) (Sigma, Cat#P2308). The tubes were shaken with an Eppendorf ThermoMixer at 300 rpm at 56 °C overnight. Then, 200 mL of isopropanol were added to precipitate DNA after digestion. The samples were stored at −20 °C for 3 to 4 hours. The DNA was pelleted at 12,000 ×g at 4 °C for 15 min.
Third, for the detection of the exon 9 of Ddr2 in mice after injection, PCR was performed with the genomic DNA isolated from the articular cartilage; the forward primer was 5'-AGTAGGTGCTAGCTACCTCCCACC-3', and the reverse primer was 5'-GATGGGAGTCCATTGTTGTC-3'. For the quantitative measurement of the exon 9 of Ddr2, duplex real-time PCR was performed using TaqMan Copy Number Assay. For the target gene, Ddr2 exon 9 (Transcript: ENSMUST00000027985), TaqMan probe (Cat# 4400291), was labeled with FAM; for the reference gene, Tfrc, TaqMan probe (Cat# 4458366) was labeled with VIC. PCR was performed in 20 ml of reaction buffer containing 20 ng of genomic DNA according to the company’s protocol. Real-time PCR reaction was carried out at 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s, using StepOnePlus real-time PCR System (ThermoFisher Scientific). Each sample was tested in triplicate.
Histology
Acan+/CreERT2;Ddr2flox/flox;Col11a1+/− mice were injected peritoneally with tamoxifen or oil at 10 weeks of age, and knee joints were collected at 3, 9, and 15 months of age. There were eight joints collected from each experimental group, with one joint being collected from each chosen mouse. Knee joints were then fixed and processed for paraffin embedding, and 6-µm thick serial sections were cut from the anterior to posterior side of each joint. Every tenth section was stained with Safranin O/Fast green.
Knee joint morphology was evaluated by a modified Mankin score system as recommended by the Osteoarthritis Research Society International (15). We made minor modifications to this scoring system. We added a chondrocyte cluster and/or considered an increase in Safranin O staining as score 1 and a loss of Safranin O staining as score 2 since these morphological appearances are the earliest pathological signs in articular cartilage degeneration. We then ranked fibrillation above the tidemark as 3, fibrillation reaching to the tidemark as 4, cartilage missing less than 25% of the cartilage surface as 5, cartilage missing less than 50% of the cartilage surface as 6, cartilage missing less than 75% of the cartilage surface as 7, and cartilage missing more than 75% of the cartilage surface as 8. The morphological conditions of meniscus, subchondral bone, and synovial membrane were also evaluated to assess the overall condition of the joint.
Immunohistostaining
Unstained paraffin sections were used for immunohistostaining. Four animals were randomly selected from three groups at the age of 9 months old. Eight to ten paraffin sections from each joint were used for immunohistostaining with a polyclonal antibody. The sections were incubated with the antibody against Ddr2 (1:300 dilution, Abcam, cat. no. ab203219), the antibody against MMP-13 (1:300 dilution, Abcam, cat. no. ab84594), or the antibody against degraded collagen type II (1:400 dilution, IBEX Pharmaceuticals Inc, cat. no. 50–1053) at 4 °C, overnight. The samples were then incubated with a biotinylated secondary antibody (goat anti-rabbit IgG). After that, the samples were treated with a mixture of avidin and biotinylated horseradish peroxidase (VECTASTAIN ABC Kit, Vector Laboratories, cat. no. PK-400). NovaRED peroxidase substrate (Vector Laboratories, cat. no. SK-4800) was used for color detection. Sections were counterstained with 0.2% Fast Green solution. For negative controls, staining with isotype-matched normal IgG (Vector Laboratories) and staining without primary antibodies were also performed.
Statistics
For histology, all of the sections stained by Safranin O/Fast green were examined. From each knee joint, ten to twelve paraffin sections were scored, and the worst score was selected to represent that joint. Six to eight scores were obtained, and an average score was calculated from each group. Normally distributed data were analyzed by the Student’s t-test, and are presented as mean ± 95% confidence intervals (CI). A P value <0.05 was used to determine the presence of statistically significant differences between any two scores.
Results
The genetic removal of Ddr2 from the articular cartilage of knee joints in Col11a1+/− mice
The average size of cartilage pieces was about 1.5×1.5×0.15 mm3. From each knee joint, 1.5 to 2.0 mg of the cartilages were collected. For detection of the exon 9 of Ddr2, PCR was carried out with the genomic DNA. PCR product, 1.43-kb in size, was detected in the DNA of Acan+/CreERT2;Ddr2flox/flox;Col11a1+/− mice treated with oil, whereas a size of 0.5-kb was detected in the DNA of Acan+/CreERT2;Ddr2flox/flox;Col11a1+/− mice treated with tamoxifen; this indicated that the exon 9 of Ddr2 was deleted in tamoxifen-treated Acan+/CreERT2;Ddr2flox/flox;Col11a1+/− mice (Figure 1B).
For the quantitative measurement of the exon 9 of Ddr2 in chondrocytes, genomic DNA was also used. About 75% of chondrocytes in Acan+/CreERT2;Ddr2Δ/Δ;Col11a1+/− mice did not have the exon 9 of Ddr2 (Figure 1C). The number of cells with the exon 9 of Ddr2 was significantly lower in Acan+/CreERT2;Ddr2Δ/Δ; Col11a1+/− mice than in Acan+/CreERT2;Ddr2flox/flox;Col11a1+/− mice.
Delay of the articular cartilage degeneration of knee joints in Acan+/CreERT2;Ddr2Δ/Δ;Col11a1+/− mice
The progressive process of articular cartilage degeneration was significantly delayed in Col11a1+/− mice without Ddr2. At the age of 3 months old, the major pathological indicator in the articular cartilage of Col11a1+/− mice with or without Ddr2 was chondrocyte clusters (Figure 2A). The scores for the evaluation of cartilage condition indicated no significant difference between these two groups (Figure 2B). At the age of 9 months old, fibrillation was only seen in the articular cartilage of knee joints in Col11a1+/− mice with Ddr2. The score was significantly lower in Col11a1+/− mice without Ddr2 than in Col11a1+/− mice with Ddr2. At the age of 15 months, the missing cartilage was observed only in Col11a1+/− mice with Ddr2. Again, the score was significantly lower in Col11a1+/− mice without Ddr2 than in Col11a1+/− mice with Ddr2. The morphological analyses demonstrated that the deletion of Ddr2 could significantly delay the progression of Col11a1+/− mouse knee joints into OA-like joints. There were no abnormal morphological changes in the wild-type groups with the exception of a slight degradation of proteoglycans in the cartilage at the age of 15 months old.
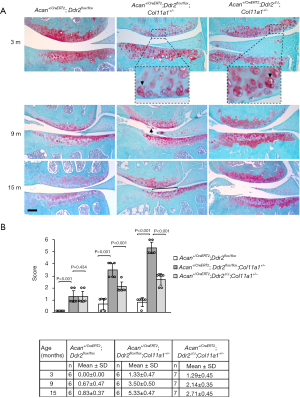
With regard to other joint tissues, synovial tissue inflammation and osteophyte formation were seen in Col11a1+/− mice at the age of 15 months (12). However, we did not observe these changes in Col11a1+/− mice without Ddr2. We did not detect any pathological change in the subchondral bone of both Col11a1+/− mice with and without Ddr2.
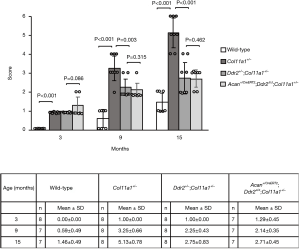
In one of our previous investigations, we found that the process of articular cartilage degeneration was significantly attenuated in Ddr2+/−;Col11a1+/− mice (conventional heterozygous knockout Ddr2 in Col11a1+/− mice) in which 50% of the Ddr2 was removed in Col11a1+/− mice (15). In this present study, we re-evaluated the condition of the articular cartilages of knee joints in Col11a1+/− and Ddr2+/−;Col11a1+/− mice. Results showed that there were no significant differences in the scores between Ddr2+/−;Col11a1+/− and Acan+/CreERT2;Ddr2Δ/Δ;Col11a1+/− mice at the ages of 3, 9, and 15 months (Figure 3).
Decrease in Ddr2 and MMP-13 expression and the amount of the degraded collagen type II in Acan+/CreERT2;Ddr2Δ/Δ;Col11a1+/− mice
The protein expression of Ddr2 could barely be detected in the articular cartilage of knee joints in Acan+/CreERT2;Ddr2Δ/Δ;Col11a1+/− mice (Figure 4), which is consistent with the result of the removal efficiency, indicating that Ddr2 was deleted in majority of articular chondrocytes. We also found that the protein expression of MMP-13 the degraded collagen type II could also barely be detected in the articular cartilages in Acan+/CreERT2;Ddr2Δ/Δ;Col11a1+/− mice. The protein expression of Ddr2 and MMP-13 and the amount of the degraded collagen type II, were dramatically increased in Acan+/CreERT2;Ddr2flox/flox;Cl11a1+/− mice (Figure 4). This is in line with our previous observation that the expression of Ddr2 and MMP-13 and the amount of the degraded collagen type II were increased in Col11a1+/− mice (5).

Discussion
Results from investigations by numerous research groups have confirmed that the increase in the expression of Ddr2 is associated with the acceleration of articular cartilage degeneration, which eventually leads to OA (2,5,8,16). In one of our previous studies, we conventionally removed one copy of Ddr2 in Col11a1+/− mice (Ddr2+/−;Col11a1+/− mice) by crossing heterozygous Ddr2-knockout (Ddr2+/−) mice with Col11a1+/− mice. The result indicated that the removal of one copy of Ddr2 in Col11a1+/− mice could delay the progression of articular cartilage degeneration in knee joints. In addition, the result from one of our other investigations demonstrated that the conditional deletion of Ddr2 in the articular cartilage of adult mouse knee joints significantly attenuated the cartilage degeneration induced by post-traumatic injury (the destabilization of the medial meniscus, DMM). One question that remains is the exact biological effect conferred by the conditional removal of Ddr2 in the articular cartilage of adult knee joints in Col11a1+/− mice. In the present study, we conditionally deleted Ddr2 in the articular cartilage of knee joints in adult Col11a1+/− mice. We found that the deletion of Ddr2 significantly delayed the progressive process of the cartilage degeneration in Col11a1+/− mice, suggesting that the inhibitory activity of Ddr2 can protect knee joints against the development of OA initiated by post-traumatic injury or non-traumatic factors. We noticed that there was no significant difference in the deceleration of articular cartilage degeneration between Ddr2+/−;Col11a1+/− mice and Acan+/CreERT2;Ddr2Δ/Δ;Col11a1+/− mice. We speculate that one reason for this was that Ddr2 was only deleted in 75% of chondrocytes in Acan+/CreERT2;Ddr2Δ/Δ;Col11a1+/- mice. Another possibility is that more time is required, for example 24 months old or longer, for the mice to display a marked difference.
Results from immunohistostaining demonstrated that the removal of Ddr2 in the knee joints of Col11a1+/− mice could dramatically downregulate the expression of MMP-13. The reduction in the amount of the degraded collagen type II in Col11a1+/− mice without Ddr2 also indicated a decreased activity of MMP-13 in the cartilage. This suggests that the chondro-protective effect in the articular cartilage of knee joints in Col11a1+/− mice without Ddr2 may be due to inhibiting the expression of MMP-13 by the deletion of Ddr2. With regard to the mechanism by which the cartilage is protected by the removal of Ddr2, based on our previous investigations and other studies, we find that TGF-β1 and HTRA1 also play important roles in the pathological development of OA. Therefore, we hypothesize the possible molecular pathway underlying articular cartilage degeneration as follows: excessive mechanical stresses induced by aging-related overloading of normal joints can stimulate chondrocytes and other joint tissues to synthesize and release latent TGF-β1 into synovial fluid. The latent TGF-β1 is then activated and binds to TGFβ receptor II which induces expression of HTRA1 in chondrocytes, leading to degradation of the pericellular matrix and enhanced exposure of chondrocytes to type II collagen. As the pericellular matrix of chondrocytes plays significant roles in keeping Ddr2 inactive. The degradation of the pericellular matrix can expose chondrocytes to collagen type II. The interaction of chondrocytes with collagen type II activates Ddr2, resulting in induction of MMP-13 as well as expression of DDR2 itself. The result is the degradation of collagen type II and proteoglycans, leading to type II collagen and aggrecan fragments, which in turn activates signals that further increase the synthesis of MMP-13. This feedback amplification loop eventually results in irreversible articular cartilage degeneration, which eventually leads to OA (1,5-9,12,16). We observed that Ddr2 was not completely deleted from adult articular chondrocytes in Acan+/CreERT2;Ddr2Δ/Δ;Col11a1+/− mice. This might be due to the difficulty in removing Ddr2 from every aggrecan-producing chondrocyte via tamoxifen injection. In addition, our previous experiment showed that articular chondrocytes below the tidemark in adult knee articular cartilages do not express aggrecan (1). In the examination of the efficiency of Ddr2 removal experiment, the collection of articular cartilage included the cartilage below the tidemark. This might partly account for the non-aggrecan–expressing cells (about 25%) that contained Ddr2. We also noticed that the articular cartilage was continually damaged in Acan+/CreERT2;Ddr2Δ/Δ;Col11a1+/− mice as the mice aged. This suggests that there may be other extracellular matrix–degrading enzymes, apart from MMP-13, that cause the degradation of the cartilage in Acan+/CreERT2;Ddr2Δ/Δ;Col11a1+/− mice.
We are aware that in the last 15 years, results from other research groups have demonstrated that the genetic inactivation or inhibition of genes can protect articular cartilage from being degraded. These genes include hypoxia-inducible factor-2α (HIF-2α), Indian hedgehog (IHH), the Zn2+ importer ZIP8, toll-like receptor, Wnt/b-Catenin signaling, and others. The genetic removal of Hif-2α or Ihh can inhibit chondrocyte hypertrophy, resulting in the inhibition expression of MMP-13 (17,18). The delay of the OA progression by the deletion of Zip8 may be due to a downregulation of the expression of extracellular matrix-degrading enzymes, MMP3 and MMP-13 (19). Toll-like receptor and Wnt/β-Catenin signaling may also be involved in the initiation and progression of articular cartilage degeneration (20). Taken together, these studies suggest that numerous molecular pathways interact with each other, eventually leading to MMP-13 induction in chondrocytes. Thus, the removal of any one of these pathways can significantly inhibit induction of MMP-13 in chondrocytes. Obviously, as this study is a preliminary investigation of aging-related OA mouse model, more indepth experiments are needed to understand the exact mechanisms by which these genes contribute to the development of OA.
In conclusion, the study presented here provides additional data to support our observation that the increase of the activity and expression of Ddr2 may accelerate the progressive process of articular cartilage degeneration of joints, which eventually results in OA. Thus, biological reagents, such as large- or small-molecule inhibitors of Ddr2, may be used as disease-modifying OA drugs to treat the disease.
Acknowledgments
The authors acknowledge Prof. Yefu Li and Dr. Lin Xu from the Harvard School of Dental Medicine, for their technical support.
Funding: This work was supported by a grant from the National Natural Science Foundation of China (no. 81571004) and a grant from West China Hospital of Stomatology, Sichuan University (no. WCHS-201704).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5786
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5786
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5786). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal work described in this study was approved by and was conducted in accordance with the Institutional Animal Care and Use Committee of Harvard Medical School.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Manning LB, Li Y, Chickmagalur NS, et al. Discoidin Domain Receptor 2 as a Potential Therapeutic Target for Development of Disease-Modifying Osteoarthritis Drugs. Am J Pathol 2016;186:3000-10. [Crossref] [PubMed]
- Holt DW, Henderson ML, Stockdale CE, et al. Osteoarthritis-like changes in the heterozygous sedc mouse associated with the HtrA1-Ddr2-Mmp-13 degradative pathway: a new model of osteoarthritis. Osteoarthritis Cartilage 2012;20:430-9. [Crossref] [PubMed]
- Klatt AR, Zech D, Kühn G, et al. Discoidin domain receptor 2 mediates the collagen II-dependent release of interleukin-6 in primary human chondrocytes. J Pathol 2009;218:241-7. [Crossref] [PubMed]
- Vonk LA, Doulabi BZ, Huang C, et al. Collagen-induced expression of collagenase-3 by primary chondrocytes is mediated by integrin α1 and discoidin domain receptor 2: a protein kinase C-dependent pathway. Rheumatology (Oxford) 2011;50:463-72. [Crossref] [PubMed]
- Xu L, Peng H, Wu D, et al. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem 2005;280:548-55. [Crossref] [PubMed]
- Xu L, Peng H, Glasson S, et al. Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum 2007;56:2663-73. [Crossref] [PubMed]
- Sunk IG, Bobacz K, Hofstaetter JG, et al. Increased expression of discoidin domain receptor 2 is linked to the degree of cartilage damage in human knee joints: a potential role in osteoarthritis pathogenesis. Arthritis Rheum 2007;56:3685-92. [Crossref] [PubMed]
- Suutre S, Kerna I, Lintrop M, et al. Evaluation of correlation of articular cartilage staining for DDR2 and proteoglycans with histological tissue damage and the results of radiographic assessment in patients with early stages of knee osteoarthritis. Int J Clin Exp Pathol 2015;8:5658-65. [PubMed]
- Xu L, Polur I, Servais JM, et al. Intact pericellular matrix of articular cartilage is required for unactivated discoidin domain receptor 2 in the mouse model. Am J Pathol 2011;179:1338-46. [Crossref] [PubMed]
- Seegmiller R, Fraser FC, Sheldon H. A new chondrodystrophic mutant in mice. Electron microscopy of normal and abnormal chondrogenesis. J Cell Biol 1971;48:580-93. [Crossref] [PubMed]
- Li Y, Lacerda DA, Warman ML, et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell 1995;80:423-30. [Crossref] [PubMed]
- Xu L, Flahiff CM, Waldman BA, et al. Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho). Arthritis Rheum 2003;48:2509-18. [Crossref] [PubMed]
- Hamerman D. The biology of osteoarthritis. N Engl J Med 1989;320:1322-30. [Crossref] [PubMed]
- Henry SP, Liang S, Akdemir KC, de Crombrugghe B. The postnatal role of Sox9 in cartilage. J Bone Miner Res 2012;27:2511-25. [Crossref] [PubMed]
- Glasson SS, Chambers MG, Van Den Berg WB, et al. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 2010;18 Suppl 3:S17-23. [Crossref] [PubMed]
- Xu L, Servais J, Polur I, et al. Attenuation of osteoarthritis progression by reduction of discoidin domain receptor 2 in mice. Arthritis Rheum 2010;62:2736-44. [Crossref] [PubMed]
- Saito T, Fukai A, Mabuchi A, et al. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med 2010;16:678-86. [Crossref] [PubMed]
- Zhou J, Chen Q, Lanske B, et al. Disrupting the Indian hedgehog signaling pathway in vivo attenuates surgically induced osteoarthritis progression in Col2a1-CreERT2; Ihhfl/fl mice. Arthritis Res Ther 2014;16:R11. [Crossref] [PubMed]
- Kim JH, Jeon J, Shin M, et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell 2014;156:730-43. [Crossref] [PubMed]
- Hou Y, Lin H, Zhu L, et al. Lipopolysaccharide increases the incidence of collagen-induced arthritis in mice through induction of protease HTRA-1 expression. Arthritis Rheum 2013;65:2835-46. [Crossref] [PubMed]
(English Language Editor: J. Gray)


