Effects of radioactive iodine treatment on cardiovascular disease in thyroid cancer patients: a nationwide cohort study
Introduction
Thyroid cancer (TC) is the most common malignancy of the endocrine system and has a rapidly increasing global incidence (1-4). Despite this, the American Cancer Society reported that the 5-year relative survival rate in TC between 2008 and 2014 was 98% (5). A large number of living survivors, especially those with papillary thyroid cancer (PTC), the most common TC, inevitably face issues of overdiagnosis and overtreatment (6-8). Additionally, treatment-related adverse outcomes, including cardiovascular disease (CVD) due to levothyroxine replacement therapy (9,10) or second primary cancer due to radioactive iodine (RAI) therapy (11,12), have been reported as patients with TC have good survival with low disease-specific mortality.
The conventional treatment of differentiated thyroid cancer (DTC) is composed of initial thyroidectomy, followed by thyroid-stimulating hormone (TSH) suppression therapy and adjuvant radioactive iodine (RAI) therapy. RAI therapy using iodine-131 (131I) is a well-established treatment that can be administered to reduce disease recurrence after thyroidectomy in patients with DTC and aggressive histopathologic features or distant metastasis (13,14). Although RAI therapy is generally safe and effective, there are several adverse effects to be mentioned, for example, sialadenitis, gastrointestinal discomfort, second primary malignancy and infertility. Particularly, in previous studies, patients receiving RAI therapy for hyperthyroidism or toxic adenoma showed an increased risk of cerebrovascular diseases (15-17). In la Cour et al.’s study (18), the calculated radiation dose to the carotid artery after RAI therapy for benign thyroid disease was high enough to facilitate endothelial dysfunction and lead to atherosclerosis. However, several years later, la Cour et al. reported that RAI therapy had no or minimal effects on the atherosclerotic burden of the carotid arteries (19). These inconsistent results can be explained by the differences in the sample size, RAI dosage, underlying thyroid disease, and duration of follow-up; they further highlight the importance of concluding the debate concerning RAI treatment and CVD risk. Moreover, previous studies have primarily focused on stroke and cerebrovascular diseases in benign thyroid disease patients, who receive a relatively low cumulative RAI dosage. Another study, albeit with a small number of patients and short-term follow-up, investigated the adverse effects of severe hypothyroid state, induced by the withdrawal of thyroid hormone replacement therapy before RAI therapy, on the metabolic parameters, suggesting an increased risk of CVD in patients with RAI therapy (20).
In view of this, it is a salient point of our study to compare the cardiovascular events between patients with TC who received RAI treatment and those who did not using real-world data based on the Korean National Health Insurance-Health Screening Cohort (NHIS-HEALS, 2002-2015). We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5222).
Methods
Data source and study population
The Korean NHIS is the sole mandatory public medical insurance system managed by the South Korean government and covers all citizens of South Korea. The NHIS recently released the second version of the NHIS-HEALS database (2002–2015), which included information from 512,568 randomly selected South Koreans aged 40–79 years who were eligible for the National Health Screening Program. The longitudinal information of the participants’ demographic, medical, and pharmaceutical records based on the International Classification of Disease, 10th revision (ICD-10) were accessed, and data on the medical procedures, hospitalization, biochemical laboratory results (such as fasting glucose and lipid profiles), blood pressure, and drug prescriptions were recorded. The detailed cohort protocol has been published previously (21-23).
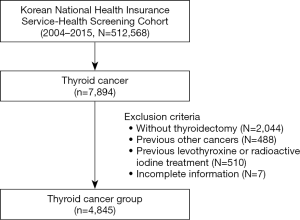
This study originally included 7,894 patients newly diagnosed with TC of all types (ICD-10 C73) between January 1, 2004 to December 31, 2015. We excluded patients who met the following criteria: (I) patients who did not undergo thyroidectomy after TC diagnosis or had already undergone thyroidectomy 6 months before the diagnosis, (II) those with prior diagnosis of other malignancies (C00–C97, except C73), (III) those with a history of levothyroxine or RAI treatment, or (IV) those with incomplete information regarding levothyroxine or RAI treatments. A total of 4,845 patients with TC who underwent thyroidectomy after diagnosis and had no history of other malignancies and levothyroxine prescription or RAI treatment were finally evaluated. Figure 1 illustrates the schematic design of this study. All the 4,845 cases were analyzed without additional data handing since there was no missing data.
This study was approved by the Institutional Review Board of the Korea University Anam Hospital (IRB number: ED16255). Informed consent was not required because this study was based on the NHIS database, which includes fully anonymized and de-identified data. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study definition and outcomes
To minimize false-positive detection, which the national insurance claims database inevitably includes, we defined the primary CVD outcome only as a composite of ischemic stroke (IS), ischemic heart disease (IHD), hemorrhagic stroke (HS), or heart failure (HF), which account for most, but not all, CVD events, and we supplemented the ICD-10 codes of the primary CVD outcomes with hospitalization history. Based on the ICD-10 codes, IHD was defined as hospitalization for I20–I25; IS was defined as hospitalization for I63; HS was defined as hospitalization for I60–I62 or I64; cerebrovascular disease was defined as hospitalization for I60–I69; and HF was defined as hospitalization for I42–I43 or I50. We performed a subgroup analysis of the following specific CVD outcomes: IHD; IS; HS; cerebrovascular disease composed of IS, HS; and HF. We further used specific criteria for reducing false-positive diagnoses of previous diseases. Hypertension was defined as codes I10–I15 with concurrent hypertension medication prescription or codes I10–I15 with systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg. Diabetes mellitus (DM) was defined as codes E10–E14 with concurrent diabetes medication prescription or fasting glucose level ≥126 mg/dL, and dyslipidemia was defined as code E78 with total cholesterol level >240 mg/dL. Previous CVD history was defined as at least one event of IHD, IS, or HS before the index date. Socioeconomic status (SES) at baseline was categorized into three groups based on the income level of an individual: lower, 30%; middle, 40%; and upper, 30%.
We defined follow-up duration as the time between the date of TC claim to the earliest date of incident-specific CVD outcome or the last date of data collection in this cohort (December 31, 2015).
Statistical analysis
Categorical data are presented as frequencies and percentages. Continuous data are presented as mean and standard deviation (SD) for normally distributed variables and median and interquartile range (IQR) for non-normally distributed variables. The Kaplan-Meier analysis followed by a log-rank test was used to examine difference of cumulative incidence between RAI and non-RAI treatments for all CVD outcomes. Adjusted effect of RAI treatment on CVD outcomes was evaluated using a multivariable Cox’s PH regression model, whose effect was presented in terms of hazard ratio (HR) and its 95% confidence interval (CI). Variables adjusted for were age, sex, body mass index, socioeconomical status, smoking, alcohol consumption, levothyroxine dosage, and previous history of hypertension, DM, dyslipidemia, and CVD. All reported p-values were two-sided, and a P value <0.05 was considered significant. Statistical analyses were performed using SAS v9.4 (SAS Institute Inc., Cary, NC, USA) and R Statistical Software v3.3.3 (Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics of the study population
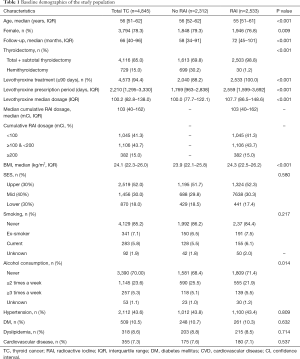
This study included 4,845 patients with TC (3,794 women and 1,051 men) with a median age of 56 years (IQR, 51–62) (Table 1). Of them, 2,533 (52.3%) patients had undergone thyroidectomy and RAI treatment. The median follow-up was 58 (IQR, 34–91) and 72 (IQR, 45–101) months in the non-RAI and RAI treatment groups, respectively. Almost all patients in the RAI group underwent total or subtotal thyroidectomy (98.8%), and the median cumulative RAI dose was 103 mCi (IQR, 40–162 mCi). Levothyroxine treatment for at least 90 days was prescribed in 100% and 88.2% patients in the RAI and non-RAI groups, respectively. The median levothyroxine dose prescribed in the RAI and non-RAI groups was 107.7 (IQR, 86.5–148.6) mcg/day and 100.0 (IQR, 77.7–122.1) mcg/day, respectively. Previous histories of hypertension (43.8% vs. 43.4%), DM (10.7% vs. 10.3%), dyslipidemia (8.8% vs. 8.5%), upper SES levels (51.7% vs. 52.3%), and CVD (7.6% vs. 7.1%) were comparable, with no statistical significance between the non-RAI and RAI groups. However, the proportion of patients who had never consumed alcohol was significantly lower (68.4% vs. 71.4%) and the BMI was slightly lower (23.9 vs. 24.3 kg/m2) in the non-RAI treatment group compared to the RAI group. In summary, the patients who received RAI treatment were younger, were composed of more males, and received total or subtotal thyroidectomy with longer follow-up than those who did not receive RAI treatment. However, previous histories of HTN, DM, dyslipidemia, and major CVD, which were important risk factors of CVD outcomes, were comparable between the two groups.
Full table
CVD risks in patients with TC according to RAI treatment
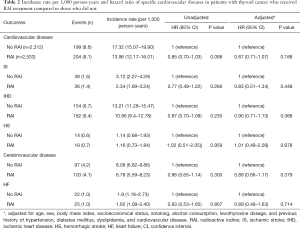
Table 2 summarizes the incidence and risks of composite CVD outcome and specific CVDs between the RAI and non-RAI groups. Between 2004 and 2015, the incidence of composite CVD (per 100 person-years) in the non-RAI and RAI treatment groups was 17.32 and 13.96, respectively, with an unadjusted HR of 0.85 (95% CI, 0.70–1.03), which remained at 0.87 (95% CI, 0.71–1.07) after adjustments for age, sex, BMI, SES, smoking, alcohol consumption, levothyroxine dosage, and histories of hypertension, DM, dyslipidemia, and CVD. The risks of IS (adjusted HR, 0.83; 95% CI, 0.51–1.34), IHD (adjusted HR, 0.90; 95% CI, 0.71–1.13), HS (adjusted HR, 1.01; 95% CI, 0.49–2.09), cerebrovascular disease (adjusted HR, 0.88; 95% CI, 0.66–1.17), and HF (adjusted HR, 0.89; 95% CI, 0.49–1.63) were not significantly different between the two groups. The Kaplan-Meier curves for the incidence of composite CVD outcome and each specific CVDs in patients with and without RAI treatment are shown in Figure 2.
Full table

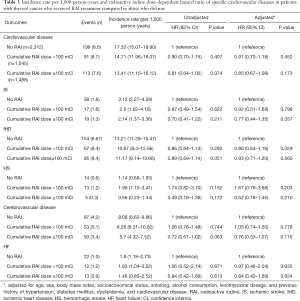
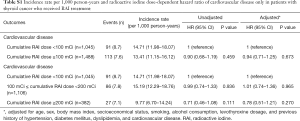
We analyzed whether the cumulative RAI dosage was associated with CVD outcomes in patients with TC (Table 3). We divided the patients into the following three groups according to the cumulative RAI dosage: no RAI, <100, and ≥100 mCi. After adjustments for age, sex, BMI, SES, smoking, alcohol consumption, levothyroxine dosage, and histories of hypertension, DM, dyslipidemia, and CVD, the HR for the incidence of composite CVD was 0.91 (95% CI, 0.70–1.18) for cumulative RAI dosage <100 mCi, and 0.85 (95% CI, 0.67–1.08) for cumulative RAI dosage ≥100 mCi compared with no RAI. The risks of incident IS, IHD, HS, cerebrovascular disease, and HF were not significantly different with a higher cumulative RAI dosage. In addition, we analyzed the effect of the cumulative RAI dosage with CVD outcomes only in patients who received RAI therapy (Table S1). Higher cumulative RAI dosages did not significantly increase the risks of composite CVD outcomes.
Full table
Full table
Discussion
Over the past several decades, RAI therapy has been largely used as an essential treatment modality in DTC as it allows for accurate postoperative staging and postsurgical follow-up while yielding better clinical outcomes (13,14). However, the adverse effects of RAI treatment have been much debated, and the RAI therapy-related CVD adverse effects, in particular, have yet to be fully elucidated. To this end, we used a large nationwide cohort database to compare patients who received RAI therapy with those who did not, and found that the risks of composite CVD were not significantly increased in TC patients with RAI therapy.
Previously, la Cour et al. (16) reported an increased risk of cerebrovascular events by comparing >4,000 hyperthyroid and 1,022 euthyroid patients treated with RAI for benign thyroid disorders with 20,540 age- and sex-matched controls over a median follow-up of 11.5 years. In multivariate analyses after adjustments, the HR for cerebrovascular events was 1.17 (95% CI, 1.07–1.28) in hyperthyroid patients and 1.21 (95% CI, 1.02–1.44) in euthyroid patients. It should be noted, however, that the actual values of thyroid stimulating hormone (TSH), which are important discriminative factors between RAI-treated patients and controls for cerebrovascular events, were not included in this study, and the median total dosage of RAI therapy (approximately 11 mCi) was relatively low compared to that used in other studies of patients with TC (9,24). We also postulate baseline patient age, extent of residual thyroid tissue as well as differences in underlying disease contribute discrepancy between two studies. Another recent nationwide cohort study with a 10-year follow-up from Taiwan (24) reported an HR of 0.89 (95% CI, 0.60–1.33) after adjustments for age, sex, and comorbidities, suggesting that 131I treatment for TC did not increase the risk of stroke. These discrepancies are likely due to the studies’ different underlying thyroid diseases, population parameters, and design; therefore, the inconsistent findings concerning higher cerebrovascular disease risk at lower cumulative RAI dosage and no increase risk at higher cumulative RAI dosage requires standardized international studies to acquire definite conclusions.
Unlike previous research, which focused on cerebrovascular diseases, especially IS, our study analyzed the specific CVDs, such as IHD and HF. Ryödi et al. (25) recently reported that hyperthyroid patients treated with RAI remained at higher CVD risk, particularly for HF, compared with patients treated with thyroidectomy. Furthermore, there have been few studies that have focused on coronary artery disease and HF induced by RAI treatment even with higher cumulative RAI dosage. Although more evidence is required, our findings demonstrated that a higher cumulative dosage did not significantly increase the risk of IHD and HF.
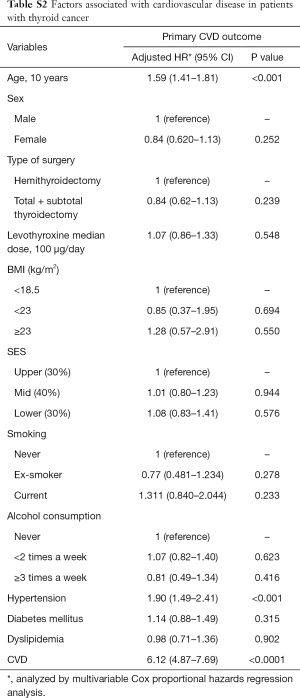
In the further analysis, old age and previous histories of HTN and major CVD, which have been well established as risk factors of CVD, were identified as independent risk factors of CVD (Table S2). Interestingly, the levothyroxine median dosage and type of surgery were not related with CVD risk in the patients with thyroid cancer.
Full table
Some limitations of this study should also be addressed. First, the relatively short follow-up might have masked the latent adverse effects of RAI therapy that occur 5 or 10 years after exposure, and more careful interpretation is required before reaching definite conclusions because of possible false-negative effects. Another major limitation of this study is the lack of clinicopathological information, such as the tumor stage, pathological subtype, and thyroid-stimulating hormone (TSH) levels, which can affect the treatment course and prognosis in TC. Recently, TSH levels have been reported to be associated with CVD events and CVD-related mortality (9,10,26); therefore, the effects of long-term TSH suppression therapy on CVD risks in patients with TC might have been overlooked. Third, our results cannot be generalized to patients aged <40 and ≥80 years because the NHIS-HEALS cohort only includes patients aged 40–79 years. However, a previous report has demonstrated that the incidence of newly diagnosed TC markedly increases after the age of 45 years (27), and a majority of CVD outcomes usually occur in this population (9,28). Lastly, as a common caveat of claims data, the primary outcomes were based on ICD-10 codes. To reduce the false-positive detection bias, we defined specific primary outcomes along with hospitalization history for each CVD ICD-10 code.
In this large nation-wide cohort study, we demonstrated that the risk of IS, IHD, HS, and HF was not significantly increased in patients with TC who received RAI treatment compared with those who did not, and that the cumulative RAI dosage was not associated with increased CVD risks in patients with TC. These results, together with those of previous studies, provide invulnerable evidence regarding the long-term effects of RAI therapy in patients with TC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STORBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5222
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5222
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5222). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of the Korea University Anam Hospital (IRB number: ED16255). Informed consent was not required because this study was based on the NHIS database, which includes fully anonymized and de-identified data. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 2017;317:1338-48. [Crossref] [PubMed]
- SEER [Internet]. SEER Cancer Statistics Review, 1975-2017 [cited April 21, 2020]. Available online: https://seer.cancer.gov/csr/1975_2017/index.html
- Vaccarella S, Franceschi S, Bray F, et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med 2016;375:614-7. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69:363-85. [Crossref] [PubMed]
- Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer "epidemic"--screening and overdiagnosis. N Engl J Med 2014;371:1765-7. [Crossref] [PubMed]
- Park S, Oh CM, Cho H, et al. Association between screening and the thyroid cancer “epidemic” in South Korea: evidence from a nationwide study. BMJ 2016;355:i5745. [Crossref] [PubMed]
- Lortet‐Tieulent J, Franceschi S, Dal Maso L, et al. Thyroid cancer “epidemic” also occurs in low- and middle-income countries. Int J Cancer 2019;144:2082-7. [Crossref] [PubMed]
- Suh B, Shin DW, Park Y, et al. Increased cardiovascular risk in thyroid cancer patients taking levothyroxine: a nationwide cohort study in Korea. Eur J Endocrinol 2019;180:11-20. [Crossref] [PubMed]
- Klein Hesselink EN, Klein Hesselink MS, de Bock GH, et al. Long-term cardiovascular mortality in patients with differentiated thyroid carcinoma: an observational study. J Clin Oncol 2013;31:4046-53. [Crossref] [PubMed]
- Yu CY, Saeed O, Goldberg AS, et al. A systematic review and meta-analysis of subsequent malignant neoplasm risk after radioactive iodine treatment of thyroid cancer. Thyroid 2018;28:1662-73. [Crossref] [PubMed]
- Molenaar RJ, Sidana S, Radivoyevitch T, et al. Risk of hematologic malignancies after radioiodine treatment of well-differentiated thyroid cancer. J Clin Oncol 2018;36:1831-9. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Yi KH, Lee EK, Kang HC, et al. 2016 revised Korean thyroid association management guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol 2016;9:59-126. [Crossref]
- Franklyn JA, Maisonneuve P, Sheppard MC, et al. Mortality after the treatment of hyperthyroidism with radioactive iodine. N Engl J Med 1998;338:712-8. [Crossref] [PubMed]
- la Cour JL, Jensen LT, Vej-Hansen A, et al. Radioiodine therapy increases the risk of cerebrovascular events in hyperthyroid and euthyroid patients. Eur J Endocrinol 2015;172:771-8. [Crossref] [PubMed]
- Metso S, Auvinen A, Salmi J, et al. Increased long-term cardiovascular morbidity among patients treated with radioactive iodine for hyperthyroidism. Clin Endocrinol (Oxf) 2008;68:450-7. [Crossref] [PubMed]
- la Cour JL, Hedemann-Jensen P, Søgaard-Hansen J, et al. Modeling the absorbed dose to the common carotid arteries following radioiodine treatment of benign thyroid disease. Ann Nucl Med 2013;27:862-6. [Crossref] [PubMed]
- la Cour JL, Andersen UB, Sørensen CH, et al. Radioiodine therapy does not change the atherosclerotic burden of the carotid arteries. Thyroid 2016;26:965-71. [Crossref] [PubMed]
- An JH, Song KH, Kim DL, et al. Effects of thyroid hormone withdrawal on metabolic and cardiovascular parameters during radioactive iodine therapy in differentiated thyroid cancer. J Int Med Res 2017;45:38-50. [Crossref] [PubMed]
- Seong SC, Kim YY, Park SK, et al. Cohort profile: the national health insurance service-national health screening cohort (NHIS-HEALS) in Korea. BMJ Open 2017;7:e016640. [Crossref] [PubMed]
- Kim NH, Han KH, Choi J, et al. Use of fenofibrate on cardiovascular outcomes in statin users with metabolic syndrome: propensity matched cohort study. BMJ 2019;366:l5125. [Crossref] [PubMed]
- Kim KJ, Jang S, Kim KJ, et al. Actual causes of death in thyroid cancer patients in Korea: a nationwide case control cohort study. Eur J Endocrinol 2020;182:103-10. [Crossref] [PubMed]
- Lin CY, Lin CL, Lo YC, et al. Association between radioiodine treatment for thyroid cancer and risk of stroke. Head Neck 2017;39:2311-8. [Crossref] [PubMed]
- Ryödi E, Metso S, Huhtala H, et al. Cardiovascular morbidity and mortality after treatment of hyperthyroidism with either radioactive iodine or thyroidectomy. Thyroid 2018;28:1111-20. [Crossref] [PubMed]
- Akirov A, Gimbel H, Grossman A, et al. Elevated TSH in adults treated for hypothyroidism is associated with increased mortality. Eur J Endocrinol 2017;176:57-66. [Crossref] [PubMed]
- Jung KW, Won YJ, Hong S, et al. Prediction of cancer incidence and mortality in Korea, 2020. Cancer Res Treat 2020;52:351-8. [Crossref] [PubMed]
- Kim H, Kim S, Han S, et al. Prevalence and incidence of atherosclerotic cardiovascular disease and its risk factors in Korea: a nationwide population-based study. BMC Public Health 2019;19:1112. [Crossref] [PubMed]
(English Language Editor: J. Gray)