
Association of circulating 25-Hydroxyvitamin D and its related genetic variations with hepatocellular carcinoma incidence and survival
Introduction
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related death worldwide, accounting for >80% of primary malignancy of the liver (1). An estimated 782,500 new liver cancer cases and 745,500 deaths occurred worldwide during 2012, with China alone constitutes about 50% of the total number of cases and deaths (2,3). Risk factors for HCC include chronic hepatitis B virus and hepatitis C virus, alcohol consumption, non-alcohol fatty liver disease, but there is still 15–50% HCC patients not having these risk factors (1,4). Although surgical resection is a potentially curative treatment that could prolong survival time, management of HCC remains disappointing due to high frequency of metastasis and recurrence (5). Several recent studies have examined the role of serum 25-hydroxyvitamin D [25(OH)D] concentrations with HCC risk or mortality (6-8).
When 7-dehydrocholesterol in the skin is exposed to Ultraviolet B radiation, vitamin D is produced and then transported to the liver, where it is hydroxylated to become 25(OH)D. Serum 25(OH)D, as a primary circulating form of vitamin D, makes a useful indicator of vitamin D stores (9). In vitro and animal studies suggest that vitamin D has a variety of anti-cancer effects, including the prevention of cell disjunction and overgrowth, and exerting multiple anti-proliferative and anti-inflammatory effects (9,10). The association between vitamin D and HCC is controversial. Higher 25(OH)D levels were associated with a 49% reduction in the risk of HCC in a nested case-control study within EPIC cohort study (6). And patients with 25(OH)D deficiency had an increased mortality risk (HR =2.225) (7). However, findings from EPIC cohort showed that vitamin D from dairy sources was associated with an increased HCC risk, whereas vitamin D from nondairy sources showed null associations (8).
The controversy may be caused by study’s latitudes which only focused on specific populations and the variation of different 25(OH)D measurement techniques. More fundamentally, a limitation of observational studies is that confounding and reverse causation may barricade reliable results. Randomized clinical trials (RCT) are an attractive alternative to observational studies, as these remove biases from confounding and reverse causation. However, RCTs are costly and logistically cumbersome, and there are only three undergoing RCTs assessing the relationship between 25(OH)D levels and risk of HCC [NCT01575717, NCT02779465 and NCT01956864]. Mendelian randomization (MR) is an approach for evaluating associations of an exposure with a disease (11,12). This technique utilizes the fact that allelic variants are assigned at random during meiosis, making them potentially robust and unbiased (free from confounding effects) instruments to gauge the effect of an exposure (e.g., low vitamin D) on a trait (e.g., HCC) (13).
Herein, in the current study, we first assess the association of serum 25(OH)D concentration with HCC risk in 100 HCC patients and 8,242 healthy controls, and test a potential causal inference in an MR framework using publicly available GWAS summary data. We further estimate the hazard ratio of 25(OH)D related genetic variation and its interaction with circulating 25(OH)D concentration for HCC prognosis. We present the following article in accordance with the REMARK reporting checklist (14) (available at http://dx.doi.org/10.21037/atm-20-1637).
Methods
Study population and design
The study protocol was approved by the Ethics Committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University (No. 20170222-60). All participants gave their written informed consent prior to study inclusion. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013) for prospective biomarker studies. HCC was defined as first incident tumor in the liver [C22.0 as per the 10th Revision of the International Statistical Classification of Diseases, Injury and Causes of Death (ICD-10)]. For each identified case, the histopathological examination of biopsies taken from liver tumors was reviewed to exclude metastatic cases or other types of liver cancers. We excluded patients who were an age below 18 years, had a history of cancer other than HCC. During Sep 2013 to April 2016, a total of 109 HCC cases who underwent liver resection were identified. Nine cases had no available serum samples for vitamin D analyses and so could not be included; however, they did not differ by lifestyle and demographic characteristics from those cases with available serum sample. Thus, a final series of 100 HCC with available serum sample were identified. Cancer-free participants containing 4,884 males and 3,358 females who took annual check-ups during 2018 were selected as healthy controls. General information (e.g., age, sex, body mass index (BMI), smoke and alcohol status) and clinical characteristics [e.g., blood collection time, fasting glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alpha fetoprotein (AFP), Child-Pugh score, transarterial chemoembolization (TACE) treatment and hepatitis B surface antigen (HBsAg)] of participants were obtained from Sir Run Run Shaw Hospital Bigdata Database.
HCC cases who underwent hepatic resection in our hospital were required to take outpatient follow-up every 3 months during the first year and every 6 months subsequently. We also conducted regular telephone interview (every 6 month) to check their health status. Recurrence of HCC after resection, recurrence time, cause of death, and death time were recorded. Cases were followed up until death or up to Oct 2018.
25(OH)D assessment
At recruitment, blood samples were collected from each participant. After centrifugation, serum was aliquoted and stored at ‒80 °C. Serum 25(OH)D2 and 25(OH)D3 concentrations were determined using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (ABSciex 4500), which is the gold criteria for vitamin D detection. The average intra-assay coefficient of variation for serum 25(OH)D was 5%. Total 25(OH)D concentration, which equals to the sum of 25(OH)D2 and 25(OH)D3 concentrations, was used in this study.
SNP selection and genotype
Six genetic loci (rs3755967 in Group-Specific Component (GC) gene, rs12785878 in NAD Synthetase 1 (NADSYN1) gene, rs10741657 in Cytochrome P450 family 2, subfamily R, polypeptide 1 (CYP2R1) gene, rs17216707 in Cytochrome P450 family 24, subfamily A, polypeptide 1 (CYP24A1) gene, rs10745742 in Amidohydrolase Domain Containing 1 (AMDHD1) gene and rs8018720 in Sec23 Homolog A (SEC23A) gene were identified by a GWAS with a 79,366 discovery sample and a 40,562 replication sample in association with serum levels of 25(OH)D (15). Genomic DNA was prepared from EDTA anti-coagulated peripheral blood by using Tiangen DNA kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s instructions. Genotypes were determined by the KASP genotyping assay, a unique form of competitive allele-specific PCR combined with a novel, homogeneous, fluorescence-based reporting system for identification and measurement of genetic variation (16).
Statistical analysis
Continuous variables with normal distribution were shown as mean ± standard deviation, with abnormal distribution shown as median (interquartile range), and categorical variables were reported as frequency (proportion). Differences in the general characteristics between HCC cases and healthy controls were determined using the Chi-squared test, Student’s t-test or non-parametric Wilcoxon-Mann-Whitney test. Serum 25(OH)D concentrations <20 ng/mL were considered as vitamin D insufficiency, and serum 25(OH)D levels ≥20 ng/mL were considered vitamin D sufficiency. Multivariate Logistic regression model was built to determine odds ratios (ORs) and 95% CIs for HCC risk after adjustment for age at diagnosis, sex, BMI, blood collection time (January-March regarded as spring, April-June as summer, July-September as autumn, and October-December as winter), and fasting glucose. Since it’s unbalanced between HCC cases and controls, 1:4 propensity score matching according to age and sex was performed to test the robustness of results.
These six index vitamin D proxy single nucleotide polymorphisms (SNP) are used as instrumental variables (IVs) in our MR analyses. assessing the association between vitamin D and HCC. Applying a two-sample MR approach, the association of index SNPs with HCC risk was obtained from a GWAS study including 721 individuals with HCV-induced HCC and 2,890 HCV-negative controls. Three core assumptions need to be satisfied to ensure a valid IV (17,18): (I) IV should be associated with the exposure; (II) No association of IV and exposure-outcome relationship; (III) IV should affect the outcome only through the exposure, this also called no pleiotropy assumption. When all MR assumptions are met, a causal relationship of exposure and outcome can be made since SNPs are randomly allocated at conception and always precede disease onset. Beta coefficient and standard error (SE) of index SNPs with 25(OH)D was extracted from a GWAS with a 79,366 European ancestry (15). Besides, beta coefficient and standard error of index SNPs with HCC risk was obtained from a GWAS study including 721 individuals with HCV-induced HCC and 2,890 HCV-negative controls (19).We applied a two-sample MR approach to determine the causal relationship between circulating 25(OH)D concentration and the risk of HCC incidence. We applied several MR methods including an inverse variance-weighted average approach (IVW) (20), a weighted median approach (21) and MR-Egger regression (22)(20). In addition, a sensitivity analysis in which one SNP was excluded at a time was performed to test the potential influence of outlying variants on the estimates.
Death because of HCC was treated as events. Accordingly, alive at the most recent follow-up (Oct 2018) or deaths from other causes were treated as censored observation. The overall survival (OS) was derived from the date of the diagnosis to the date of death. The disease-free survival (DFS) was defined as interval from the date of the diagnosis to the date of cancer recurrence. Linear regression analyses were used to determine the association of the six SNPs identified by Genome-wide Association Study and log transformed 25(OH)D concentration. The beta coefficient of each SNP in linear regression model was ensured in the same direction (the vitamin D increasing allele is the effect allele), then used to construct weighted genetic risk score (GRS). Due to the very low allele frequency of rs17216707 wild-type, this SNP was not included in the survival analyses. Multivariate Cox proportional hazard model was built to determine hazard ratios (HRs) and 95% CIs for HCC OS and DFS after adjustment for age at diagnosis, sex, BMI, HBsAg status, Child-Pugh score and TACE treatment. The interaction items were calculated based on dichotomized SNPs with either a continuous 25(OH)D variable or dichotomized 25(OH)D variable. Random-effect meta-analysis with inverse-variance weights was also performed to pool the effect of each SNP on HCC prognosis. We designated the vitamin D increasing allele is the effect allele when performing meta-analysis.
Statistical analyses were performed using the SAS 9.2 software (SAS Institute Inc., Cary, NC, USA) and R version 3.6.1 (R Development Core Team 2011). The R package “MatchIt” was used for PSM and “Mendelian Randomization” for MR analysis. P value less than 0.05 with two tailed was considered to be statistically significant.
Results
Participants’ baseline characteristics are presented in Table 1. HCC cases were older, had more male subjects, higher fasting glucose level, and greater prevalence of liver injury (higher ALT and AST level) than healthy subjects. Mean serum 25(OH)D was higher in cancer cases versus control subjects (24.1 versus 15.4 ng/mL, P<0.001). But no differences were observed in blood collection season and BMI between cancer cases and control subjects (P>0.05).
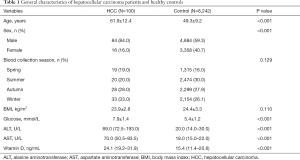
Full table
As shown in Table 2, sufficient circulating 25(OH)D level was associated with an increased risk of HCC (OR =7.56, 95% CI: 4.58–12.50). Statistically significant associations were observed of older age, higher fasting glucose level and Spring/Winter blood collection time with the higher risk of HCC, however, inverse associations of female and higher BMI with HCC risk were also observed. Because of the unparalleled distribution of age and sex between HCC cases and controls, we performed PSM using age and sex as the matching variable (Table S1). Table 3 presented the finding of the sub-analysis in HCC patients and 1:4 matched controls which confirmed that high 25(OH)D level was in relation to high risk of HCC (OR =5.48, 95% CI: 2.91–10.30).
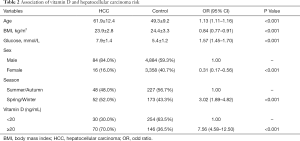
Full table

Full table
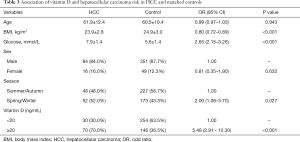
Full table
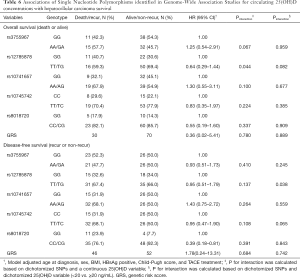
Table 4 shows the general information of the six index SNPs identified in genome-wide analyses for circulating 25(OH)D level, including the beta coefficient and standard error of these SNPs with 25(OH)D and HCC risk respectively. No evidence showed that any of the individual index SNPs were associated with the risk of HCC. Applied IVW approach, the magnitude of association was 0.028 (SE =0.620) for the instrumental variables, with corresponding OR of 1.03 (95% CI: 0.31–3.47) for HCC risk of continuous 25(OH)D concentration increasing. We identified no pleiotropy using MR-Egger (Pintercept =0.06). Furthermore, there was no evidence of a causal relationship between vitamin D and HCC by weighted-median and MR-Egger methods. The results did not change in the leave-one-out analyses where one SNP was removed at a time.

Full table
The general features of HCC patients were presented in Table 5. The distribution of demographic, smoking and alcohol drinking habit, and clinical features were comparable between insufficient (<20 ng/mL) and sufficient circulating 25(OH)D concentration (≥20 ng/mL). With regard to HCC cases with lower serum 25(OH)D, they were slightly older, had a lower BMI, greater proportion of female and blood collection time in Spring or Winter, but a smaller proportion of smoking and drinking. In addition, patients with insufficient plasma 25(OH)D had higher ALT and AST levels, a larger percentage of Child-Pugh B/C grade, but a smaller percentage of higher AFP level and HBsAg positive status. HCC cases with lower 25(OH)D levels had a significant less rate of TACE treatment (20% vs. 44.3%).
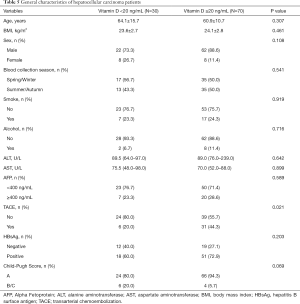
Full table
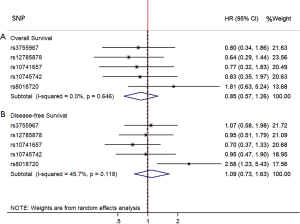
Multivariable hazard ratios for SNPs related with 25(OH)D levels on HCC overall survival and disease-free survival were summarized in Table 6. All other five index SNPs and GRS were not significantly associated with HCC mortality after adjustment for age at diagnosis, sex, BMI, HBsAg positive, Child-Pugh score, and TACE treatment. However, patients with rs8018720 CC/CG genotype had longer disease-free survival months (HR =0.39, 95% CI: 0.18–0.81) compared with GG genotype. Similarly, an interaction between rs12785878 and a continuous 25(OH)D variable was presented a reduced risks of HCC mortality with HR =0.91 (95% CI: 0.83–0.99). The multivariate hazard ratio of an interaction between rs12785878 and a dichotomized 25(OH)D variable for HCC disease-free survival was 0.23 (95% CI: 0.06–0.92). Furthermore, weighted GRS was not associated with the HCC mortality and disease-free survival, hazard ratios were 0.36 (95% CI: 0.02–5.41) and 1.78 (95% CI: 0.24–13.31), respectively. As shown in Figure 1, the hazard ratio for the effect of combining the individual SNP using random-effect meta-analysis with inverse-variance weights on HCC mortality was 0.85 (95% CI: 0.57–1.26), and on HCC disease-free survival was 1.09 (95% CI: 0.73–1.63).
Full table
Discussion
Vitamin D has been brought into focus in cancers within the last years. In this study, a significant association was observed for HCC risk in subjects with sufficient circulating 25(OH)D levels, but genetic variations related with 25(OH)D concentration were not remarkably associated with HCC recurrence and mortality. Furthermore, the interaction between rs12785878 genotype and circulating 25(OH)D concentration notably prolonged the HCC overall survival and disease-free survival time.
We observed a significant positive association for serum 25(OH)D concentration greater than 20 ng/mL with the risk of HCC (OR =7.56, 95% CI: 4.58–12.50). A nested case-control study embedded in EPIC cohort found that vitamin D from dairy source per 10% increase was related with an increased risk of HCC (HR =1.02, 95% CI: 1.01–1.04) (8). However, vitamin D from nondairy sources per 10% increase was not associated with HCC risk (HR =0.99, 95% CI: 0.97–1.02). It’s noteworthy that serum 25(OH)D per 10 nmol/L increase was associated with a 20% reduction in the risk of HCC (IRR=0.80, 95% CI: 0.68–0.94) in another study nested in EPIC cohort (6). Within ATBC study, lower serum 25(OH)D concentrations were associated with higher risk of liver cancer (<25 versus ≥50 nmol/L, OR =1.91, 95% CI: 1.16–3.15) (23). Nevertheless, evidence from the Linxian Nutrition Intervention Trials showed there were no significant associations between serum 25(OH)D and risk of liver cancer incidence, although risk estimates decreased across increasing quartiles of vitamin D (24). The findings of MR approach in this study also validated the null relationship between serum 25(OH)D concentration and HCC risk.
Our findings were inconsistent with previous studies that have reported significant inverse associations between 25(OH)D and HCC incidence. Populations in our study and the EPIC cohort were very different with respect to diet, lifestyle, environmental exposures, and HCC risk factor profiles (6), since they recruited European populations. For example, the levels of circulating vitamin D were lower in our study than in EPIC cohort (15.4 versus 20.0 ng/mL among controls), and had a narrow range of values (interquartile range 11.5 to 20.7 ng/mL). In addition, the outcome of ATBC study and Linxian trails was liver cancer, this would also cause the inconsistency of results.
In the past decade, increasing evidence has demonstrated that vitamin D levels are significantly associated with chronic liver disease (CLD) mortality (23,24). Within ATBC study, multivariate logistic regression model showed that low serum 25(OH)D concentrations were associated with higher risk of CLD mortality (OR =1.67, 95% CI: 1.02–2.75) (23). Another nested case-control study within the Linxian Nutrition Intervention Trials also demonstrated that compared with the lowest quartile, subjects in the fourth quartile had lower risk of CLD death (OR =0.34, 95% CI: 0.21–0.55) (24). A study including 200 HCC patients also found patients with severe 25(OH)D3 deficiency was associated with the highest risk of mortality (HR =2.225, 95% CI: 1.331–3.719), independently from the MELD score and high AFP levels in a multivariate Cox regression model (7). Our study found the potential benefits of rs8018720 CC/CG genotype for HCC recurrence (HR =0.39, 95% CI: 0.18–0.81). There was also supporting evidence that the interaction between rs12785878 and vitamin D was related with the prognosis of HCC patients. However, we found no evidence that vitamin D was associated with HCC prognosis. The inconsistency of findings could be explained by the population difference that Finkelmeier et al. and our study recruited HCC patients (7), another two studies selected CLD patients (23,24). Future study including a large sample size patients needs to be conducted to confirm the effect of vitamin D on HCC prognosis.
Key advantages of the present study were its MR design, which allows to make the causal estimation of vitamin D status and cancer development, and measurement of 25(OH)D using the state-of-the-art, gold standard LC-MS/MS method rather than enzyme-linked immunosorbent assay (ELISA) methods (25). This study also incorporates biomarkers of liver function (Child-Pugh score) into the analysis allowing the consideration of the 25(OH)D-HCC prognosis association for adjustment by potential liver injury. The liver plays a central role in the vitamin D metabolism (26), therefore, the metabolism leading to 25(OH)D generation is disturbed in patients with poor liver function. Lastly, the outcome of our study not only focused on HCC incidence, but also HCC prognosis containing overall survival and disease-free survival.
Meanwhile, our study still had some limitations. First, vitamin D concentration was measured once and only at the time of diagnosis. Second, the SNPs chosen in our study only explained a small fraction of the variance of 25(OH)D concentration since only six SNPs were included. Furthermore, only small sample size of HCC cases was recruited into the analyses, however, no substantial changes were observed in the sub-analysis after PSM. Lastly, vitamin D status might be susceptible to confounding, because high vitamin D concentration might reflect a healthier lifestyle in general. Additional confounding variables such as time spent outdoors, socio-economic status, vitamin D supplementation were not adjusted in our model, as this information was not available on all individuals in our dataset.
Conclusions
In summary, the findings from this study suggest that higher circulating 25(OH)D levels increased the risk of HCC incidence, however, the interaction between circulating 25(OH)D concentration and its related genetic variation reduced the hazards for HCC recurrence and mortality. Further research is needed to confirm or refute these findings in other populations and understand the underlying mechanisms of vitamin D’s actions.
Acknowledgments
We gratefully acknowledge staff from Bigdata platform and sample bank of Sir Run Run Shaw Hospital for providing subjects clinical information and blood samples.
Funding: This study was supported by Natural Science Foundation of Zhejiang Province, China [LQ17H260001 to HL, LY18H160029 to XL]; and National Natural Science Foundation of China [81772546 to XC].
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1637
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1637
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1637). All authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Ethics Committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University (No. 20170222-60). All participants gave their written informed consent prior to study inclusion. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589-604. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019;156:477-91.e1. [Crossref] [PubMed]
- Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015;261:947-55. [Crossref] [PubMed]
- Fedirko V, Duarte-Salles T, Bamia C, et al. Prediagnostic circulating vitamin D levels and risk of hepatocellular carcinoma in European populations: a nested case-control study. Hepatology 2014;60:1222-30. [Crossref] [PubMed]
- Finkelmeier F, Kronenberger B, Koberle V, et al. Severe 25-hydroxyvitamin D deficiency identifies a poor prognosis in patients with hepatocellular carcinoma - a prospective cohort study. Aliment Pharmacol Ther 2014;39:1204-12. [Crossref] [PubMed]
- Duarte-Salles T, Fedirko V, Stepien M, et al. Dairy products and risk of hepatocellular carcinoma: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2014;135:1662-72. [Crossref] [PubMed]
- Wu DB, Wang ML, Chen EQ, et al. New insights into the role of vitamin D in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol 2018;12:287-94. [Crossref] [PubMed]
- Chiang KC, Yeh CN, Chen MF, et al. Hepatocellular carcinoma and vitamin D: a review. J Gastroenterol Hepatol 2011;26:1597-603. [Crossref] [PubMed]
- Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res 2017;26:2333-55. [Crossref] [PubMed]
- Yarmolinsky J, Wade KH, Richmond RC, et al. Causal Inference in Cancer Epidemiology: What Is the Role of Mendelian Randomization? Cancer Epidemiol Biomarkers Prev 2018;27:995-1010. [Crossref] [PubMed]
- Sheehan NA, Didelez V, Burton PR, et al. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med 2008;5:e177. [Crossref] [PubMed]
- McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005;97:1180-4. [Crossref] [PubMed]
- Jiang X, O'Reilly PF, Aschard H, et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat Commun 2018;9:260. [Crossref] [PubMed]
- He C, Holme J, Anthony J. SNP genotyping: the KASP assay. Methods Mol Biol 2014;1145:75-86. [Crossref] [PubMed]
- Zheng J, Baird D, Borges MC, et al. Recent Developments in Mendelian Randomization Studies. Curr Epidemiol Rep 2017;4:330-45. [Crossref] [PubMed]
- Teumer A. Common Methods for Performing Mendelian Randomization. Front Cardiovasc Med 2018;5:51. [Crossref] [PubMed]
- Kumar V, Kato N, Urabe Y, et al. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat Genet 2011;43:455-8. [Crossref] [PubMed]
- Burgess S, Scott RA, Timpson NJ, et al. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol 2015;30:543-52. [Crossref] [PubMed]
- Bowden J, Davey Smith G, Haycock PC, et al. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol 2016;40:304-14. [Crossref] [PubMed]
- Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512-25. [Crossref] [PubMed]
- Lai GY, Wang JB, Weinstein SJ, et al. Association of 25-Hydroxyvitamin D with Liver Cancer Incidence and Chronic Liver Disease Mortality in Finnish Male Smokers of the ATBC Study. Cancer Epidemiol Biomarkers Prev 2018;27:1075-82. [Crossref] [PubMed]
- Wang JB, Abnet CC, Chen W, et al. Association between serum 25(OH) vitamin D, incident liver cancer and chronic liver disease mortality in the Linxian Nutrition Intervention Trials: a nested case-control study. Br J Cancer 2013;109:1997-2004. [Crossref] [PubMed]
- Saleh L, Mueller D, von Eckardstein A. Analytical and clinical performance of the new Fujirebio 25-OH vitamin D assay, a comparison with liquid chromatography-tandem mass spectrometry (LC-MS/MS) and three other automated assays. Clin Chem Lab Med 2016;54:617-25. [Crossref] [PubMed]
- Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med 2011;364:248-54. [Crossref] [PubMed]


