
Association of ABO blood groups and non-culprit plaque characteristics in acute coronary syndrome: an optical coherence tomography study
Introduction
Cardiovascular disease (CVD) poses a serious threat to human health, with extremely high global morbidity, mortality, and disability rates (1). In 2017, approximately 17.8 million lives were lost to CVD worldwide (2). Recent research has suggested that CVD is a growing health concern, especially in developing countries, which account for almost 80% of CVD-related mortality globally (3).
The pathogenesis of CVD is much disputed, although it is known to be related to many factors. Over the last century, the relationship between ABO blood type and CVD has drawn considerable attention (4,5). In the 1990s, patients with type A blood were found to be more susceptible to CVD, while those with type O blood were less susceptible (6). Akhund et al. found a high incidence of myocardial infarction and angina among individuals with type A blood, but these conditions were relatively rare in individuals with type O blood (7). A large-scale general population study in the United States revealed ABO blood group to be significantly related the incidence of CVD, with patients with non-O blood types more likely to have CVD than patients with type O blood (8). Moreover, cross-sectional studies have indicated that non-O blood types are a risk factor for CVD complications, severity, and mortality (9-11). Previous studies mainly focused on the relationship between blood type and the characteristics of culprit coronary plaques, while this study focused on the relationship between different blood types and the characteristics of non-culprit coronary plaques, as the non-culprit lesion of coronary artery can represent the progress of coronary artery. Non-culprit lesion is relative to culprit lesion of coronary artery. It refers to all lesions in coronary artery except those that can cause ACS. ACS is often associated with multiple vascular lesions. In addition to criminal lesions with vulnerability, non-criminal Lesions also have vulnerability. In patients with ACS, MACE was found to be associated with a higher risk of major cardiac events in patients with multivessel lesion than in patients with single-vessel lesion (12). In recent years, with the development of intracoronary imaging technology, the application of CAG technology to guide the treatment of CAD, including the intervention treatment of the coronary lesion, its deficiency is more and more obvious. OCT can detect human coronary artery plaques, especially for the analysis of plaque characteristics and stability evaluation, greatly improve the diagnosis and treatment of CHD, especially ACS (13). Moreover, the advent of a new generation of OCT will not only improve scanning speed and imaging, but also assess peripheral coronary blood flow (FFR), especially in guiding the critical lesions of coronary artery lumen whether PCI treatment and assessment of microvascular blood flow, has shown great advantages, making OCT applications more extensive (14,15). In this study, we aimed to compare features of non-culprit plaques in patients with acute cardiac syndrome (ACS) with non-O and O blood types using optical coherence tomography (OCT). We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5381).
Methods
Study subjects
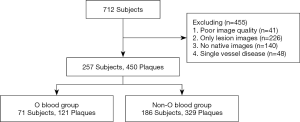
OCT images were taken from the image database of the Department of Cardiology of The Second Affiliated Hospital of Harbin Medical University. Between December, 2015, and November, 2016, 712 patients with ACS were registered. The exclusion criteria were as follows: poor OCT image quality, images only of the lesion, no native images, or single vessel disease. Non-culprit lesions were defined as untreated lesions in coronary angiography. Of the non-culprit lesions, plaques were included only when the narrow diameter was greater than 30% compared to the reference vessel on OCT (16,17). Finally, 257 ACS patients with 450 non-culprit plaques were included for analysis (Figure 1).
The patients’ demographic and clinical data including age, sex, comorbidity history (including hypertension, diabetes mellitus, and dyslipoproteinemia), smoking history, ABO blood type, serum lipid levels [including total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apoprotein A, apoprotein B, and triglycerides], and high-sensitivity C-reactive protein (hs-CRP) were collected. The diagnoses of ACS were established based on the criteria of the World Health Organization (WHO). The patients’ ABO blood types and blood biochemical indicators were determined in the Clinical Chemistry Department of our hospital.
This study was approved by the Ethics Committee of The Second Affiliated Hospital of Harbin Medical University, Harbin, China. Written informed consent was obtained from all patients before their inclusion in the study. The procedures followed were in accordance with the principles of the Declaration of Helsinki (as revised in 2013).
Acquisition of OCT images
OCT measurements were made using the Time Domain M2/M3 OCT System Operating Platform (SaintJude Medical, Westford, MA, USA). The guide wires used for OCT detection were from LightLab and St. Jude Medical. OCT-specific operating procedures were performed as previously described (18,19). Briefly, the frequency-domain system included a 2.7-F OCT catheter, which was introduced distal to the damage; as the blood was cleared, the imaging catheter was introduced automatically, and the image was acquired. In the time-domain system, an occlusion balloon was used to inflate the proximal damage at 0.4–0.6 atm. The guide wire was then automatically pulled back, and the image was acquired. Whole images were stored for later analysis.
Analysis of OCT images
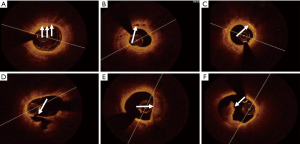
Plaques in OCT images were divided into fibrous and lipid plaques. Fibrous plaques were characterized by a uniform signal, high intensity, and weak attenuation. Lipid plaques displayed diffuse borders with low signals as well as strong attenuation regions. Lipid-rich plaques included those with a lipid arc (LA) of >90° and fibrous cap thickness (FCT) of <120 µm (20-22). The LA, lipid length (LL), and FCT of the lipid-rich plaques were measured. The LA was measured using a cross-section at each 1-mm interval, and the mean and maximum values were calculated; LL was measured using the longitudinal view; FCT was obtained using the average of three measurements at the thinnest section of the lipid-rich plaque. Thin-cap fibro atheromas (TCFAs), lipid-rich patches with a FCT of <65 µm, were identified from the OCT images (23). Macrophage infiltration was displayed as highly reflective region, with strongly attenuated point-like or banded structures, and often formed radial light and shadows after a high-signal point-like area (Figure 2A). Microchannels with diameters of 50 to 300 µm had signal-poor intensity (Figure 2B). Cholesterol crystals presented as thin and linear structures with high signal intensity and were usually located on the fibrous cap or necrotic core of plaques (Figure 2C). Plaque rupture in OCT was a continuous interruption of the fibrous cap accompanied by a ruptured cavity (Figure 2D). Calcification manifested as a high-signal area with sharp edges (Figure 2E). Thrombi appeared as irregular masses bonding with the facial part of the lumen or drifting in the lumen (Figure 2F) (24-27). The contents of macrophage infiltration, micro channels, plaque disruption, cholesterol crystals, and calcifications, as well as thrombi, were qualitatively analyzed. OCT image analysis was performed by two professional technicians using LightLab Imaging. When disagreements arose between the two analysts, the opinion of a third professional technician was sought to achieve a consensus.
Angiographic analysis
Coronary angiograms were analyzed by two senior angiographers using Offline Quantitative Coronary Angiography Software (Quantcor QCA 5.0). Along with the reference diameter and minimum lumen diameter, the stenosis diameter, lesion location, and lesion length were also measured. The images were simultaneously analyzed independently by two analysts. When disagreements arose during the analysis, the opinion of a third analyst was sought to achieve a consensus.
Statistical analyses
The D’Agostino-Pearson test was used to determine the normality of the distribution of continuous variables. Continuous variables were expressed as mean ± SD. Categorical variables were expressed as percentages or numbers. Chi-squared and Student’s t-tests were used to compare continuous and categorical variables between blood type groups. Analysis of variance (ANOVA) and chi-squared tests were used to compare continuous and categorical variables among three blood groups (types A, B, and AB). The Bonferroni correction was applied for multiple hypothesis tests among the three groups. Non-normally distributed measurement data were described as medians. Comparisons between groups were made using the Kruskal-Wallis test. The important factors were included into the logistic regression analysis to determine factors associated with the incidence of TCFA. One-tailed P values <0.05 were considered to represent statistical significance. All data were analyzed using SPSS 20.0 software (International Business Machines Corporation, Armonk, New York, USA). The sample size was estimated using PASS software (NCSS Limited Liability Company, Kaysville, Utah, USA). The significance level was set at α=0.05 and the test efficiency power was 0.08. The sample size was calculated based on two independent samples t-tests and corrected with the pooled variance method. The minimum sample size (n=105, 35 cases in the control group and 70 cases in the experimental group) was obtained. In total, 71 patients with 121 plaques were included in the O blood type group and 186 patients with 329 plaques were included in the non-O blood type group, which comprised sufficient numbers according to our power calculation.
Results
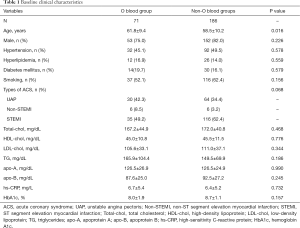
Baseline clinical features
Table 1 displays the baseline features and laboratory parameters. There were 186 ACS patients with non-O blood types and 71 subjects with type O blood. The non-O blood type group was significantly younger than the type O blood group (61.8±9.4 versus 58.5±10.2; P=0.016). No significant differences were found between groups with respect to other baseline clinical features.
Full table
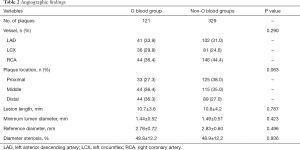
Angiographic findings
Table 2 displays the characteristics of the angiographic findings. A total of 450 non-culprit plaques were detected in 257 ACS patients. Subjects with non-O blood types had 329 plaques, and those with blood type O had 121 plaques. In the patients with blood type O, 33.9% of the plaques were on the left anterior descending artery (LAD), 29.8%were on the left circumflex artery (LCX), and 36.4% were on the right coronary artery (RCA). In the patients with non-O blood groups, 31.0% of the plaques were located in the LAD, 24.6% were located in the LCX, and 44.4% were in the RCA. No significant differences existed between the groups with respect to the reference diameter, minimum diameter, or percent diameter of the stenoses.
Full table
OCT findings
Table 3 shows the plaque characteristics identified by OCT. No statistically significant differences existed in the incidence of lipid-rich plaques among subjects with non-O and O blood types (56.8% vs. 60.3%, P=0.192), or in the prevalence of disruptions, calcifications, macrophage accumulation, microvessels, cholesterol crystals, or thrombi. However, the non-O blood type group had significantly more TCFAs than the O blood type group (24.3% vs. 5.0%; P<0.001). Quantitative OCT findings showed that, compared with the plaques of type O blood subjects, those of non-O type subjects had larger lipid arcs (maximum lipid arc: 288.57°±63.51° vs. 263.69°±59.40°; P=0.004), mean lipid arcs (207.01°±43.94° vs. 182.02°±39.48°; P<0.001),and thinner fibrous caps (68.7±14.2 vs. 84.0±16.4; P<0.001). Lipid length was not significantly different between the non-O and O type subjects (11.31±4.91 vs. 10.89±4.02; P=0.521).
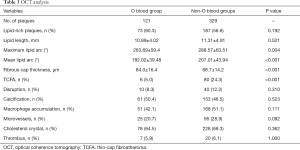
Full table
Univariate and multivariate logistic analysis
To test our hypothesis that blood groups work as a predictor of TCFAs, univariate and multivariate logistic analyses were carried out (Table 4). Univariate analysis revealed age [OR: 0.970 (95% CI: 0.943–0.998), P=0.033], hyperlipidemia [OR: 2.094 (95% CI: 1.018–4.308)], total cholesterol [OR: 1.012 (95% CI: 1.005–1.019)], LDL-C [OR: 1.016 (95% CI: 1.007–1.025)], and non-O blood group [OR: 11.891 (95% CI: 3.599–39.289)] to be indicators of TCFAs. After multivariate analysis, non-O blood group [OR: 9.421 (95% CI: 2.727–32.542)] was the only predictor of TCFA.
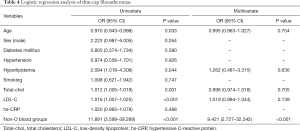
Full table
OCT subgroup analysis of different blood types
The OCT subgroup analyses of various blood types are shown in Table 5. Subjects with types A, B, and AB blood were compared with those with type O blood. The A blood type group had larger lipid arcs (maximum lipid arc: 292.89°±59.75° vs. 263.69°±59.40°; P=0.004), a larger mean lipid arc (221.55°±57.73° vs. 182.02±39.48°; P<0.001), thinner fibrous caps (64.7±14.3 vs. 84.0±16.4; P<0.001), and a greater number of TCFAs (30.5% vs. 5.0%; P<0.001). The B blood type group also had larger lipid arcs (maximum lipid arc: 289.69°±70.90° vs. 263.69°±59.40°; P=0.025), a larger mean lipid arc (207.24°±35.52° vs. 182.02°±39.48°; P=0.002), thinner fibrous caps (70.0±13.8 vs. 84.0±16.4; P<0.001), and a greater number of TCFAs (21.2% vs. 5.0%; P=0.001). The same results were obtained for the AB blood group, which also had larger lipid arcs (maximum lipid arc: 285.44°±52.85° vs. 263.69°±59.40°; P=0.047), a larger mean lipid arc (203.62°±46.33° vs. 182.02°±39.48°; P=0.006), thinner FCT (74.2±12.3 vs. 84.0±16.4; P=0.001), and a greater number of TCFAs (14.3% vs. 5.0%; P=0.039). Among the non-O blood type subjects, the type A group had thinner fibrous caps than the B and AB blood type groups (64.7±14.3 vs. 70.0±13.8 vs. 74.2±12.3; P<0.001) and a greater number of TCFAs (30.5% vs. 21.2% vs. 14.3%; P<0.001).
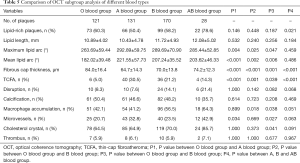
Full table
Discussion
Compared to the type O group, the non-O type group’s plaques were more vulnerable, with larger lipid arcs, thinner fibrous caps, and more TCFAs. In multiple logistic regression analysis, non-O blood type was found to be an independent predictor of TCFAs in non-culprit lesions. To our knowledge, this study is the first to compare non-culprit coronary plaque characteristics among different blood types in ACS patients using OCT.
Plaques based on larger lipid cores and thinner fibrous caps are at high risk of rupture and are considered vulnerable (28,29). The fracture of fragile plaques, together with the resulting thrombus, constitute the major causes of ACS. In recent years, non-culprit lesions have been extensively researched. Based on the autopsy results of patients who died of acute myocardial infarction, Mauriello et al. found that vulnerable plaques were present in both culprit and non-culprit lesions (30). Kato et al. reported similar results in a study of patients with ACS using intravascular ultrasound (IVUS) with coronary angiography, finding that the vulnerable plaques in non-culprit lesions were stronger than those in patients with stable angina pectoris (31). In the present study, non-culprit ACS plaques in subjects with non-O blood types had larger lipid arcs and thinner fibrous caps than those in subjects with type O blood. In subgroup analysis, blood types A, B, and AB subjects had larger lipid arcs as well as thinner fibrous caps compared to blood type O subjects. These findings are consistent with the pathological characteristics of plaque weakness. Our study is pioneering in that it analyzed detailed plaque characteristics in non-culprit lesions in patients with ACS of non-O blood types.
Patients with non-O blood types had more TCFAs than those with type O blood. Studies indicated that TCFAs are prone to plaque rupture, resulting in ACS (28,32). Tian et al. reported mild and moderate stenosis to have a higher number of TCFAs compared to severe stenosis, and both culprit and non-culprit lesions were characterized by TCFAs (33). In one multi center prospective study, 697 patients with ACS who underwent virtual histology intravascular ultrasound (VH-IVUS) of three vessels were recruited, and the relationship between non-culprit lesions and major adverse cardiac events (MACE) was assessed. The study showed that non-culprit lesions were associated with MACE, mostly because of TCFAs (34). Kubo et al. showed that TCFAs were more common in non-culprit lesions inpatients with ST-segment elevated myocardial infarction (STEMI) than in unstable patients with angina pectoris (35). These data suggested that TCFA may be an indicator of vulnerability and rupture. In the present study, we found that subjects with non-O blood types had more TCFAs than type O subjects. In subgroup analysis, the type A, B, and AB blood groups all had more TCFAs than the type O group. Further, patients with type A blood had more TCFAs than patients with types B and AB. Therefore, type A blood may be a potential risk factor for coronary events; however, more exploration is needed to verify these findings. TCFAs had a notably higher incidence in subjects with non-O blood types, especially in type A subjects.
Epidemiological studies discovered that ABO blood type, especially non-O types, had relevance to CVD. He et al. found that ABO blood type had significant relevance to CVD, and after more than 20 years’ follow-up of 62,073 women and 27,428 men, non-O type subjects had a higher incidence of CVD than those with type O (8). Zhang et al. conducted a 24.6-month follow-up study in a Chinese Han population and showed that type non-O blood increased the risk of cardiovascular events. Non-Oblood types were associated with higher incidences of CVD and myocardial infarction as well as higher Gensini scores than type O (36). Celebi et al. studied 212 patients with coronary angiography and determined coronary collateral circulation to be associated with blood type and the incidence of benign collateral circulation to be significantly higher than that of the adverse collateral circulation in patients with type O blood (37). In a microscopic analysis of plaques, Huang et al. studied 392 culprit plaques in patients with ACS using OCT and found that O types had more and thicker fibrous caps and fewer TCFAs than non-O types (38). These results are consistent with our findings regarding the association between blood type and non-culprit plaques. Furthermore, our study found that non-O blood types, especially type A, were associated with greater lipid arcs than type O blood. Previous studies have suggested that plaques in non-O blood type patients progress faster than those in patients with type O blood.
The mechanisms underlying the relationship between blood type and CVD are less clear. Chen et al. demonstrated that non-O type subjects had higher cholesterol levels, so they supposed that cholesterol levels may be a mediator between the ABO blood type and CVD (39). Timur et al. studied 206 patients with PCI, discovering that ABO blood type was closely related to platelet function, which affects platelet aggregation in the pathophysiology of myocardial infarction (40). Gong et al. investigated 3,806 patients with CVD identified using coronary angiography. They reported that non-O blood groups were significantly associated with CVD and myocardial infarction and had elevated levels of hs-CRP. Moreover, non-O blood groups were also found to possibly influence hs-CRP levels, thereby increasing the risks of CVD and myocardial infarction (41). Unlike in the previous studies, the non-O blood group patients in our study were younger than the O blood group patients, which suggest that, in the same state, the progression of CVD may be faster in patients with non-O blood types. There was no significant difference in serum cholesterol or hs-CRP levels between patients with non-O type vs. O type blood. These results do not support the mechanism previously proposed; instead, they suggest the mechanism underlying the relationship between ABO blood group and CVD is complicated and should be further explored.
In summary, the association between CVD and ABO blood type is complex. ABO blood types might, through diverse pathological processes such as atherosclerosis, plaque rupture, and thrombosis, promote the incidence of CVD and even ACS. ADAMTS7, which is at the ABO locus, is relevant to coronary atherosclerosis and myocardial infarction (42). Another study found that O blood type was correlated with reduced glycosyltransferase activity and FVIII-VWF levels in the circulation, lowering the risks of CVD and myocardial infarction (43). Genetic studies have shown that the ABO blood group locus is associated with interleukin-10 (IL-10), p-selectin, and thromboxane B2, the formation of which accelerates the process of arteriosclerosis (44,45). In the present study, OCT was used to determine that non-O type patients had more vulnerable plaque characteristics than type O patients in ACS. Non-O type subjects were also found to harbor more TCFAs than type O subjects, and non-O type blood was shown to be an independent indicator of TCFAs in non-culprit ACS lesions. Blood group can be easily determined and might useful in identifying vulnerable plaques in high-risk patients. Nevertheless, more studies are needed to clarify a potential system to verify our conclusions.
Limitations
Our study has several limitations. First, it was a single-center study, and therefore may be subject to selection bias. For this reason, we applied strict inclusion and exclusion criteria, and our findings should be validated in multi-center studies. Second, we only considered general clinical laboratory indicators such as serum lipid levels, hs-CRP, and glycated hemoglobin. In future research, the serum levels of factor VIII and von Willebrand factor (FVIII-VWF) and other coagulative factors that previous studies have indicated may be related to CVD will be analyzed. Finally, owing to the penetration depth limitations of OCT, plaque vulnerability could not be further evaluated.
Conclusions
Non-O type ACS subjects had more vulnerable non-culprit plaque characteristics and TCFAs than type O subjects according to OCT. Non-O type blood is considered to be a useful predictor of TCFAs in non-culprit ACS plaques. Among patients with non-O blood types, the type A blood group displayed more vulnerable characteristics than the type B and AB blood groups. Further studies of risk management and targeted therapies for various ABO blood groups are needed.
Acknowledgments
We wish to thank all participants and investigators for their participation in the study. We also would like to thank Editage (www.editage.cn) for English language editing.
Funding: The study was supported by National Key R&D Program of China (Grant No. 2016YFC1301100).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5381
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5381
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5381). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University, Harbin, China (No. KY2017-255). Written informed consent was obtained from all patients before their inclusion in the study. The procedures followed were in accordance with the principles of the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roth GA, Johnson C, Abajobir A, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1-25. [Crossref] [PubMed]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736-88. [Crossref] [PubMed]
- Ahmadizar F, Maitland-van der Zee AH. AdDIT Editorial comment—challenges in medication treatment of renal and cardiovascular diseases and risk factors in adolescents with type 1 diabetes. Ann Transl Med 2018;6:193. [Crossref] [PubMed]
- Garrison RJ, Havlik RJ, Harris RB, et al. ABO blood group and cardiovascular disease: the Framingham study. Atherosclerosis 1976;25:311-8. [Crossref] [PubMed]
- Medalie JH, Levene C, Papier C, et al. Blood groups, myocardial infarction and angina pectoris among 10,000 adult males. N Engl J Med 1971;285:1348-53. [Crossref] [PubMed]
- Tarján Z, Tonelli M, Duba J, et al. Correlation between ABO and Rh blood groups, serum cholesterol and ischemic heart disease in patients undergoing coronarography. Orv Hetil 1995;136:767-9. [PubMed]
- Akhund IA, Alvi IA, Ansari AK, et al. A study of relationship of ABO blood groups with myocardial infarction and angina pectoris. J Ayub Med Coll Abbottabad 2001;13:25-6. [PubMed]
- He M, Wolpin B, Rexrode K, et al. ABO blood group and risk of coronary heart disease in two prospective cohort studies. Arterioscler Thromb Vasc Biol 2012;32:2314-20. [Crossref] [PubMed]
- Carpeggiani C, Coceani M, Landi P, et al. ABO blood group alleles: A risk factor for coronary artery disease. An angiographic study. Atherosclerosis 2010;211:461-6. [Crossref] [PubMed]
- Gong P, Luo SH, Li XL, et al. Relation of ABO blood groups to the severity of coronary atherosclerosis: aGensini score assessment. Atherosclerosis 2014;237:748-53. [Crossref] [PubMed]
- Kaya A, Tanboga IH, Kurt M, et al. Relation of ABO blood groups to coronary lesion complexity in patients with stable coronary artery disease. Anadolu Kardiyol Derg 2014;14:55-60. [PubMed]
- Virmani R, Kolodgie FD, Burke AP, et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262-75. [Crossref] [PubMed]
- Gonzalo N, Tearney GJ, Serruys PW, et al. Second-generation optical coherence tomography in clinical practice. High-speed data acquisition is highly reproducible in patients undergoing percutaneous coronary intervention. Rev Esp Cardiol 2010;63:893-903. [Crossref] [PubMed]
- Zafar H, Sharif F, Leahy MJ. Feasibility of intracoronary frequency domain optical coherence tomography derived fractional flow reserve for the assessment of coronary artery stenosis. Int Heart J 2014;55:307-11. [Crossref] [PubMed]
- Prati F, Regar E, Mintz GS, et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 2010;31:401-15. [Crossref] [PubMed]
- Kato K, Yonetsu T, Kim SJ, et al. Comparison of nonculprit coronary plaque characteristics between patients with and without diabetes: a 3-vessel optical coherence tomography study. JACC Cardiovasc Interv 2012;5:1150-8. [Crossref] [PubMed]
- Kato K, Yonetsu T, Jia H, et al. Nonculprit coronary plaque characteristics of chronic kidney disease. Circ Cardiovasc Imaging 2013;6:448-56. [Crossref] [PubMed]
- Hasegawa T, Otsuka K, Iguchi T, et al. Serum n-3 to n-6 polyunsaturated fatty acids ratio correlates with coronary plaque vulnerability: an optical coherence tomography study. Heart Vessels 2014;29:596-602. [Crossref] [PubMed]
- Lee SY, Ahn JM, Mintz GS, et al. Characteristics of Earlier Versus Delayed Presentation of Very Late Drug-Eluting Stent Thrombosis: An Optical Coherence Tomographic Study. J Am Heart Assoc 2017;6:e005386. [Crossref] [PubMed]
- Yabushita H. Characterization of Human Atherosclerosis by Optical Coherence Tomography. Circulation 2002;106:1640-5. [Crossref] [PubMed]
- Jang IK, Tearney GJ, MacNeill B, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation 2005;111:1551-5. [Crossref] [PubMed]
- Jia H, Abtahian F, Aguirre AD, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol 2013;62:1748-58. [Crossref] [PubMed]
- De Rosa R, Vasa-Nicotera M, Leistner DM, et al. Coronary Atherosclerotic Plaque Characteristics and Cardiovascular Risk Factors- Insights From an Optical Coherence Tomography Study. Circ J 2017;81:1165-73. [Crossref] [PubMed]
- Tearney GJ. Quantification of Macrophage Content in Atherosclerotic Plaques by Optical Coherence Tomography. Circulation 2003;107:113-9. [Crossref] [PubMed]
- Tearney GJ, Regar E, Akasaka T, et al. Consensus Standards for Acquisition, Measurement, and Reporting of Intravascular Optical Coherence Tomography Studies. J Am Coll Cardiol 2012;59:1058-72. [Crossref] [PubMed]
- Kitabata H, Tanaka A, Kubo T, et al. Relation of microchannel structure identified by optical coherence tomography to plaque vulnerability in patients with coronary artery disease. Am J Cardiol 2010;105:1673-8. [Crossref] [PubMed]
- Kume T, Akasaka T, Kawamoto T, et al. Assessment of Coronary Arterial Thrombus by Optical Coherence Tomography. Am J Cardiol 2006;97:1713-7. [Crossref] [PubMed]
- Bom MJ, van der Heijden DJ, Kedhi E, et al. Early Detection and Treatment of the Vulnerable Coronary Plaque. Circ Cardiovasc Imaging 2017;10:e005973. [Crossref] [PubMed]
- Bentzon JF, Otsuka F, Virmani R, et al. Mechanisms of Plaque Formation and Rupture. Circ Res 2014;114:1852-66. [Crossref] [PubMed]
- Mauriello A, Sangiorgi G, Fratoni S, et al. Diffuse and active inflammation occurs in both vulnerable and stable plaques of the entire coronary tree: a histopathologic study of patients dying of acute myocardial infarction. J Am Coll Cardiol 2005;45:1585-93. [Crossref] [PubMed]
- Kato K, Yonetsu T, Kim SJ, et al. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non-acute coronary syndromes: a 3-vessel optical coherence tomography study. Circ Cardiovasc Imaging 2012;5:433-40. [Crossref] [PubMed]
- Virmani R, Burke AP, Farb A, et al. Pathology of the Vulnerable Plaque. J Am Coll Cardiol 2006;47:C13-8. [Crossref] [PubMed]
- Tian J, Dauerman H, Toma C, et al. Prevalence and characteristics of TCFA and degree of coronary artery stenosis: an OCT, IVUS, and angiographic study. J Am Coll Cardiol 2014;64:672-80. [Crossref] [PubMed]
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226-35. [Crossref] [PubMed]
- Kubo T, Imanishi T, Kashiwagi M, et al. Multiple coronary lesion instability in patients with acute myocardial infarction as determined by optical coherence tomography. Am J Cardiol 2010;105:318-22. [Crossref] [PubMed]
- Zhang Y, Li S, Zhu CG, et al. Risk Factors, Coronary Severity, Outcome and ABO Blood Group. Medicine 2015;94:e1708. [Crossref] [PubMed]
- Celebi S, Celebi OO, Berkalp B, et al. Blood Group Types O and Non-O Are Associated With Coronary Collateral Circulation Development. Clin Appl Thromb Hemost 2020;26:1076029619900544. [Crossref] [PubMed]
- Huang X, Zou Y, Li L, et al. Relation of ABO Blood Groups to the Plaque Characteristic of Coronary Atherosclerosis. BioMed Res Int 2017;2017:2674726.
- Chen Y, Chen C, Ke X, et al. Analysis of circulating cholesterol levels as a mediator of an association between ABO blood group and coronary heart disease. Circ Cardiovasc Genet 2014;7:43-8. [Crossref] [PubMed]
- Timur AA, Barnard J, Murugesan G, et al. The relation between ABO blood types and clinical and platelet function parameters in patients who underwent percutaneous coronary intervention. Coron Artery Dis 2019;30:51-8. [Crossref] [PubMed]
- Gong P, Li S, Luo SH, et al. High-sensitivity C-reactive protein mediates in part the impact of ABO blood group on coronary artery disease. Int J Cardiol 2014;177:641-3. [Crossref] [PubMed]
- Reilly MP, Li M, He J, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet 2011;377:383-92. [Crossref] [PubMed]
- Roest M, Voorbij HA, Barendrecht AD, et al. Risk of acute ischemic heart disease in postmenopausal women depends on von Willebrand factor and fibrinogen concentrations, and blood group genotype. J Thromb Haemost 2007;5:189-91. [Crossref] [PubMed]
- Johansson Å, Alfredsson J, Eriksson N, et al. Genome-Wide Association Study Identifies That the ABO Blood Group System Influences Interleukin-10 Levels and the Risk of Clinical Events in Patients with Acute Coronary Syndrome. PLoS One 2015;10:e0142518. [Crossref] [PubMed]
- Christiansen MK, Larsen SB, Nyegaard M, et al. The ABO locus is associated with increased platelet aggregation in patients with stable coronary artery disease. Int J Cardiol 2019;286:152-8. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)


