
Clinical features and the efficacy of adjuvant chemotherapy in resectable small bowel adenocarcinoma: a single-center, long-term analysis
Introduction
Cancer of the small bowel is so rare that it only comprise less than 5% of gastrointestinal cancers. Of tumors diagnosed in the small intestine, small bowel adenocarcinoma (SBA) has been reported as the most common histological type, accounting for 30% to 50% of cases (1,2). Approximately 10,000 new cases of small intestine tumor were estimated in the United States in 2018, of which more than 3,000 cases were SBA (3).
Complete resection with regional lymph node dissection is the only promising way to cure small SBA. However, unfortunately, locoregional recurrences and distant metastasis can occur (4,5). Local recurrence, typically in the surgical bed and lymph nodes, has been reported in 8% to 48% of SBA cases (6). Because of its rarity, there are currently no credible guidelines for SBA. The role of adjuvant chemotherapy and regimen selection has mainly been addressed in retrospective reports.
Only limited data exists on the role of chemotherapy in adjuvant treatment, and retrospective studies have found contradictory results. In several studies, adjuvant chemotherapy was not found to improve overall survival (OS) (7-9). However, retrospective evidence to support the use of adjuvant chemotherapy, particularly in patients with regional lymph node involvement, has been increasingly presented (10,11).
To facilitate a better understanding of the role of adjuvant chemotherapy for treating SBA, this retrospective study set out to evaluate the survival advantage of adjuvant chemotherapy after completed resection and to investigate better adjuvant chemotherapy regimens at a single institution.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1503).
Methods
Patients
Following approval from the hospital’s Ethics Review Committee, the medical records of 148 SBA patients who received radical surgical resection at Henan Cancer Hospital between 2008 and 2018 were retrospectively reviewed.
The criteria for inclusion were as follows: patients diagnosed as SBA without distant metastasis who underwent radical surgical resection. TNM stages were classified according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system. Available clinical details in relation to adjuvant therapy were collected. Only patients who had finished at least four cycles of adjuvant chemotherapy were included, and those who had underwent other anti-cancer therapies simultaneously were excluded. Follow up was performed every 3–6 months for the first 2 years after the operation and 6–12 months thereafter. Each patient had a physical examination and regular abdominal CT scan. The median follow-up period was 49 months (range, 10–90 months).
This was a retrospective study approved by the ethics committee of Henan Cancer Hospital (2019111815), and the requirement for informed consent was waived. The study conformed to the provisions of the Declaration of Helsinki, as revised in 2013.
Treatment and assessment
The patients were divided into three groups: no adjuvant chemotherapy; fluoropyrimidine [including 5-fluorouracil (5-FU) or capecitabine or S1] alone; and a combination of oxaliplatin and fluoropyrimidine-based chemotherapy. The doses of regimens were showed in Table 1. Disease-free survival (DFS) time was defined as the period of time from surgery to relapse, metastasis, or last follow-up. The OS time was calculated from the date of diagnosis to the date of death or last follow-up.

Full table
Statistical analysis
The Kaplan-Meier method and log-rank test were used to analyze DFS and OS. Multivariate survival analysis in the form of Cox proportional hazards regression was performed to estimate factors related to DFS and OS. All statistical analyses were performed using SPSS 23.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was considered to exist when P<0.05.
Results
Patient characteristics
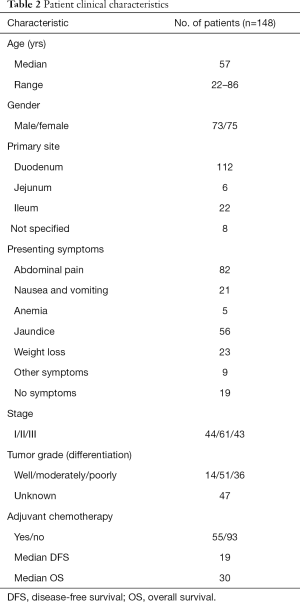
Between January 2008 and December 2018, 148 patients with SBA were treated at the hospital. The patient characteristics were summarized in Table 2. The median age of the patients was 57 years (range, 22–86 years). In most cases, the primary tumor was located in the duodenum (112/148, 75.68%). The proportion of lesions located in the jejunum and the ileum was 4.05% and 14.86%, respectively. While in 5.40% of cases, the location was not specified. At the time of diagnosis, 129 patients (129/148, 87.16%) had tumor-related symptoms, the most common of which were pain (55.40%), jaundice (37.84%), weight loss (15.54%), and nausea and vomiting (14.19%), while 19 patients (12.84%) had no symptoms at the time of diagnosis. Among the 55 patients who received adjuvant chemotherapy, 43 received the combined regimen and 12 received single-agent chemotherapy.
Full table
Kaplan-Meier analysis
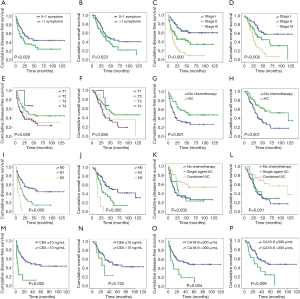
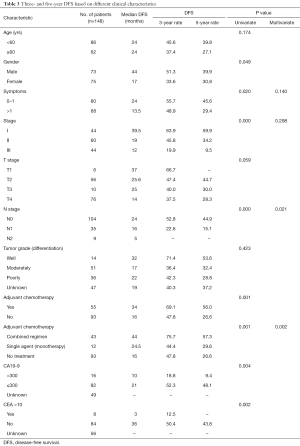
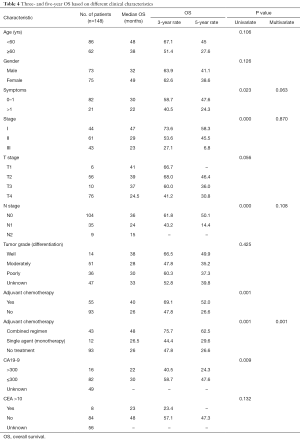
During the follow-up period, 87 patients (58.78%) experienced relapse. The median DFS and median OS were 19 and 32 months, respectively, for all 148 patients. TNM stage, N-stage, and more than one symptom at the time of diagnosis were associated with poor prognosis. The 3-year DFS rate was 63.9%, 45.8%, and 19.9%, for patients with stage I, II, III, respectively (Figure 1, Table 3). Notably, as N stage increased, the DFS and OS were shortened accordingly. The histological grading of all patients was available, but no association was found with DFS or OS. In addition, the median DFS and OS in patients with CA19-9 greater than 300 µ/mL were 10 and 23 months, respectively. The median OS of patients with CA19-9 less than 300 µ/mL was significantly longer. The same trend was observed when the cutoff value of CEA was 10 ng/mL.
Full table
Furthermore, there were also statistical differences in DFS and OS between patients with or without chemotherapy. Patients who received adjuvant chemotherapy had longer DFS and OS than who did not receive chemotherapy after curable resection (DFS: 34 vs. 16 months; OS: 40 vs. 26 months; Figure 1, Tables 3,4). Further analysis showed that the survival benefit was mainly associated with postoperative combined chemotherapy. The 43 patients who received combined adjuvant chemotherapy tended to have preferable 3- and 5-year DFS (75.7% and 57.3%, respectively) and OS (75.7% and 62.5%, respectively). However, single-agent chemotherapy was not superior to no chemotherapy in terms of DFS and OS.
Full table
Multivariate analysis
To identify independent risk factors for DFS and OS, the statistically significant factors whose P values were less than 0.05 by performing the univariate Cox regression were selected for subsequent multivariate analyses. The multivariate analysis showed that only N stage and combined adjuvant chemotherapy were statistically significant predictors of DFS. Nevertheless, only patients who received combined adjuvant chemotherapy had prolonged OS.
Discussion
SBA is a rare type of gastrointestinal tumor. Because of its rarity, no randomized phase III trials have been conducted to evaluate the potential of adjuvant chemotherapy for treating SBA patients, nor has a standard chemotherapy regimen been established. As a consequence, the treatment regimens for SBA commonly imitate those of colorectal cancer. However, whether adjuvant chemotherapy is beneficial as an SBA treatment is debatable.
After curative resection, the combination of fluoropyrimidine and oxaliplation are standard treatment for stage II (with high risk) and stage III colon cancer. This regimen of combined chemotherapy, which has an approximate overall response rate of 11–50%, has served as the optimal choice for advanced SBA in phase II and phase III studies (11-13). Currently, data regarding adjuvant chemotherapy for SBA are almost entirely limited to retrospective reports and the effect of adjuvant chemotherapy is still not conclusive. However, several retrospective studies have reported adjuvant chemotherapy to be associated with improved OS compared to no chemotherapy in both univariate and multivariate analysis (14,15).
In our retrospective, single-center, observational study, data from 148 patients with SBA were analyzed. At the time of diagnosis, more than 70% SBA was located in the duodenum and 129 (87.16%) patients were symptomatic. Although the patients in our study experienced similar symptoms to those in previous retrospective studies, the survival time of patients with different symptoms differed. The number of symptoms a patient experienced was an independent predictor of DFS (P=0.019) and OS (P=0.039). Patients with fewer symptoms had better therapeutic outcomes.
Based on univariate analysis, no association with survival time was found with gender, T-stage, and tumor grade. By contrast, N stage, CA19-9 or CEA status, no adjuvant chemotherapy, and a higher cancer stage could predict a decreased survival time. Although the univariate analysis revealed higher levels of CEA and/or CA19-9 to be of great significance to poor survival, in the multivariate analysis, only combined adjuvant chemotherapy was found to be an independent predictor of survival.
Diverse survival rates had been reported in different studies. In one retrospective analysis, the 5-year OS rate for stages I, II and III was shown to be 57%, 43%, and 42%, respectively (16). However, other studies found the 5-year survival rate to be around 30% (17,18). In other retrospective observational studies, the median OS ranged between 28.6 (19) and 36.9 (20) months in all stage I–III patients. In this study, the 5-year OS rate was shown to be 58.3%, 45.5%, and 6.8% for stages I, II and III, respectively. The median OS was 30 months, similar to those reported by other studies. Notably, patients who received adjuvant chemotherapy after curable resection achieved better DFS and OS compared with those who did not receive adjuvant chemotherapy. Survival analysis showed that the benefit of adjuvant chemotherapy could mainly be attributed to a combination of 5-FU and oxaliplatin. Combination chemotherapy was more effective in prolonging DFS and OS than 5-flurouracil alone. Patients treated with single-agent chemotherapy after surgery had the same DFS and OS as those who received no chemotherapy. Therefore, combined chemotherapy could improve the DFS and OS of SBA patients and should be recommended as an adjuvant therapy.
For early SBA, there may exist more risk factors to be found, such as dietary habits, history of smoking and alcohol abuse, cardiovascular diseases, diabetes, etc. However, they cannot be well controlled in this retrospective analysis. We expect more prospective studies to provide us with more accurate results in the future. Our study had limitations, including its retrospective design, single-center approach, and the absence of detailed information of the adverse events of adjuvant chemotherapy. Notwithstanding these limitations, our study may provide new evidence of the superiority of combined adjuvant chemotherapy over single-agent chemotherapy for extending survival time after radical resection.
Conclusions
Postoperative recurrence of small SBA was found to be influenced by both N stage and combined adjuvant chemotherapy. Moreover, combined adjuvant chemotherapy may serve as an independent prognostic factor of DFS and OS.
Acknowledgments
Funding: This study was supported by Key Scientific and Technological Projects in Henan Province (202102310111) and Key projects of Henan Provincial Department of Education (13A320440).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1503
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1503
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1503). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This is a retrospective study approved by the ethics committee of Henan Cancer Hospital (2019111815), and the requirement for informed consent was waived. The study conformed to the provisions of the Declaration of Helsinki, as revised in 2013.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 2009;249:63-71. [Crossref] [PubMed]
- Mitselos IV, Christodoulou DK. What defines quality in small bowel capsule endoscopy. Ann Transl Med 2018;6:260. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Ali WA, Liu W, Xiao Y, et al. A case report of small bowel adenocarcinoma with liver metastases: genetic profiling and clinical management. Transl Cancer Res 2019;8:1624-9. [Crossref]
- Mizushima T, Tamagawa H, Mishima H, et al. The effects of chemotherapy on primary small bowel cancer: A retrospective multicenter observational study in Japan. Mol Clin Oncol 2013;1:820-4. [Crossref] [PubMed]
- Ecker BL, McMillan MT, Datta J, et al. Adjuvant chemotherapy versus chemoradiotherapy in the management of patients with surgically resected duodenal adenocarcinoma: A propensity score-matched analysis of a nationwide clinical oncology database. Cancer 2017;123:967-76. [Crossref] [PubMed]
- Ye X, Zhang G, Chen H, et al. Meta-analysis of postoperative adjuvant therapy for small bowel adenocarcinoma. PLoS One 2018;13:e0200204. [Crossref] [PubMed]
- Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 2004;101:518-26. [Crossref] [PubMed]
- Rompteaux P, Gagnière J, Gornet JM, et al. Resection of small bowel adenocarcinoma metastases: Results of the ARCAD-NADEGE cohort study. Eur J Surg Oncol 2019;45:331-5. [Crossref] [PubMed]
- Halfdanarson TR, McWilliams RR, Donohue JH, et al. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am J Surg 2010;199:797-803. [Crossref] [PubMed]
- Aparicio T, Zaanan A, Mary F, et al. Small Bowel Adenocarcinoma. Gastroenterol Clin North Am 2016;45:447-57. [Crossref] [PubMed]
- Horimatsu T, Nakayama N, Moriwaki T, et al. A phase II study of 5-fluorouracil/L-leucovorin/oxaliplatin (mFOLFOX6) in Japanese patients with metastatic or unresectable small bowel adenocarcinoma. Int J Clin Oncol 2017;22:905-12. [Crossref] [PubMed]
- Li X, Ying H, Cheng Y, et al. Clinicopathological features and treatment outcomes of metastatic or locally unresectable small bowel adenocarcinoma. J BUON 2019;24:2539-45. [PubMed]
- Hirao M, Komori M, Nishida T, et al. Clinical use of molecular targeted agents for primary small bowel adenocarcinoma: A multicenter retrospective cohort study by the Osaka Gut Forum. Oncol Lett 2017;14:1628-36. [Crossref] [PubMed]
- Akce M, Jiang R, Zakka K, et al. Clinical Outcomes of Small Bowel Adenocarcinoma. Clin Colorectal Cancer 2019;18:257-68. [Crossref] [PubMed]
- Huffman BM, Jin Z, Yadav S, et al. Novel Prognostic Factors in Resected Small Bowel Adenocarcinoma. Clin Colorectal Cancer 2019;18:218-25. [Crossref] [PubMed]
- Young JI, Mongoue-Tchokote S, Wieghard N, et al. Treatment and Survival of Small-bowel Adenocarcinoma in the United States: A Comparison With Colon Cancer. Dis Colon Rectum 2016;59:306-15. [Crossref] [PubMed]
- Takayoshi K, Kusaba H, Uenomachi M, et al. Suggestion of added value by bevacizumab to chemotherapy in patients with unresectable or recurrent small bowel cancer. Cancer Chemother Pharmacol 2017;80:333-42. [Crossref] [PubMed]
- Aparicio T, Zaanan A, Svrcek M, et al. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis 2014;46:97-104. [Crossref] [PubMed]
- Rompteaux P, Gagnière J, Gornet JM, et al. Resection of small bowel adenocarcinoma metastases: Results of the ARCAD-NADEGE cohort study. Eur J Surg Oncol 2019;45:331-5. [Crossref] [PubMed]


