
Curcumol enhances the anti-tumor effects of metformin via suppressing epithelial-mesenchymal transition in triple-negative breast cancer
Introduction
Triple-negative breast cancer (TNBC) is an aggressive form of breast cancer with a high rate of metastasis and poor prognosis (1). TNBC lacks the expression of estrogen receptors, progesterone receptors, and human epidermal growth factor receptor 2 (HER2) (2). TNBC comprises 10–20% of breast cancer cases and is responsible for 25% of breast cancer-related deaths (3). Numerous studies exploring potentially effective medicines and therapeutic strategies for TNBC have been performed (2-4); however, conventional chemotherapy is still the most common method for treating TNBC patients (5). Besides, clinical trials for other novel approaches, such as molecular alterations (6), immune checkpoint inhibitors, and antiandrogen therapies, are ongoing (7). However, these options are still far from satisfactory, and an effective therapeutic strategy for TNBC is urgently needed.
Curcuma, commonly known as turmeric, has long been used in traditional Chinese herbal medicine. Curcumol, an active ingredient extracted from curcuma (8), has been investigated for its effects on various cancers, including gastric adenocarcinoma (9), colorectal cancer (10), and nasopharyngeal carcinoma (11). Importantly, the previous study reported curcumol to exert a protective effect in TNBC cells by triggering apoptosis via regulating the activation of p73 and p53 upregulated modulator of apoptosis (PUMA) (12). Besides, curcumol increased the sensitivity of TNBC cells to doxorubicin via regulating miR-181b-2-3p-ATP Binding Cassette Subfamily C Member 3 (ABCC3) axis (13). The drug metformin is approved for the treatment of diabetes. However, in recent years, it has also attracted attention for its anti-cancer properties (14). Metformin was reported to inhibit TNBC cell proliferation, colony formation and induce apoptosis through activating the intrinsic and extrinsic signaling pathways (15). The effects of metformin combined with curcumin, another active ingredient isolated from curcuma, on the progression of some tumors, including breast cancer, have been investigated (16-18). Structurally, however, curcumol and curcumin differ, which suggests that they might possess different bioactivity (19). For example, the inhibitory effect of curcumol on the production of inflammatory factors in RAW246.7 cells was more significant than that of curcumin (20). Currently, the effects of the combination of curcumol and metformin on the progression of TNBC are unclear. Therefore, more in-depth studies are needed to investigate the effects of curcumol and metformin in TNBC.
Previous studies have revealed that epithelial-mesenchymal transition (EMT) performs a vital role in cell remodeling for embryonic growth and cellular differentiation (21). The EMT process was accompanied by the loss of epithelial markers (e.g., vimentin and E-cadherin) and the gain of mesenchymal markers (e.g., N-cadherin and Twist 1) (22). Therefore, epithelial cells present strong metastatic potential due to the deficiency of epithelial cell polarities (23). Furthermore, EMT was shown to be related to cancer development and metastasis, with the transformation between mesenchymal and epithelial often leading to different results based on the different conversion directions (24). Moreover, accumulating evidence has demonstrated that the EMT process plays crucial roles in TNBC (25,26). The previous study found that the metastasis of TNBC was suppressed after inhibiting EMT process by Luteolin (27). However, the effect of curcumol administration on the EMT process in TNBC has yet to be illuminated. Hence, our study investigated the effects of curcumol combined with metformin in TNBC progression in vitro and in vivo. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5438).
Methods
Cell culture
MDA-MB-231, HCC1806, and MDA-MB-468 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, HyClone; GE Healthcare Life Sciences, Logan, UT, USA) with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher Scientific, Inc., MA, USA), 100 µg/mL streptomycin, and 100 U/mL penicillin (HyClone; GE Healthcare Life Sciences). All cells were maintained at 37 °C in a cell incubator containing 5% CO2. Subconfluent cells were treated with different concentrations of curcumol, 10 µM metformin, or 5 µM rucaparib (Merck KGaA, Darmstadt, Germany).
Cell viability
Cell viability was detected using Cell Counting Kit-8 (CCK-8, MCE, Shanghai, China). After treatment with different concentrations of curcumol, 10 µM metformin, or 5 µM rucaparib, cells were collected and plated into 96-well plates at a density of 2×105 cells per well. The cells were cultured in an incubator at 37 °C with 5% CO2. After 24 hours of culture, 10 µL CCK-8 was added to each well, and the cells were incubated with CCK-8. After 1 hour, the absorption values were detected with a Microplate Reader (Bio-Rad, Hercules, CA, USA) at 450 nm.
Cell apoptosis assay
Cell apoptosis was determined using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. MDA-MB-231 and HCC 1,806 cells were treated with 50 µM curcumol, 10 µM metformin, or 5 µM rucaparib, and then cell apoptosis was determined using a commercial TUNEL assay kit (Thermo Fisher Scientific, Waltham, MA, USA) following the instructions of the manufacturer. The cell nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI). The rate of apoptosis was defined as the number of cells with positive TUNEL staining divided by the number of total cells.
Wound healing assay
Cells were seeded into 6-well plates and cultured until they reached confluency. Afterward, a pipette tip was used to make a straight scratch to simulate a wound. The detached cells and debris were removed by washing the cells twice. The size of the wounds was measured at 0 and 24 hours.
Invasion assay
Transwell invasion assays were performed using 8.0-µm pore inserts (BD Biosciences, San Jose, CA, USA). A 200 µL cell suspension made using serum-free medium (2.5×104 cells) was loaded into the upper wells, and 600 µL complete medium with 10% FBS was added to the lower chambers as a chemoattractant. After 48 hours of incubation at 37 °C, the invasive cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet solution (Sigma-Aldrich, St. Louis, MO, USA). The number of invasive cells was calculated by counting five random areas.
Western blot
Cells were lysed for 30 min using radioimmunoprecipitation (RIPA) buffer (Sigma-Aldrich, St. Louis, MO, USA). Then, the cells were centrifuged at 10,000 ×g for 10 min at 4 °C, and the supernatants were collected. After that, cell lysates (50 µg) were resolved in 8–10% SDS-PAGE gels and then transferred to polyvinylidene difluoride (PVDF) membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked with 4% non-fat milk and then probed at 4 °C for 12 hours with the following primary antibodies: anti-cleaved Caspase-3 (1:500), Ki-67 (1:500), PCNA (1:500), MMP-9 (1:500), MMP-14 (1:500), E-cadherin (1:500), N-cadherin (1:500), Twist1 (1:500), β-Catenin (1:500), Wnt2 (1:500), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:1,000) (Abcam, Cambridge, UK). The membranes were then incubated with the corresponding secondary antibodies at room temperature for 1 hour. GAPDH was used as the internal control.
In vivo experiments
A tumor model was established in specific-pathogen-free (SPF) nude mice via subcutaneous implantation. Thirty-two 4-week-old nude mice were obtained from Beijing Laboratory Animal Research Center (Beijing, China). All of the mice were raised in an SPF environment at an animal facility at Capital Medical University. The mice were randomly divided into four groups (each group, n=8) including control group, curcumol group, metformin group and curcumol + metformin group. Then, TNBC MDA-MB-231 cells (1×107) were injected subcutaneously into the right flank of the mice. After developing measurable tumors, the mice in the control group were administered daily injections of Phosphate-buffered saline (PBS). The mice in the curcumol group were administered daily injections of curcumol 60 mg/kg. The mice in the metformin group were administered daily injections of metformin 100 mg/kg. The mice in the curcumol + metformin group were administered daily injections of Curcumol 60 mg/kg and metformin 100 mg/kg. The tumor volumes were detected every 7 days for 28 days. Four weeks later, the mice were sacrificed by the method of cervical dislocation, and the tumors were dissected and weighed. Experiments were performed under a project license (NO: SYXK [BEI JING] 2018-0002) granted by the Medical Ethics Committee for animal experimentation of Capital Medical University, in compliance with Chinese guidelines for the care and use of animals.
Immunohistochemistry
The tumor tissues were extracted from the mice, fixed with formaldehyde, and embedded using paraffin. The tissues were then cut into 4-µm sections. Endogenous peroxidase was blocked with 3% hydrogen peroxide and antigen retrieval was performed using citrate buffer with pH 6.0. Then, the sections were incubated with anti-Ki-67 antibody (1:200) and anti-VEGF antibody (1:200) (Abcam, Cambridge, UK) at 4 °C overnight. Following that, the sections were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (Abcam, Cambridge, UK) at 37 °C for 1 hour. The stained tissues were detected under light microscopy (Olympus, Japan).
Statistical analysis
Data were shown as mean ± standard deviation (SD). Statistical analyses were performed using Graphpad 6.0 statistical software (GraphPad Software, San Diego, CA, USA). Differences between groups were compared using one-way analysis of variance (ANOVA). A P value of <0.05 was considered to be statistically significant. All experiments were repeated at least three times.
Results
Curcumol enhanced the anti-proliferative effect of metformin in TNBC cells
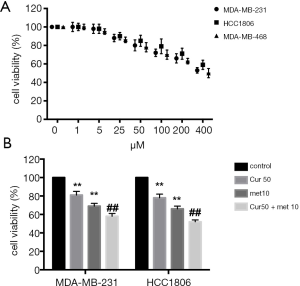
To ascertain the optimal concentration of curcumol for treating TNBC cells, 3 cell lines (MDA-MB-231, HCC1806, and MDA-MB-468) were treated with different concentrations of curcumol (1, 5, 25, 50, 100, 200, and 400 µM) and the effects on cell viability were detected. The results of the CCK-8 assay showed that 50 µM curcumol was the lowest concentration that significantly inhibited cell viability; consequently, this concentration was chosen for the following experiments (Figure 1A). Besides, MDA-MB-231 and HCC 1806 cells were used in the subsequent experiments. After treatment of MDA-MB-231 and HCC 1806 cells with 50 µM curcumol or 10 µM metformin, the cell viability was determined. The results showed that the cell viability of MDA-MB-231 and HCC 1,806 cells were slightly decreased after treatment with 50 μM curcumol; however, 10 µM metformin significantly reduced cell viability (P<0.01, Figure 1B). Moreover, the combination of 50 µM curcumol and 10 µM metformin further inhibited the viability of MDA-MB-231 and HCC 1806 cells (P<0.01, Figure 1B). These results indicated that curcumol enhanced the anti-proliferative functions of metformin in TNBC cells.
Curcumol promoted the apoptotic effect of metformin in TNBC cells
To explore the effects of curcumol and metformin on TNBC progression, we investigated TNBC cell apoptosis after treatment with 50 µM curcumol or 10 µM metformin. The TUNEL assay results showed that cell apoptosis was promoted by treatment with 50 µM curcumol alone as well as by treatment with 10 µM metformin alone (both P<0.01). Furthermore, 50 µM curcumol combined with 10 µM metformin further increased the cell apoptosis of MDA-MB-231 and HCC 1806 cells compared to 10 µM metformin alone group (both P<0.01, Figure 2A,B). These results revealed that curcumol could promote metformin-induced cell apoptosis.
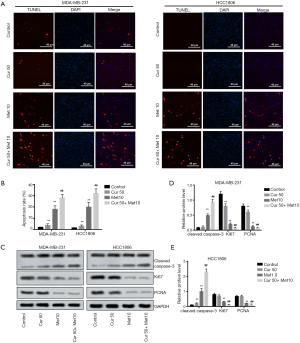
Additionally, we detected several related proteins: cleaved-Caspase-3, Ki67, and proliferating cell nuclear antigen (PCNA). Cleaved caspase-3 is one of the most crucial factors involved in the regulation of cell apoptosis (28). Ki67 is the most commonly used marker for evaluating the proliferative index in breast cancer, and TNBC has previously been shown to have higher levels of Ki67 expression (29). Meanwhile, PCNA is closely related to cell proliferation (30). As shown in Figure 2C,D,E, the levels of cleaved caspase-3 increased after treatment with curcumol alone and metformin alone, while the level of cleaved caspase-3 was further enhanced by the combination of curcumol and metformin (all P<0.01). The expression levels of Ki67 and PCNA were decreased by metformin, and inhibited further by the combination of curcumol and metformin (all P<0.01). Taken together, curcumol alone did not affect cell proliferation, but the combination of curcumol and metformin decreased cell proliferation and induced cell apoptosis; this suggested that curcumol enhanced the effect of metformin on the cell proliferation and apoptosis of TNBC cells.
Curcumol enhanced the anti-metastatic and anti-EMT effect of metformin in TNBC cells
To further investigate the effects of curcumol and metformin in TNBC, the migration and invasion abilities of TNBC cells were studied after treatment with 50 µM curcumol or 10 µM metformin. As shown in Figure 3A,B,C,D, no suppressive effects were observed in cell migration and invasion after treatment with curcumol alone; however, metformin inhibited cell migration and invasion ability (both P<0.01). Furthermore, the combination of curcumol and metformin significantly enhanced the inhibitory effect of metformin on the migration and invasion abilities of TNBC cells (both P<0.01).
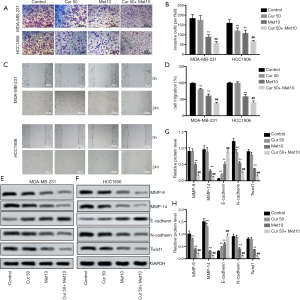
Then, we detected the expression of the cell migration and invasion-related proteins MMP-9, and MMP-14. The results of western blot showed that the levels of MMP-9 and MMP-14 in TNBC cells were decreased by metformin, and inhibited further by the combination of curcumol and metformin (all P<0.01, Figure 3E,F,G,H). Furthermore, the expression of the EMT-related proteins E-cadherin, N-cadherin, and Twist1, were also detected. Interestingly, the effects of metformin on the expression of E-cadherin, N-cadherin, and Twist1 levels in TNBC cells were also enhanced by the combination of curcumol and metformin (all P<0.01, Figure 3E,F,G,H). Therefore, curcumol could enhance the inhibitive effects of metformin on metastasis and EMT in TNBC.
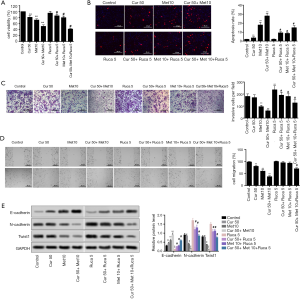
Curcumol reversed proliferation, migration, invasion, and EMT induced by rucaparib, and enhanced the effect of metformin in TNBC cells
To investigate the effect of curcumol on enhancing the anti-metastasis and anti-EMT properties exhibited by metformin in TNBC, the EMT inducer rucaparib was used to treat TNBC cells. The results showed that rucaparib significantly inhibited the effect of metformin. However, after the administration of curcumol, cell viability was decreased, which indicated that curcumol reversed the inhibitive effect of rucaparib on metformin (P<0.05, Figure 4A). Similarly, the function of metformin in promoting cell apoptosis was statistically decreased by rucaparib; however, the administration of curcumol increased the cell apoptosis rate and reduced the influence of rucaparib to a certain degree (P<0.05, Figure 4B). Meanwhile, we also observed the effects of curcumol on cell invasion and migration of rucaparib-induced TNBC cells. Rucaparib increased cell invasion (Figure 4C) and migration (Figure 4D), which were decreased by metformin; however, the administration of curcumol reversed this phenomenon (P<0.05, Figure 4C,D). Furthermore, the expression levels of EMT-related proteins E-cadherin, N-cadherin, and Twist1 followed the same trend (Figure 4E). Hence, curcumol could reverse rucaparib-induced proliferation, migration, invasion, and EMT, and enhanced the effects of metformin.
Curcumol enhanced the anti-growth and anti-EMT effect of metformin in vivo
To confirm our hypothesis based on the in vivo experiments, a tumor model was established via subcutaneous implantation, and tumor growth was measured. The results showed that the nude mice treated with metformin developed smaller tumors than the control mice, and the combination of curcumol and metformin further inhibited tumor growth when compared to metformin (P<0.01, Figure 5A,B). Moreover, immunohistochemistry was performed to determine the expression of Ki67 and VEGF in tumors (Figure 5C,D). The results of immunohistochemistry demonstrated that the tumors of the mice treated with curcumol combined with metformin had the lowest expression of Ki67 (P<0.01) and VEGF (P<0.01, Figure 5E). Furthermore, the expression of EMT-related proteins E-cadherin, N-cadherin, and Twist1 indicated that curcumol promoted the anti-EMT effect of metformin in vivo (P<0.01, Figure 5F,G,H).
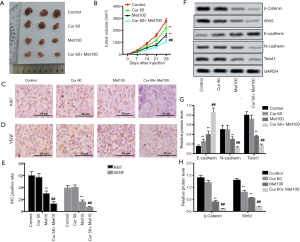
Finally, we detected Wnt/β-Catenin pathway-related proteins, including β-catenin and Wnt2. Interestingly, we found that the combination of curcumol and metformin enhanced the inhibitory effects of metformin on the expression levels of β-catenin and Wnt2 (both P<0.01, Figure 5F,G,H).
Discussion
TNBC is a life-threatening disease with a high metastasis rate and poor prognosis (5). Our study investigated the effects of curcumol and metformin on TNBC in respect to cell proliferation, migration, invasion, and EMT, and tumor growth in vitro and in vivo.
Cell proliferation and apoptosis are two vital biological processes in the development and growth of cells (31). Therefore, to investigate the effects of curcumol and metformin on TNBC cells, we first explored cell proliferation and apoptosis. Interestingly, we found that the administration of curcumol could enhance the effects of metformin in inhibiting cell proliferation and promoting cell apoptosis. Numerous experiments have demonstrated that metformin can suppress cancer cell proliferation and induce cell apoptosis (32,33). A previous study pointed out that co-treatment of hepatocellular carcinoma cells with metformin and sorafenib suppressed cell proliferation (34). Our study firstly revealed the anti-tumor effects of the combination of curcumol and metformin in TNBC cells. Besides, metformin also was found to regulate cell cycle in breast cancer (35). However, the effect of curcumol and metformin on TNBC cell cycle was not explored. We will perform cell cycle detection in the future study.
Furthermore, the administration of curcumol and metformin increased the expression of cleaved caspase-3 but inhibited the expression of Ki67 as well as PCNA, which further confirmed that curcumol improved the effects of metformin on cell proliferation and apoptosis in the TNBC cell lines MDA-MB-231 and HCC1806. Zhang et al. reported that curcumol induced apoptosis in osteosarcoma cells (36), which was consistent with the results in our research that the administration of curcumol strengthened the inhibitive effects of metformin.
Moreover, the migration and invasion of cancer cells to the surrounding area is a crucial part of metastasis (37). In this study, we determined the effects of metformin and the administration of curcumol on cell migration and invasion. Accordingly, our results demonstrated that metformin inhibited the migration and invasion abilities of TNBC cells, while the addition of curcumol enhanced these effects. Trinh et al. and He et al. demonstrated that metformin was found to exert a suppressive effect on cell migration and invasion in various cancers, such as cholangiocarcinoma (38) and esophageal squamous cell carcinoma (39), which was in agreement with the findings of the present study.
MMP-9 and MMP-14 are two vital proteins associated with cell migration and invasion. In our study, we found that metformin decreased the expression of MMP-9 and MMP-14, which was consistent with previous findings that metformin could inhibit endothelial progenitor cell migration by decreasing MMP-9 expression (40). Similarly, another study also pointed out that curcumol could suppress cell migration via inhibiting MMP-9 expression in breast cancer cells (41). Interestingly, the results in our study also pointed to a similar conclusion. Meanwhile, we discovered that the combination of curcumol and metformin could further decrease the expression of MMP-9 and MMP-14, which indicated that the addition of curcumol enhanced the anti-migration and anti-invasion effects of metformin in TNBC cells.
We also explored whether the administration of curcumol could affect the EMT process, and found that co-treatment with curcumol and metformin enhanced the effect seen with metformin on the expression of EMT-related proteins N-cadherin and Twist 1. These results indicate that curcumol enhances the anti-tumor function of metformin, possibly via changing EMT. In subsequent experiments, the EMT inducer rucaparib was used to determine the effect of curcumol and metformin in EMT regulation. Rucaparib is an inhibitor of poly (ADP-ribose) polymerase (PARP). Han et al. reported that rucaparib could induce the EMT process in TNBC cells (16). Therefore, rucaparib was used as an EMT inducer in this study. Our results indicated that curcumol reversed rucaparib-induced proliferation, migration, invasion, and EMT, and enhanced the protective effect of metformin in TNBC cells. Previous studies have shown that curcumol could induce EMT arrest in nasopharyngeal carcinoma (42) and breast cancer (43) cells, which supports the results of the current study. Taken together, we found that the combination of curcumol and metformin could strongly inhibit cell migration, invasion, and induce EMT arrest.
In vivo experiments were carried out to confirm the results obtained in vitro. Curcumol was found to have an anti-proliferative effect in colorectal cancer in vivo (10). In our study, the combination of curcumol and metformin enhanced the anti-tumor effect of metformin in inhibiting tumor growth, which was consistent with the previous findings.
The Wnt/β-Catenin pathway is closely related to TNBC. Previous studies have demonstrated that the inactivation of the Wnt/β-Catenin pathway is usually accompanied by anti-tumor effects, while the activation of the Wnt/β-Catenin pathway often leads to tumor growth and cancer development (44,45). Our study revealed that metformin decreased the expression of Wnt2 and β-Catenin, and the combination of curcumol and metformin enhanced the inhibitory effects compared with treatment with metformin alone, which suggests that curcumol can play a possible role in regulating Wnt/β-Catenin pathway in TNBC. A previous study pointed out that metformin inactivated the Wnt/β-Catenin pathway in colorectal cancer cells (46). Furthermore, the inactivation of the Wnt/β-Catenin pathway mediated the suppression of cell growth and metastasis in various cancer types, including TNBC (47,48). Therefore, curcumol might improve the antitumor effects of metformin via suppressing the Wnt/β-Catenin pathway.
In conclusion, this study investigated the anti-tumor effects of curcumol combined with metformin in TNBC cells. Curcumol alone did not significantly affect TNBC progression; however, curcumol improved the anti-tumor effects of metformin in TNBC via regulating the EMT and Wnt/β-Catenin pathways. Therefore, the combination of curcumol and metformin may be a promising therapeutic strategy for TNBC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5438
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5438
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5438). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (NO: SYXK [BEI JING] 2018-0002) granted by the Medical Ethics Committee for animal experimentation of Capital Medical University, in compliance with Chinese guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- EL Baiomy MA. El Kashef WF. ERCC1 Expression in Metastatic Triple Negative Breast Cancer Patients Treated with Platinum-Based Chemotherapy. Asian Pac J Cancer Prev 2017;18:507-13. [PubMed]
- Yang K, Zeng L, Ge A, et al. Investigating the regulation mechanism of baicalin on triple negative breast cancer’s biological network by a systematic biological strategy. Biomed Pharmacother 2019;118:109253. [Crossref] [PubMed]
- El Majzoub R, Fayyad-Kazan M, El Dine AN, et al. A thiosemicarbazone derivative induces triple negative breast cancer cell apoptosis: possible role of miRNA-125a-5p and miRNA-181a-5p. Genes Genomics 2019;41:1431-43. [Crossref] [PubMed]
- Castelli V, Piroli A, Marinangeli F, et al. Local anesthetics counteract cell proliferation and migration of human triple‐negative breast cancer and melanoma cells. J Cell Physiol 2020;235:3474-84. [Crossref] [PubMed]
- Naito Y, Urasaki T. Precision medicine in breast cancer. Chin Clin Oncol 2018;7:29. [Crossref] [PubMed]
- Botti G, Cantile M, Collina F, et al. Morphological and pathological features of basal-like breast cancer. Transl Cancer Res 2019;8:S503-9. [Crossref]
- Wang RX, Xu XE, Huang L, et al. eEF2 kinase mediated autophagy as a potential therapeutic target for paclitaxel-resistant triple-negative breast cancer. Ann Transl Med 2019;7:783. [Crossref] [PubMed]
- Wang J, Li X-m, Bai Z, et al. Curcumol induces cell cycle arrest in colon cancer cells via reactive oxygen species and Akt/GSK3β/cyclin D1 pathway. J Ethnopharmacol 2018;210:1-9. [Crossref] [PubMed]
- Zang S, Tang Q, Dong F, et al. Curcumol inhibits the proliferation of gastric adenocarcinoma MGC-803 cells via downregulation of IDH1. Oncol Rep 2017;38:3583-91. [PubMed]
- Liu H, Wang J, Tao Y, et al. Curcumol inhibits colorectal cancer proliferation by targeting miR-21 and modulated PTEN/PI3K/Akt pathways. Life Sci 2019;221:354-61. [Crossref] [PubMed]
- Li X, Liu H, Wang J, et al. Curcumol induces cell cycle arrest and apoptosis by inhibiting IGF‐1R/PI3K/Akt signaling pathway in human nasopharyngeal carcinoma CNE‐2 cells. Phytother Res 2018;32:2214-25. [Crossref] [PubMed]
- Huang L, Li A, Liao G, et al. Curcumol triggers apoptosis of p53 mutant triple-negative human breast cancer MDA-MB 231 cells via activation of p73 and PUMA. Oncol Lett 2017;14:1080-8. [Crossref] [PubMed]
- Zeng C, Fan D, Xu Y, et al. Curcumol enhances the sensitivity of doxorubicin in triple-negative breast cancer via regulating the miR-181b-2-3p-ABCC3 axis. Biochem Pharmacol 2020;174:113795. [Crossref] [PubMed]
- Podhorecka M, Ibanez B, Dmoszyńska A. Metformin-its potential anti-cancer and anti-aging effects. Postepy Hig Med Dosw (online) 2017;71:170-5. [Crossref] [PubMed]
- Liu B, Fan Z, Edgerton SM, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle 2009;8:2031-40. [Crossref] [PubMed]
- Han Y, Li CW, Hsu JM, et al. Metformin reverses PARP inhibitors-induced epithelial-mesenchymal transition and PD-L1 upregulation in triple-negative breast cancer. Am J Cancer Res 2019;9:800-15. [PubMed]
- Siddappa G, Kulsum S, Ravindra DR, et al. Curcumin and metformin-mediated chemoprevention of oral cancer is associated with inhibition of cancer stem cells. Mol Carcinog 2017;56:2446-60. [Crossref] [PubMed]
- Lindsay C, Kostiuk M, Conrad D, et al. Antitumour effects of metformin and curcumin in human papillomavirus positive and negative head and neck cancer cells. Mol Carcinog 2019;58:1946-59. [Crossref] [PubMed]
- Itokawa H, Shi Q, Akiyama T, et al. Recent advances in the investigation of curcuminoids. Chin Med 2008;3:11. [Crossref] [PubMed]
- Li N, Liu TH, Yu JZ, et al. Curcumin and Curcumol Inhibit NF-κB and TGF-β1/Smads Signaling Pathways in CSE-Treated RAW246.7 Cells. Evid Based Complement Alternat Med 2019;2019:3035125.
- Chen T, You Y, Jiang H, et al. Epithelial-mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis. J Cell Physiol 2017;232:3261-72. [Crossref] [PubMed]
- Liu CY, Lin HH, Tang MJ, et al. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 2015;6:15966-83. [Crossref] [PubMed]
- Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol 2016;43:7-13. [Crossref] [PubMed]
- Montanari M, Rossetti S, Cavaliere C, et al. Epithelial-mesenchymal transition in prostate cancer: an overview. Oncotarget 2017;8:35376. [Crossref] [PubMed]
- Jang MH, Kim HJ, Kim EJ, et al. Expression of epithelial-mesenchymal transition-related markers in triple-negative breast cancer: ZEB1 as a potential biomarker for poor clinical outcome. Hum Pathol 2015;46:1267-74. [Crossref] [PubMed]
- Qin H, Liu X, Li F, et al. PAD1 promotes epithelial-mesenchymal transition and metastasis in triple-negative breast cancer cells by regulating MEK1-ERK1/2-MMP2 signaling. Cancer Lett 2017;409:30-41. [Crossref] [PubMed]
- Lin D, Kuang G, Wan J, et al. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol Rep 2017;37:895-902. [Crossref] [PubMed]
- Glushakova OY, Glushakov AO, Borlongan CV, et al. Role of Caspase-3-Mediated Apoptosis in Chronic Caspase-3-Cleaved Tau Accumulation and Blood-Brain Barrier Damage in the Corpus Callosum after Traumatic Brain Injury in Rats. J Neurotrauma 2018;35:157-73. [Crossref] [PubMed]
- Hashmi AA, Hashmi KA, Irfan M, et al. Ki67 index in intrinsic breast cancer subtypes and its association with prognostic parameters. BMC Res Notes 2019;12:605. [Crossref] [PubMed]
- Boehm EM, Gildenberg MS, Washington MT. The many roles of PCNA in eukaryotic DNA replication. The Enzymes. Elsevier, 2016:231-54.
- Ye K, Wei Q, Gong Z, et al. Effect of norcantharidin on the proliferation, apoptosis, and cell cycle of human mesangial cells. Ren Fail 2017;39:458-64. [Crossref] [PubMed]
- Chen HL, Ma P, Chen YL, et al. Effect of Metformin on Proliferation Capacity, Apoptosis and Glycolysis in K562 Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2019;27:1387-94. [PubMed]
- Kim MY, Kim YS, Kim M, et al. Metformin inhibits cervical cancer cell proliferation via decreased AMPK O-GlcNAcylation. Anim Cells Syst (Seoul) 2019;23:302-9. [Crossref] [PubMed]
- Ling S, Song L, Fan N, et al. Combination of metformin and sorafenib suppresses proliferation and induces autophagy of hepatocellular carcinoma via targeting the mTOR pathway. Int J Oncol 2017;50:297-309. [Crossref] [PubMed]
- Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 2009;8:909-15. [Crossref] [PubMed]
- Zhang C, Wang LM. Inhibition of autophagy attenuated curcumol-induced apoptosis in MG-63 human osteosarcoma cells via Janus kinase signaling pathway. Oncol Lett 2017;14:6387-94. [PubMed]
- Duff D, Long A. Roles for RACK1 in cancer cell migration and invasion. Cell Signal 2017;35:250-5. [Crossref] [PubMed]
- Trinh SX, Nguyen HT, Saimuang K, et al. Metformin Inhibits Migration and Invasion of Cholangiocarcinoma Cells. Asian Pac J Cancer Prev 2017;18:473-7. [PubMed]
- He Y, Tan X, Hu H, et al. Metformin inhibits the migration and invasion of esophageal squamous cell carcinoma cells by downregulating the protein kinase B signaling pathway. Oncol Lett 2018;15:2939-45. [PubMed]
- Li WD, Li NP, Song DD, et al. Metformin inhibits endothelial progenitor cell migration by decreasing matrix metalloproteinases, MMP-2 and MMP-9, via the AMPK/mTOR/autophagy pathway. Int J Mol Med 2017;39:1262-8. [Crossref] [PubMed]
- Ning L, Ma H, Jiang Z, et al. Curcumol Suppresses Breast Cancer Cell Metastasis by Inhibiting MMP-9 Via JNK1/2 and Akt-Dependent NF-κB Signaling Pathways. Integr Cancer Ther 2016;15:216-25. [Crossref] [PubMed]
- Yan D, Deng S, Gan W, et al. Curcumol attenuates epithelial-mesenchymal transition of nasopharyngeal carcinoma cells via TGF-β1. Mol Med Rep 2018;17:7513-20. [PubMed]
- Li Z, Sun X, Liu X, et al. Antitumor Effects of Ruyiping on Cell Growth and Metastasis in Breast Cancer. Cancer Biother Radiopharm 2019;34:297-305. [Crossref] [PubMed]
- Liu S, Wang Z, Liu Z, et al. miR-221/222 activate the Wnt/β-catenin signaling to promote triple-negative breast cancer. J Mol Cell Biol 2018;10:302-15. [Crossref] [PubMed]
- Xie W, Zhang Y, Zhang S, et al. Oxymatrine enhanced anti-tumor effects of Bevacizumab against triple-negative breast cancer via abating Wnt/β-Catenin signaling pathway. Am J Cancer Res 2019;9:1796-814. [PubMed]
- Amable G, Martínez-León E, Picco ME, et al. Metformin inhibits β-catenin phosphorylation on Ser-552 through an AMPK/PI3K/Akt pathway in colorectal cancer cells. Int J Biochem Cell Biol 2019;112:88-94. [Crossref] [PubMed]
- Nie J, Jiang HC, Zhou YC, et al. MiR-125b regulates the proliferation and metastasis of triple negative breast cancer cells via the Wnt/β-catenin pathway and EMT. Biosci Biotechnol Biochem 2019;83:1062-71. [Crossref] [PubMed]
- Yu L, Wang C, Pan F, et al. HePTP promotes migration and invasion in triple-negative breast cancer cells via activation of Wnt/β-catenin signaling. Biomed Pharmacother 2019;118:109361. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)


