
Antibacterial peptides inhibit MC3T3-E1 cells apoptosis induced by TNF-α through p38 MAPK pathway
Introduction
Periodontitis and peri-implantitis are chronic infectious diseases caused by pathogenic microorganisms. The bacteria, inflammatory factors produced by bacterial metabolism and their inducing immune response may lead to alveolar bone resorption and even implant failures (1). Except for periodontal scaling and root planning procedure, local usage of antibiotics can effectively inhibit bacteria and reduce the inflammation response. Unfortunately, the susceptibility to drug-resistance of antibiotics and the inflammatory cytokines released from dead bacteria will aggravate the inflammatory response (2). Current therapies could not thoroughly control the development of inflammation and recover the damaged alveolar bone tissue. How to prevent alveolar bone loss and increase the amount of regenerated bone has been an important research topic.
Alveolar bone is considered as a major supporting structure of periodontal tissues. And osteoblasts are the important functional cells for bone formation, which is involved in early osteogenesis and late bone reconstruction (3). Lipopolysaccharide (LPS) produced by dead bacteria and its inducing inflammatory cytokines (4), such as tumor necrosis factor-a (TNF-a) and interleukin-1 play a significant role in alveolar bone resorption, and they can increase osteoclasts production and promote osteoblast apoptosis (5,6). Studies have shown that LPS could affect the proliferation and apoptosis of osteoblasts through multiple signaling pathways transductions, such as the induction of proliferation through Notch pathway, the inhibition of apoptosis through JNK signaling pathway (7,8). P38MAPK signaling pathway is also shown to participate in regulating the apoptosis of osteoblasts induced by LPS (9).
Antimicrobial peptides (AMP) is a small cationic peptide with broad-spectrum antibacterial activity and minor drug-resistance, have definite clinical value in inhibiting pathogenic microorganisms and cancer cells (10,11). Also, antimicrobial peptides can inhibit the production of inflammatory factors, including TNF-α or IL-1, and promote tissue healing in damaged areas (12). In recent years, antimicrobial peptides have gradually attracted widespread attention of clinical scholars, and the development of new antimicrobial drugs has become a research hot spot.
Recent research has found that antimicrobial peptides could promote the differentiation, migration, proliferation of mesenchymal stem cells, also inhibit the formation of osteoclasts induced by LPS (13,14). It has also been reported that antimicrobial peptides enhanced the osteogenic differentiation of bone marrow mesenchymal stem cells by activating the bone morphogenetic protein-2/smad axis (15). However, the effect of antimicrobial peptides on the bioactivity of osteoblasts in the inflammatory environment and its related mechanism is still rarely reported. In this study, MC3T3-E1 cells induced by TNF-α were used to simulate the inflammatory condition, and the role of antibacterial peptide DP7 in inhibiting cell apoptosis through the p38 MAPK signaling pathway was investigated, which will provide the theoretical basis for its use in the treatment of periodontitis and peri-implantitis.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5338).
Methods
Main reagents and equipment
Mouse MC3T3-E1 cells line (Chinese Academy of Medical Sciences), antimicrobial peptides DP7 (State Key Biological Laboratory of Sichuan University), minimum essential medium (Gibco, America), alizarin red (Shanghai Sinopharm Group Co. LTD), TNF-α (Peprotech), Annexin V-FITC/PI Apoptosis Detection Kit (Miltenyi, Germany), CellTiter96® Aqueous One Solution Cell Proliferation Assay Kit (Promega), Real-time PCR Assay Kit (TaKaRa), Anti-phospho-MAPK (Cell signaling, America), BCA Protein Assay Kit (Beyotime). Inverted microscope (Olympus, Japan), Real-time PCR Detection System (Bio-Rad, America), fluorescence microscopy (Nikon, Japan), Gel electrophoresis system (Bio-Rad).
Study methods
Culture of MC3T3-E1 cells
MC3T3-E1 cells were cultured in a CO2 incubator with MEM medium holding 10% fetal bovine serum,100 U/mL penicillin, and 100 mg/L streptomycin solution. The medium was changed every three days. When the cells were confluent to 70−80%, they were trypsinized using trypsin and subcultured.
Cell proliferation assay
MC3T3-E1 cells were seeded into 96-well plates at a density of 5×103 cells/well and divided into five groups: MEM (A), 50 ng/mL TNF-α (B), TNF-α + 10 µg/mL AMP (C), TNF-α + 20 µg/mL AMP (D) and 10 µg/mL AMP (E). After 24 hours of incubation, TNF-α was added into groups b, c, and d at a final concentration of 50 ng/mL. Twenty-four hours later, AMP at a final concentration of 10 or 20 µg/mL were added into group C, E, or D. After another 24 h of incubation, 20 µL Cell Titer 96® Aqueous One Solution Reagent was added to each well and incubated for 2 h in the incubator. Then the absorbance of the supernatant was measured at 490 nm using a spectrophotometer, n>6. After incubation for 24 h, cells were stained by Hoechst 33342 dye and counted in five random fields on each sample using a fluorescence microscope (Leica).
Cell apoptosis assay
Cells were seeded into 6-well plates at a density of 1×104 cells/well and divided into four groups: MEM (A), 50 ng/mL TNF-α (B), TNF-α + 10 µg/mL AMP (C), TNF-α + 20 µg/mL AMP (D), which were treated as described in experiment 1.2.2. After 24 h of incubation, cells were trypsinized with trypsin, washed three times with phosphate buffer saline (PBS), then stained with Annexin-V labeled by FITC and PI for 15 min under the conditions. Within one hour, the fluorescence intensity of the early and late apoptotic cells were measured by using flow cytometry.
Detection of cell migration ability
Cells were seeded, grouped, and treated as described in experiment 1.2.3. After 24 h of culture, longitudinal scratches were made with a pipette in the center of well. At 1, 2, and 3 days after scratching, cell migration distance was respectively observed under the microscope, and images were taken at five random fields of view on each sample.
Mineralized nodule staining assay
Cells were seeded at 5×105 cells/well in a 24-well plate and treated as described in experiment 1.2.3. After incubation for 21 days, extracellular matrix (ECM) mineralization by the cells on the samples was assessed by Alizarin Red staining and observed using under a microscope. The images were taken at five random fields of view on each sample.
Alkaline phosphatase activity assay
Cells were seeded, grouped, and treated as described in experiment 1.2.3, with five duplicate wells per group. After culturing for 14 days, cells were washed with PBS three times and treated with 100 µL 0.2% TritonX-100. Then 50 µL of the above liquid was mixed with 50 µL PNPP substrate solution (4.5 mmol/L). After incubation at 37 °C for another 30 min, 50 µL NaOH (0.1 mol/L) was added to stop the reaction. The absorbance of the supernatant was measured at 490 nm.
Western blot detection of p-p38 MAPK protein expression
Cells were seeded, grouped, and treated as described in experiment 1.2.3. The protein in MC3T3-E1 cells was extracted with the lysate, placed on ice for 40 min, and centrifuged at 12,000 r/min for 40 min. Then the protein taken from the supernatant was quantified by the BCA method. Twenty µg protein samples were conducted sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane. The polyvinylidene difluoride(PVDF) membrane was put into the blocking solution for 1 h (37 °C), diluted with the primary antibody, and incubated at 4 °C overnight. After being washed with PBS 3 times, the horseradish peroxidase marker was added to incubate with a diluted secondary antibody, and the membrane was shaken at 37 °C for 1 h. Enhanced chemiluminescence (ECL) exposure imaging is scanned into the computer.
The mRNA and protein expression of caspase-3
Cells were seeded, grouped, and treated as described in experiment 1.2.3. The primer was designed and synthesized with premier 5. MiniBEST Universal RNA Extraction Kit extracted total RNA and reverse-transcribed into cDNA according to the instructions of the Prime Script RT Master Mix Kit (TaKaRa). PCR reaction system included 1 µL of cDNA, 0.5 µL of upstream and downstream primers, 10 µL of SYBR Premix Ex Taq. After the reaction finished, the Mx 3000P real-time PCR system was used for quantitative analysis. Results were standardized according to the housekeeper gene and compared with the control group. Western blot detection of caspase-3 protein expression was same as the above experiment.
Elisa detection of cell apoptosis
Cells were seeded into 24-well plates at a density of 1×104 cells/well and divided into four groups: MEM, TNF-α, TNF-α + SB203580, TNF-α +AMP, TNF-α + AMP + SB203580. Cells were inoculated with AMP for 24 h and SB203580 for 3 h and then treated with TNF-α for 48 h. After the supernatant was removed, cells were treated with 0.5 mL Lysis Buffer for 30 min at room temperature and centrifuged at 1,500 rpm for 10 min. Then 20 µL of supernatant and 80 µL immunoreagent (holding anti-histone and anti-DNA) were transferred into a streptavidin-coated microplate. After microplate was washed three times with incubation buffer, 100 µL of ABTS solution was added and incubated for 15 min. At last, the absorbance of the supernatant was determined at 405 nm.
Statistical analysis
SPSS 22.0 software was used for data analysis. The measurement data were expressed as (
Results
AMP promoted the proliferation of the MC3T3-E1 cells induced by TNF-α
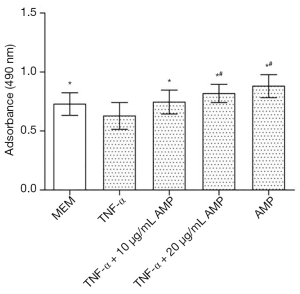
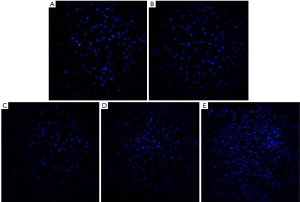
The results of the MTS assay showed that the proliferation activity of the MC3T3-E1 cells induced by TNF-α decreased significantly, while the proliferation of the cells treated with antibacterial peptides increased (P<0.05). And when the concentration of AMP went higher, the cell proliferation rate increased more obviously (Figure 1). The fluorescent staining results showed in Figure 2 also was consistent with that of MTS assay.
AMP inhibited the apoptosis of the MC3T3-E1 cells induced by TNF-α
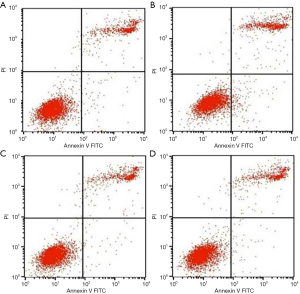
The results of fluorescent dye detected by flow cytometry (Figure 3) showed that when treated with TNF-α, the early and late apoptosis MC3T3-E1 cells increased compared to the control group (P<0.05). When treated with AMP, the early and late apoptosis rate was inhibited, but the different concentrations of antibacterial peptides changed without significance.
AMP enhanced the migration ability of MC3T3-E1 cells induced by TNF-α
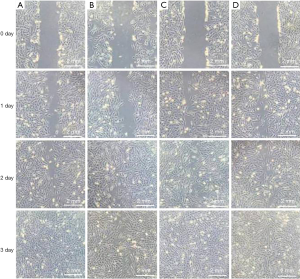
As shown in Figure 4, the migration ability of MC3T3-E1 cells treated with antibacterial peptides was higher than that treated with MEM or TNF-α, and the effect increased as the concentration increased. The migration ability of MC3T3-E1 cells induced by TNF-α was the slowest of all.
AMP promoted the osteogenesis of the MC3T3-E1 cells induced by TNF-α
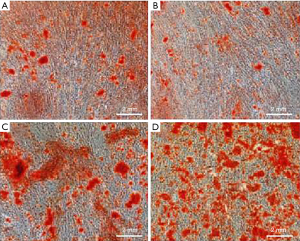
As shown in Figure 5, the formation of the mineralized nodule of cells treated with antibacterial peptides was the most, which was proportional to the concentration of antibacterial peptides. Cells induced by TNF-α formatted the least mineralized nodule. As shown in Figure 6, the alkaline phosphatase of the cells induced by TNF-α had the lowest absorbance compared with the MEM or AMP group, and the difference is statistically significant (P<0.05). There was no significant difference between the AMP groups.
AMP inhibited p38 MAPK phosphorylation in MC3T3-E1 cells induced by TNF-α
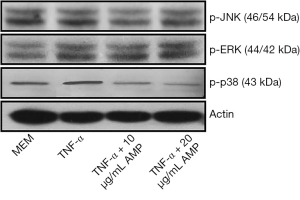
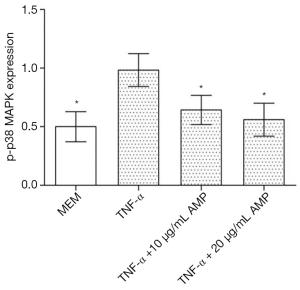
The results of Western blotting showed that when treated with TNF-α, the expression of p-p38 MAPK in MC3T3-E1 cells was significantly up-regulated compared with other groups, and the difference was statistically significant (P<0.05). After treatment with antimicrobial peptide, the phosphorylation of p-p38 MAPK was partially inhibited, whose degree negatively correlated to the concentration of AMP. But the phosphorylation of the p-JNK protein and the p-ERK protein had no significant changes (Figures 7,8).
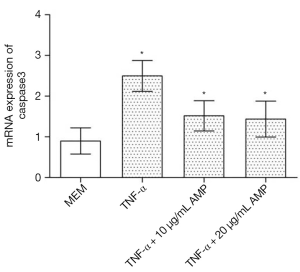
AMP inhibited caspase-3 mRNA expression in MC3T3-E1 cells induced by TNF-α
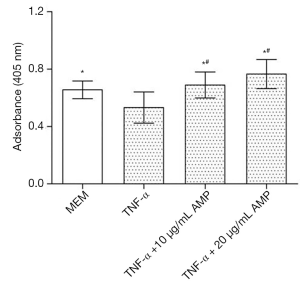
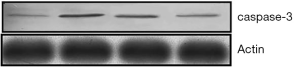
Real-time PCR analysis (Figure 9) showed that after being treated by TNF-α, the expression of caspase-3 mRNA in MC3T3-E1 cells was up-regulated. However, after antibacterial peptide treatment, caspase-3 mRNA expression was down-regulated, with significant statistical difference compared with the control group (P<0.05).As shown in Figure 10, the caspase-3 protein expression of MC3T3-E1 cells treated with TNF-α increased, but when treated with AMP DP7,the protein expression of caspase-3 reduced.
AMP inhibited MC3T3-E1 cells apoptosis induced by TNF-α via p38 MAPK pathway
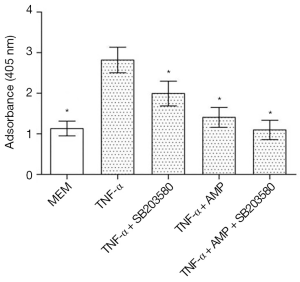
As shown in Figure 11, TNF-α treatment significantly increased MC3T3-E1 cells apoptosis, which had significant differences in statistics (P<0.05). When treated with a combination with SB203580 (a specific inhibitor of p38 MAPK), the apoptosis rate of MC3T3-E1 cells was inhibited to some extent, and there was a significant difference when compared with TNF-α treated group (P<0.05). After treated with SB203580, cell apoptosis rate in AMP treated group was back to normal, but there was no significant difference in cell apoptosis between two groups (P>0.05)
Discussion
Periodontitis is a common oral inflammatory disease, one of the leading causes of periodontal pockets, progressive attachment loss, and frontal resorption, finally leading to the loosening of teeth and loss of the tooth. It has a high prevalence and become an essential public health problem endangering people’s health. Also, peri-implant inflammation still is one of the major factors leading to implant failure. It is reported that five years after implantation, the incidence of peri-implant inflammation is as high as 14%. In addition to mechanical treatment, antibiotics are usually used to treat periodontal disease and peri-implant inflammation. However, the adverse reaction, dysbacteriosis, and resistance limited its clinical effectiveness. After antibiotics killed bacteria, substantial amounts of LPS were released, which stimulated macrophages to produce a series of inflammatory factors, including tumor necrosis factor, interleukin 6. The generation of osteoclasts and activation of osteoclasts process will be promoted directly or indirectly by inflammation factors.
Osteoblasts are the most critical functional cells for bone formation. Their proliferation and differentiation activity are the keys to osteogenesis, while their apoptosis rate increases bone resorption. The dynamic balance between osteoblasts and osteoclasts supports the relative stability of bone mass (16).
TNF-α, as important proinflammatory cytokines of periodontitis and periimplantitis, is one of the important pathogenic factors for bone resorption. It can inhibit osteoblast regeneration, reduce proliferation activity, and promote apoptosis (17). Several signaling pathways associated with inflammatory responses, including p38 MAPK, Wnt, NF-κB may, play some role in the apoptosis of osteoblasts induced by TNF-α. It is accepted that when the concentration of TNF-α was higher than 50 ng/mL, it would significantly promote cell apoptosis (18). In this study, TNF-α induced apoptosis of osteoblasts was used as an inflammatory model.
Antibacterial peptides are the first defensive line of the natural immune system and widely found in animals and plants (19). Due to their unique antibacterial mechanism, they can inhibit viruses, bacteria, fungi, protozoa, and cancer cells but have no cleavage effects on healthy mammalian cells. As one of the most promising antimicrobial drugs, antimicrobial peptides are expected to become a substitute for antibiotics (20).
DP7 is a novel 12-amino-acid cationic and hydrophilic antimicrobial peptide with the broad antibacterial spectrum and minor drug-resistance. It has a strong ability of immune regulation and inhibition of TNF-α production from LPS (21). It was reported that when the concentration of AMP was 10–20 µg/mL, it had an excellent bactericidal effect and biological activity (22).
In recent years, antibacterial peptides are shown to have the potential to promote osteogenesis of osteoblasts. Some scholars (23) found that antimicrobial peptides can markedly promote the differentiation, migration, and proliferation of mesenchymal stem cells and inhibit the formation of osteoclasts induced by LPS. Tripathi (24) reported that self-assembled antibacterial peptide KLD-12 not only had adequate antibacterial activity but also can promote rapid tissue healing. Bacitracin is also shown to promote osteogenic differentiation of human bone marrow mesenchymal stem cells by stimulating the bone morphogenetic protein-2/Smad axis (25). Another recent study suggested that cationic antibacterial peptide P15-CSP has a unique dual ability to inhibiting the formation of biofilms and increasing osteogenic activity as a hydrophilic surface coating (26).
In this study, it was found that TNF-α increased apoptosis activity MC3T3-E1 cells and decreased their migratory ability, which showed that the inflammation model was successful. AMP could inhibit the apoptosis of MC3T3-E1 cells induced by TNF-α, and they increased with the increase of concentration. Bone tissue reconstruction depends on the migration of osteoblasts. Cell migration determination suggested TNF-α treatment decreased the migratory ability of osteoblasts and thus affected bone regeneration speed. ALP activity is an early marker of osteogenic differentiation in osteoprogenitor cells and is expressed after seven days of osteogenic induction, with positive staining after osteogenic induction for 14 days (27). Mineralized nodule formation is a phenotypic marker for the last stage of mature osteoblasts. The results showed that the higher the concentration of AMP was, the stronger was the promotion of osteogenic differentiation of MC3T3-E1 cells.
To further explore the possible mechanism, we studied the role of the MAPK pathway in the inhibition of antimicrobial peptides on TNF-α induced osteoblasts apoptosis. MAPK includes p38, JNK, and ERK1/2 pathways, which play an essential role in maintaining cell morphology, proliferation, differentiation, and apoptosis. Studies showed that the p38 MAPK pathway participates in regulating the growth, development, apoptosis, and skeleton construction of mesenchymal stem cells or osteoblasts (28). Some proinflammatory cytokines were reported to activate the p38 MAPK pathway. They might be related to the suppression of p38 MAPK activation (29), which was consistent with our experimental results. Western blot showed that TNF-α activated the phosphorylation of p38 MAPK pathway in MC3T3-E1 cells, while the activation of JNK and ERK signaling pathway did not change appreciably. Moreover, antimicrobial peptides played a part in the antiapoptosis of osteoblasts by the inhibition of p38 MAPK activation to some extent.
We also found SB203580, a specific inhibitor of p38 MAPK, partially inhibited apoptosis induced by TNF-8 but did not eliminate apoptosis of osteoblasts induced by TNF-i. Therefore, we concluded that antibacterial peptides inhibit TNF-n induced apoptosis of osteoblasts via the p38 MAPK signal pathway, and there may be other mechanisms adjusting the antiapoptosis activity of antibacterial peptides on MC3T3-E1 cells. Some scholars concluded that ERK signaling pathway is involved in the proliferation, differentiation in and apoptosis of osteoblasts (30). The inhibition of ERK pathway could suppress cytochrome C release from the mitochondrion and caspase-3 activation by adjusting the down-regulated expression of Bax and up-regulated expression of Bcl-2, which led to apoptosis of osteoblasts (31,32). It is also reported that activated mitogen-activated protein kinase could transfer into the nuclear via activating NF-κB, and regulate a variety of downstream inflammatory factors, which resulted of inflammatory reaction.
There are several signaling pathways transductions mediating cell apoptosis, such as death receptor pathway, mitochondrial pathway and endoplasmic reticulum-associated pathway, among which mitochondrial pathway is the most classical one. Caspase-3 is a critical protease in apoptosis signaling pathway and plays an essential pivotal role (33). Endogenous and exogenous apoptotic signals lead to cell apoptosis by activating caspase-3 (34). In this study, we found TNF-α induced the activation of caspase-3 in osteoblasts, but antimicrobial peptides suppress the activity of caspase-3, which suggested the antiapoptosis effect of antimicrobial peptides was related to the inhibition of caspase-3 activation. After p38 MAPK was activated, nuclear translocation took place, which could also phosphorylate many protein kinases and transcription factors. Therefore, the p38 MAPK pathway may play a role in both the upstream and downstream of the caspase.
Conclusions
From the above analysis, antimicrobial peptides DP7 could inhibit the apoptosis of MC3T3-E1 cells induced by TNF-αand showed some dose-effect relationship. Its inhibiting function might be due to reducing the phosphorylation of p38 MAPK signaling pathway and thus inhibiting caspase-3 activation, but its specific mechanism needed to be further explored.
Acknowledgments
Funding: This study was financially supported by the Natural Science Foundation of Beijing (No.7182125), Beijing Excellent Talents Training Project (No. 2017000062586G232) and Military Medical Science and Technology Youth Training Program (No. 18QNP038).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5338
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5338
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5338). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dalago HR, Schuldt G, Rodrigues MAP, et al. Risk indicators for peri-implantitis. across-sectional study with 916 implants. Clin Oral Implants Res 2017;28:144-50. [Crossref] [PubMed]
- Edwardson DW, Boudreau J, Mapletoft J, et al. Inflammatory cytokine production in tumor cells upon chemotherapy drug exposure or upon selection for drug resistance. PLoS One 2017;12:e0183662. [Crossref] [PubMed]
- Chang H, Wang Y, Liu H, et al. Mutant Runx2 regulates amelogenesis and osteogenesis through a mi R-185-5p-Dlx2 axis. Cell Death Dis 2017;8:3221. [Crossref] [PubMed]
- Shu J, He X, Zhang L, et al. Human amnion mesenchymal cells inhibit lipopolysaccharide-induced TNF-α and IL-1β production in THP-1 cells. Biol Res 2015;48:69. [Crossref] [PubMed]
- Oi K, Tokunaga T, Kuranobu T, et al. Tumour necrosis factor alpha augments the inhibitory effects of CTLA-4-Ig on osteoclast generation from human monocytes via induction of CD80 expression. Clin Exp Immunol 2019;196:392-402. [Crossref] [PubMed]
- Iguchi M, Hiroi M, Kanegae H, et al. Costimulation of Murine Osteoblasts with Interferon-γ and Tumor Necrosis Factor-α Induces Apoptosis through Downregulation of Bcl-2 and Release of Cytochrome c from Mitochondria. Mediators Inflamm 2018;2018:3979606.
- Xu MX, Sun XX, Li W, et al. LPS at low concentration promotes the fracture healing through regulating the autophagy of osteoblasts via NF-κB signal pathway. Eur Rev Med Pharmacol Sci 2018;22:1569-79. [PubMed]
- Yu X, Quan J, Long W, et al. LL-37 inhibits LPS-induced inflammation and stimulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway. Exp Cell Res 2018;372:178-87. [Crossref] [PubMed]
- Ewendt F, Foller M. p38MAPK controls fibroblast growth factor 23(FGF23)synthesis in UMR106-osteoblast-like cells and in IDG-SW3 osteocytes. J Endocrinol Invest 2019;42:1477-83. [Crossref] [PubMed]
- Vrioni G, Tsiamis C, Oikonomidis G, et al. MALDI-TOF mass spectrometry technology for detecting biomarkers of antimicrobial resistance: current achievements and future perspectives. Ann Transl Med 2018;6:240. [Crossref] [PubMed]
- Cattoir V, Feldan B. Future antibacterial strategies: from basicconcepts to clinical challenges. J Infect Dis 2019;220:350-60. [Crossref] [PubMed]
- Durão J, Vale N, Gomes S, et al. Nitric Oxide Release from Antimicrobial Peptide Hydrogels for Wound Healing. Biomolecules 2018;9:4. [Crossref] [PubMed]
- Yang G, Huang T, Wang Y, et al. Sustained Release of Antimicrobial Peptide from Self-Assembling Hydrogel Enhanced Osteogenesis. J Biomater Sci Polym Ed 2018;29:1812-24. [Crossref] [PubMed]
- Makeudom A, Supanchart C, Montreekachon P, et al. The antimicrobial peptide, human beta-defensin-1, potentiates in vitro osteoclastogenesis via activation of the p44/42 mitogen-activated protein kinases. Peptides 2017;95:33-9. [Crossref] [PubMed]
- Li H, Zhang ST, Nie BE, et al. The antimicrobial peptide KR-12 promotes the osteogenic differentiation of human bone marrow stem cells by stimulating BMP/SMAD signaling. RSC Advances 2018;8:15547-57. [Crossref]
- Mammoli F, Castiglioni S, Parenti S, et al. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D-3. Int J Mol Sci 2019;20:385. [Crossref] [PubMed]
- Zheng LW, Wang WC, Mao XZ, et al. TNF-alpha regulates the early development of avascular necrosis of femoral head by mediating osteoblast autophagy and apoptosis via p38 MAPK/NF-kappa B signaling pathway. Cell Biol Int 2020. [Epub ahead of print]. [Crossref]
- Ren B, Liu J, Wu K, et al. TNF-alpha-elicited miR-29b potentiates resistance to apoptosis in peripheral blood monocytes from patients with rheumatoid arthritis. Apoptosis 2019;24:892-904. [Crossref] [PubMed]
- Zasloff M. Antimicrobial peptides of multicellular organisms: my perspective. Adv Exp Med Biol 2019;1117:3-6. [Crossref] [PubMed]
- Gong GL, Wei Y, Wang ZZ. Functional expression, purification and antimicrobial activity of a novel antimicrobial peptide MLH in Escherichia coli. Prep Biochem Biotechnol 2018;48:57-63. [Crossref] [PubMed]
- Zhang R, Wu F, Wu L, et al. Novel Self-Assembled Micelles Based on Cholesterol-Modified Antimicrobial Peptide (DP7) for Safe and Effective Systemic Administration in Animal Models of Bacterial Infection. Antimicrob Agents Chemother 2018;62:e00368-18. [Crossref] [PubMed]
- Madanchi H, Akbari S, Shabani AA, et al. Alignment-based design and synthesis of new antimicrobial Aurein-derived peptides with improved activity against Gram-negative bacteria and evaluation of their toxicity on human cells. Drug Dev Res 2019;80:162-70. [Crossref] [PubMed]
- Liu Z, Yuan X, Liu M, et al. Antimicrobial peptide combined with BMP2-modified mesenchymal stem cells promotes calvarial repair in an osteolytic model. Mol Ther 2018;26:199-207. [Crossref] [PubMed]
- Tripathi JK, Pal S, Awasthi B, et al. Variants of self-assembling peptide, KLD-12 that show both rapid fracture healing and antimicrobial properties. Biomaterials 2015;56:92-103. [Crossref] [PubMed]
- Li H, Nie B, Du Z, et al. Bacitracin promotes osteogenic differentiation of human bone marrow mesenchymal stem cells by stimulating the bone morphogenetic protein-2/Smad axis. Biomed Pharmacother 2018;103:588-97. [Crossref] [PubMed]
- Li X, Contreras-Garcia A, LoVetri K, et al. Fusion peptide P15-CSP shows antibiofilm activity and pro-osteogenic activity when deposited as a coating on hydrophilic but not hydrophobic surfaces. J Biomed Mater Res A 2015;103:3736-46. [Crossref] [PubMed]
- Lu RJ, Wang X, He HX, et al. Tantalum-incorporated hydroxyapatite coating on titanium implants: its mechanical and in vitro osteogenic properties. J Mater Sci Mater Med 2019;30:111. [Crossref] [PubMed]
- Soundharrajan I, Kim DH, Srisesharam S, et al. Limonene promotes osteoblast differentiation and 2-deoxy-D-glucose uptake through p38 MAPK and Akt signaling pathways in C2C12 skeletal muscle cells. Phytomedicine 2018;45:41-8. [Crossref] [PubMed]
- Menon MB, Gropengiesser J, Fischer J, et al. p38(MAPK)/MK2-dependent phosphorylation controls cytotoxic RIPK1 signalling in inflammation and infection. Nat Cell Biol 2017;19:1248. [Crossref] [PubMed]
- Lv M, Liu Y, Xiao TH, et al. GYY4137 stimulates osteoblastic cell proliferation and differentiation via an ERK1/2-dependent antioxidant mechanism. Am J Transl Res 2017;9:1183-92. [PubMed]
- Cheng D, Li J, Zhang L, et al. miR-142-5p suppresses proliferation and promotes apoptosis of human osteosarcoma cell line, HOS, by targeting PLA2G16 through the ERK1/2 signaling pathway. Oncol Lett 2019;17:1363-71. [PubMed]
- Liu H, Zheng X, Chen L, et al. Negative pressure wound therapy promotes muscle-derived stem cell osteogenic differentiation through MAPK pathway. J Cell Mol Med 2018;22:511-20. [Crossref] [PubMed]
- Pasquinelli V, Rovetta AI, Alvarez IB, et al. Phosphorylation of mitogen-activated protein kinases contributes to interferon γ production in response to Mycobacterium tuberculosis. J Infect Dis 2013;207:340-50. [Crossref] [PubMed]
- Pandey V, Ranjan N, Narne P, et al. Roscovitine effectively enhances antitumor activity of temozolomide in vitro and in vivo mediated by increased autophagy and Caspase-3 dependent apoptosis. Sci Rep 2019;9:5012. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


