
Cancer-associated fibroblasts promote the migration and invasion of gastric cancer cells via activating IL-17a/JAK2/STAT3 signaling
Introduction
Gastric cancer (GC) is the fifth most common malignant tumor and the third leading cause of cancer-related death all over the world (1). Currently, surgery with lymph node dissection is still the primary treatment for GC, and postoperative adjuvant chemotherapies are recommended for stage II or III. However, the 5-year overall survival rate of GC patients is less than 30% (2). Although after radical resection, about 35–70% of patients have distant metastasis or local and systemic recurrence within 5 years (3). Various adjuvant chemotherapy regimens have been designed and tested in the past few decades to reduce postoperative recurrence (4). The fluoropyrimidine-based chemotherapy is the cornerstone of chemotherapy for patients with advanced GC, and with capecitabine and platinum regimen, the overall survival of patients with locally advanced GC is prolonged (5). Also, patients with tumor recurrence or metastasis receiving chemotherapy combined with targeted therapy may have a better prognosis (6). However, nearly 50% of GC patients do not respond to the chemotherapy, and disease stabilization or partial response to the treatment are acquired by a few patients (6). Therefore, it is necessary to explore a prognostic tool that can identify a subgroup of patients with a higher risk of relapse and metastasis and afford tailor-made reasonable future treatments for these patients.
The tumor stroma includes extracellular matrix (ECM), fibroblasts, immune cells, and microvasculature. Tumor microenvironment (TME) is mainly regulated by a variety of stroma and tumor cells and has been considered to be involved in facilitating the tumorigenesis, proliferation, migration, invasion, and angiogenesis of tumors (7,8). As the main component of TME, fibroblasts from the supportive stroma with other cells, such as inflammatory cells and mesenchymal stem cells (9). They can be identified by specific biomarkers, including α-smooth muscle actin (α-SMA), fibroblast activation protein (FAP), fibroblast specific protein 1 (FSP1), and platelet-derived growth factor receptor (PDGFR) (10). More and more studies confirm that activated fibroblasts play a crucial role in the carcinogenesis of various solid cancers (11-13). Activated fibroblasts associated with carcinoma cells are known as carcinoma-associated fibroblasts (CAFs). According to reports, isolated human prostate CAFs can promote tumor growth in mice (14,15). CAFs may contribute to promote the progression of cancer (10). When co-injected with non-invasive cancer cells, CAFs can increase their aggressiveness (16). Further studies on the effects of CAFs on human breast cancer have shown that CAFs promote tumor growth and angiogenesis by secreting interstitial cell-derived factor-1 (SDF-1) (17). Matrix metalloproteinase (MMP) from the CAFs can regulate the motility of tumors and affect multiple signaling pathways of tumors (18). However, the specific activation mechanism of CAFs has not been elucidated very well.
CAFs alter the microenvironment by directly interacting with cancer cells and regulating paracrine signaling via inflammatory cytokines, control the immune response to neoplasia, deposit diverse ECM components, stimulate angiogenesis, and provide a scaffold for tumor metastasis and invasion (19,20). In inflammation and autoimmune diseases, neutrophils are the source of IL-17a (21). As an immune-inflammatory mediator, IL-17a can take part in all kinds of biological activities. Widely present in the inflammatory microenvironment of various tumors, including GC, IL-17a is involved in enhancing the invasion and migration of tumor cells, chemotherapy resistance, and immunosuppression, eventually leading to tumor invasion and metastasis (22,23). Clinical research has found that the increase in the number of IL-17a-producing cells is an independent biomarker for poor prognosis (24). IL-17a acts by binding to IL-17Rα, a common cytokine receptor, leading the activation of the Janus kinases (JAKs) family of tyrosine kinases and the signal transduction and activating agent of transcription (STAT) family, especially STAT3 (25). The activation of the IL-17a-JAK2-STAT3 signaling pathway plays an essential role in the development of various tumors (25,26). A few studies have found that both STAT3 and phosphorylated STAT3 increased expression levels in intestinal-type GC compared to adjacent healthy gastric tissues (27).
Additionally, phosphorylated STAT3 had a positive correlation with lymph node metastasis, poorly differentiated adenocarcinoma, and adverse prognosis (28). Nevertheless, the effects of CAFs and IL-17a in GC have not been well illuminated. Hence, the purpose of this study was to clarify how CAFs can accelerate migration and invasion of GC cells, and the correlation between CAFs and activation of the IL-17a/JAK2/STAT3 signaling pathway in the progression of GC cells.
Through the research, we found that CAFs were a source of IL-17a in GC. CAFs-derived IL-17a enhanced the migration and invasion of GC cells by activating the JAK2/STAT3 signaling pathway while using a neutralizing antibody of IL-17a or suppression of JAK2/STAT3 signaling pathway with AG490 dramatically repressed these phenotypes of GC cells induced by CAFs. All these results hint that inhibition of IL-17a or its downstream effectors may be an effective therapeutic strategy against GC by exerting its effect on interstitial CAFs.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4843).
Methods
Study population
Two hundred and twenty-seven GC patients who underwent surgical resection at the Department of General Surgery of The Affiliated Cancer Hospital of Zhengzhou University from 2010 to 2012 were enrolled. In the present study, all subjects were patients with gastric adenocarcinoma, and none received neoadjuvant chemotherapy. Patients with cardiopulmonary insufficiency, autoimmune diseases, or multiple primary tumors were excluded from this study. GC tissues were formalin fixed and paraffin-embedded. We extracted medical data and follow-up information related to GC patients from our prospective database. The size of the tumor is determined based on the longest diameter of the sample. The tumor stage is determined according to the eighth edition of the AJCC TNM staging classification for carcinoma of the stomach. Lauren’s classification includes intestinal type, diffuse type, and mixed type. The histological grade was classified as G1, G2, G3 adenocarcinoma, mucinous adenocarcinoma, and signet ring cell carcinoma. The H&E-stained slides were used to diagnose the lymphovascular invasion and perineural invasion. Disease-free survival (DFS) and disease-specific survival (DSS) were defined as previously described (23). Patients who died for causes unrelated to the disease were excluded from this study during the recent follow-up. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The research protocol was reviewed and approved by the institutional review board of The Affiliated Cancer Hospital of Zhengzhou University and obtained informed consent from all patients.
Immunostaining
Immunohistochemical staining was carried out by the avidin-biotin-peroxidase complex method. In short, formalin-fixed, paraffin-embedded surgically resected tumor tissue samples were cut into 4-µm sections. The sections were heated at 95 °C for 20 minutes and dewaxed. Deparaffinated in xylene and received gradient elution in ethanol. Then the slides were incubated in 3% H2O2 to block the endogenous peroxidase activity. Antigen retrieval was autoclaved for 2 min in citrate buffer (pH 6.0). Primary antibodies against α-SMA (1:400 dilution, 555723, BD) or IL-17a (1:200 dilution, ARG55256, Arigo) were dripped to the slides, incubated at 4 °C overnight, and Envision-plus detection system was applied with anti-mouse polymer (dilution 1:500, ab205719, Abcam) or anti-rabbit polymer (dilution 1:500, ab6721, Abcam) at 37 °C for 30 minutes. Each slide was reacted with a 3-3'-diaminobenzidine reagent solution for 2 minutes and counterstained with hematoxylin for 20 seconds. In all assays, we included negative control slides with the primary antibodies omitted.
For immunofluorescence analysis, fresh frozen sections of GC tissues were fixed with acetone. After blocked with normal nonimmune goat serum for 30 min, tissue sections were incubated with α-SMA and IL-17a, and were stained with appropriate Alexa Fluor 555-conjugated anti-mouse IgG and Alexa Fluor 488-conjugated anti-rabbit IgG (Molecular Probes, Carlsbad, CA). Negative control staining was performed by omission of the primary antibody.
Tumor cell lines
GC cell lines (GES-1, AGS, and SGC7901) were obtained from Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences, Shanghai, China) and were cultured as previously described (23).
CAFs isolation and culture
CAFs were isolated from fresh GC tissues. The GC tissues were washed with PBS with 100 U/mL penicillin and 100 U/mL streptomycin and cut into small pieces. Tenfold volume of 0.1% type II collagenase (GIBCO, Carlsbad, California, USA) was added to the fragments. Digestion was performed at 37 °C for 2 h and resuspended with pipette every 20 minutes. After stewing, the supernatant was collected and mixed with DMEM holding 10% FBS to stop the digesting process. The sediment was re-digested as earlier until clear cell clusters were observed. All collected supernatants were filtered through a 200-mesh cell filter and centrifuged at 300 g for 5 min. Cells were grafted in a 6-well plate and incubated at 37 °C under 5% CO2 with saturated humidity.
Quantitative real-time PCR (QRT-PCR)
Total RNA was isolated by Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s operating steps. RNA was reverse transcribed into cDNA using oligo-dT primers and SuperScript II reverse transcriptase (Promega, Madison, WI, US). cDNA was used for later qRT-PCR using the FAST SYBR Green Master Kit (Applied Biosystems, Foster City, CA, USA). Each reaction was performed on the Mastercycler PCR machine (Eppendorf, Hamburg, Germany). The relative expression level of the gene of interest was normalized to the housekeeping gene GAPDH. Data were analyzed using the comparative Ct method. The specificity of resulting PCR products was confirmed by melting curves. The primers used in this assay were: IL-17a: 5'-CGGTCCAGTTGCCTTCTCCC-3' (upper) and 5'-GAGTGGCTGTCTGTGTGGGG-3' (lower); GAPDH: 5'-GGACCTGACCTGCCGTCTAG-3' (upper) and 5'-GTAGCCCAGGATGCCCTTGA-3' (lower).
Enzyme-linked immunosorbent assay (ELISA)
According to the manufacturer’s instruction book, the protein productions of IL-17a in plasma and tissue lysates were measured by an ELISA kit (R&D Systems, Minneapolis, MN, USA).
Transwell assay
Cell migration and invasion assays were measured by using 8 µm Transwell Chambers (Corning Life Science, MA, USA). The cells were incubated in serum-free medium for 12 h, then added to the upper chamber, and 5×104 CAFs in 500 µL RPMI-1640 holding 10% FBS were added to the lower chamber. After 48 hours, non-migrating cells were removed with cotton-tipped swabs, and cells that migrated to the bottom of the membranes were stained with 0.1% crystal violet for 30 min. The stained cells were observed and counted under a 100× microscope.
Western blot analysis
The total proteins in CAFs and GC cells were lysed with RIPA cell lysis buffer in the presence of protease inhibitor (Sigma, USA) and phosphatase inhibitor cocktail (Sigma, USA). The protein concentration was determined with a BCA Protein Assay Kit (Beyotime, Shanghai, China). Proteins were separated by 10% SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, MA, USA). After blocked by skim milk, the membranes were incubated in the primary antibodies diluted by PBST buffer overnight at 4 °C and then in the HRP-conjugated secondary antibody for 2 h at room temperature. Finally, a Tanon detection system captured the protein band images with ECL reagent (Thermo). The primary antibodies used in the experiments were anti-β-actin, anti-MMP2, anti-MMP9, anti-TIMP1, anti-TIMP2, p-JAK2, JAK2, p-STAT3, and STAT3 antibodies were bought from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Statistical analysis
SPSS 19.0 software (Version 19.0, Chicago, IL, USA) and Graphpad Prism 5.0 were used for all statistical analyses. Results were expressed as mean ± SD. Analyses of variance and Pearson chi-square tests were used to assess any associations between variables. Kaplan-Meier survival curves and Cox proportion analysis were used for survival analysis. The level of significance allowing multivariate analysis inclusion and the statistical significance for all other tests used was set at P<0.05.
Results
CAFs is associated with unfavorable clinical features of GC patients
In this study, 227 cases of GC patients were enrolled. The clinicopathological features of GC patients were shown in Table 1. The median DFS and DSS of GC patients were 20.8 and 36.5 months, respectively. One hundred thirty-one patients (57.7%) had a postoperative recurrence, and 122 cases (53.7%) died of GC at the final follow-up. The median follow-up duration was 36.7 months (range, 3.2–99.5 months), and the average age of GC patients was 62.0 years (range, 31.0–98.0 years). 125 of 203 GC patients received postoperative adjuvant chemotherapy, and the average number of harvested lymph nodes after surgical resection was 33.1 (range, 10.0–63.0). CAFs in GC tissues were detected using IHC staining of α-SMA (Figure 1), and the number of CAFs in GC tissues was 64.7±3.5. Then, GC patients were divided into low or high CAFs group according to the cutoff value, which was defined as the mean value of the cohort of patients evaluated. The correlation between the number of CAFs and clinical features of GC patients was determined. As shown in Table 1, the number of CAFs in GC tissues was significantly associated with TNM stage (P=0.001) and perineural invasion (P=0.014).
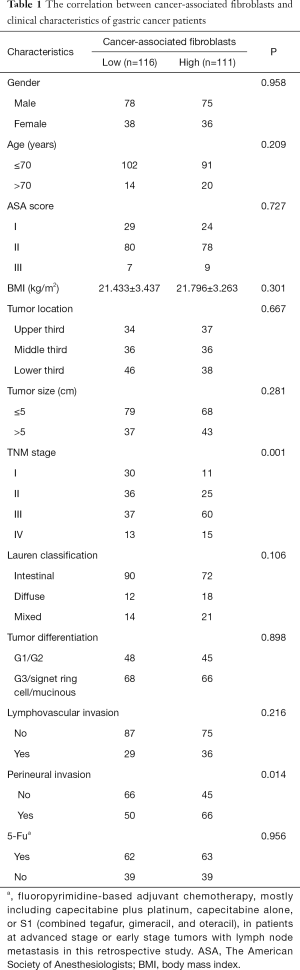
Full table
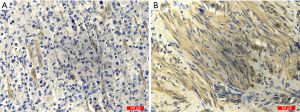
CAFs correlate with poor prognosis of GC
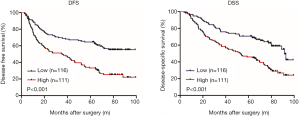
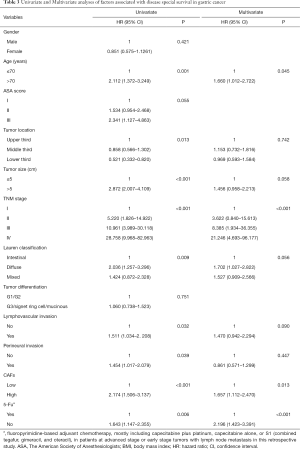
Next, the relationship between the number of CAFs and survival of GC patients was analyzed. Log-rank test showed that GC patients with high CAFs in tumor tissues had significant shorter DFS and DSS compared to those with low CAFs (P<0.001, respectively, Figure 2). Moreover, univariate analysis showed that age (P=0.001), tumor location (P=0.002), tumor size (P<0.001), TNM stage (P<0.001), Lauren classification (P=0.033), the number of CAFs (P<0.001) and postoperative chemotherapy (P=0.005) were significantly associated with DFS (Table 2). In addition, age (P=0.001), tumor location (P=0.013), tumor size (P<0.001), TNM stage (P<0.001), Lauren classification (P=0.009), lymphovascular invasion (P=0.032), perineural invasion (P=0.039), the number of CAFs (P<0.001) and postoperative chemotherapy (P=0.006) were significantly correlated with DSS (Table 3). Interestingly, multivariate analysis proved that the number of CAFs was an independent risk factor for both DFS and DSS in GC (P=0.033 and 0.013, respectively, Tables 2,3).
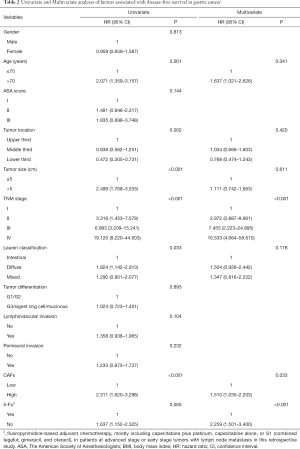
Full table
Full table
IL-17a protein is expressed by CAFs in GC
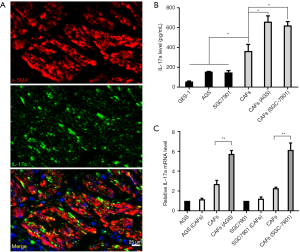
CAFs, the group of cancer cell-activated fibroblasts, contribute to the initiation and progression of GC via secreting chemokines and cytokines (29). Previous studies have revealed that IL-17a is secreted by lymphocytes and neutrophils in GC (23,30). However, whether CAFs are the source of IL-17a in GC is unclear. According to immunofluorescence staining, we found that both α-SMA and IL-17a were expressed in CAFs in peri-tumoral and intra-tumoral areas (Figure 3A). Subsequently, ELISA was performed to determine the level of IL-17a in the supernatant of cells. The levels of IL-17a in the supernatant of GES-1, AGS, and SGC7901 cells were 55.6±5.3, 154.5±4.3, and 131.2±11.5 pg/mL, respectively (Figure 3B). The level of IL-17a in the supernatant of CAFs (267.3±35.2 pg/mL) was prominently higher than that in GC cells (P<0.05, Figure 3B). Furthermore, the levels of IL-17a in the supernatant of CAFs co-cultured with AGS and SGC7901 (690.6±55.8 and 645.3±47.6 pg/mL, respectively) were significantly higher than that in CAFs (P<0.05, Figure 3B). In addition, qRT-PCR results also showed that the expression level of IL-17a mRNA in gastric cancer cell lines (AGS and SGC7901) increased slightly, after co-culture with gastric cancer cells, while the expression level of IL-17a mRNA in CAFs increased significantly in tumour microenvironment (all P<0.05) (Figure 3C). Therefore, these data showed that CAFs produce IL-17a in GC.
CAFs promote the migration and invasion of GC cells through IL-17a
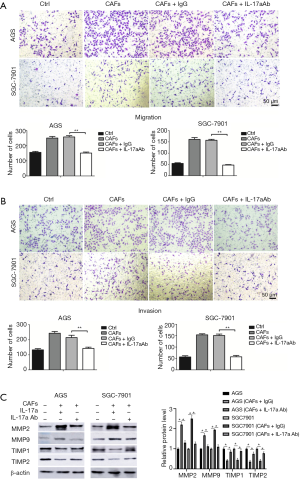
An IL-17a neutralizing antibody was used to determine the effects of IL-17a on CFAs-induced GC cell migration and invasion. AGS or SGC7901 cells and CAFs were seeded into the Transwell co-culture chamber. Transwell migration and invasion assays revealed that CAFs co-culture increased the migration and invasion of AGS and SGC7901 cells (P<0.001, Figure 4A,B). However, the migration and invasion abilities of GC cells were significantly decreased after adding IL-17a neutralizing antibody into the Transwell co-culture chamber (P<0.001, Figure 4A,B). Since MMPs have been demonstrated to promote cancer metastasis (31,32), we next investigated the effects of IL-17a on MMPs expression in GC cell lines. As expected, CAFs co-culture resulted in increased levels of MMP-2 and MMP-9, and reduced levels of TIMP-1 and TIMP-2 (P<0.05, Figure 4C), which was subsequently reversed by the IL-17a neutralizing antibody (P<0.05, Figure 4C). These results suggested that CAFs promoted the migration and invasion of GC cells and regulated MMP/TIMP balance via IL-17a.
CAFs promote GC progression via IL-17a/JAK2/STAT3 pathway
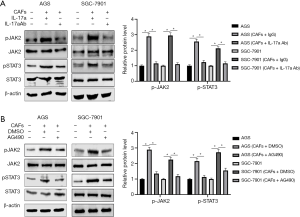
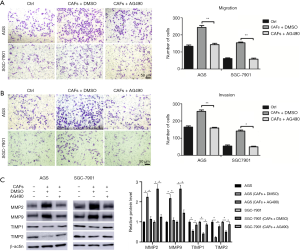
IL-17a is an established inducer to activate the JAK2/STAT3 pathway. Thus, it is necessary to determine whether the JAK2/STAT3 pathway mediates CAFs-induced GC progression. AGS or SGC7901 cells and CAFs were seeded into the Transwell co-culture chamber. The western blotting analysis showed that CAFs significantly increased the levels of p-JAK2 and p-STAT3 in GC cells (P<0.05, Figure 5A). While either the IL-17a neutralizing antibody or JAK2 protein tyrosine kinase inhibitor AG490 blocked CAFs-induced activation of the JAK2/STAT3 pathway (P<0.05, Figure 5A,B). Moreover, inactivation of the JAK2/STAT3 pathway by AG490 markedly attenuated the promoting effects of CAFs on cell migration, invasion, MMP2/9 and TIMP1/2 expression in AGS and SGC7901 cells (P<0.05, Figure 6A,B,C). Collectively, IL-17a/JAK2/STAT3 pathway mediated the role of CAFs in GC.
Discussion
As the most abundant cells in the TME and the activated fibroblasts in cancer stroma, CAFs are different from normal fibroblasts functionally and phenotypically. More and more evidence shows that CAFs have an essential effect on the growth and progression of many kinds of cancer, and could possibly be a prospective target to cancer therapeutics (10). Hence, a satisfactory comprehension of the molecular mechanism of how CAFs promote the growth and progression of tumors is significant to understand the progression of GC and find marvelous therapeutics to it. Our research found that IL-17a secreted by CAFs makes a significant contribution to the migration and invasion of GC cells. We demonstrate that CAFs derived-IL-17a facilitated the migration, invasion, and EMT of GC cells by activating the JAK2/STAT3 signaling pathway, and IL-17a neutralizing antibody or JAK2 specific inhibitor AG490 could inhibit this pathway and undermine GC metastasis induced by CAFs in vivo. Therefore, the CAFs may activate the JAK2/STAT3 signaling pathway by IL-17a, which may play a crucial part in the crosstalk between GC cells and the TME.
Importantly, our research also pays more attention to the clinical relevance of CAFs in GC. Correctly, it is observed that an increased frequency of intra-tumoral CAFs predicted a worse survival rate. Given the unsatisfactory clinical outcome for patients with GC and that few prognostic predictors for this disease after conventional surgery (33), intra-tumoral CAFs frequencies might act as a hopeful clinical biomarker in the future. Further on, CAFs may accelerate tumor progression by secreting cytokines, growth factors, or chemokines with both pro and anti-tumor functions, which is depended on the particular TME (34). Some past studies have demonstrated that CAFs could produce IL-17a in colorectal cancer (35). In this study, not only clinical samples analysis but also experimental study suggested that IL-17a is secreted chiefly by CAFs.
More research has shown that MMP regulates the TME and mediates tumor progression. Proteolytic changes are characteristic of cancer, which contributes to tissue remodeling and facilitates tumor metastasis (36). As the gelatinases of the MMP family, MMP2 and MMP9 have been well-known, and increasing expression of MMP2 and MMP9 are positively correlated to the lower survival rate of GC patients and higher levels of MMPs was found in many malignant tumors (37). In our study, overexpression of both enzymes from GC cells was discovered, hinting that the migration and invasion of the GC cells were regulated by the presence of CAFs/IL-17a/JAK2/STAT3.
After research, we found that IL-17a can promote the invasion ability of GC cells. The cancer-related death among GC patients was usually caused by metastasis. The blood or lymph vessels in the ECM are crucial for metastasis because cancer cells with loss of the ECM can invade the blood or lymphatic system and metastasize to distant organs and tissues (38). MMPs, especially of MMP-2 and MMP-9, play an essential role in the degradation of the ECM (36). IL-17a reportedly promotes cancer cell invasion using the higher expression of MMP-2 and MMP-9 (39). It has been demonstrated that MMP activities could possibly be suppressed by TIMPs to hamper the extensive degradation of ECM (40). We hypothesized whether the accelerating effects of IL-17a on migration and GC cell invasion is by way of regulation of the expressions of MMP-2, MMP-9, TIMP-1, and TIMP-2 or not to illustrate the mechanism of activation of IL-17a. In this study, we found that IL-17a enhanced the expression levels of MMP-2 and MMP-9 and degraded the expressions of TIMP-1 and TIMP-2, which further illuminated that the IL-17a accelerated cell migration and invasion in the Transwell assay. Therefore, using regulating MMP/TIMP balance, the IL-17a may have a pro-metastasis effect on GC cells.
IL-17 has a positive correlation with the degree of activation of the STAT3 signaling transduction pathway (25). The activation of STAT3 with the phosphorylation of Tyr705 was accelerated by the JAK2 signaling pathway. It has been reported previously that activation of IL-17a-JAK2/STAT3 signaling pathway by growth factors, cytokines, or chemokines plays a dominant part in tumor invasion and metastasis (23). Nevertheless, the underlying mechanism of CAFs and IL-17a in GC is still mostly unknown. Our data have demonstrated that, by secreting the IL-17a, CAFs could activate the JAK2/STAT3 signaling pathway of GC cells and inhibiting the JAK2/STAT3 signaling pathway activation with AG490 dramatically attenuated migration and invasion of GC cells, as well as EMT in vitro induced by CAFs. It is well-known that CAFs can secrete multitudinous growth factors, cytokines, and chemokines such as TNF-α, CCL2, PDGFR, IL-6, and IL-17a, which exists in the TME that enhance the proliferation and metastasis of the underlying tumor by activating multiple signaling pathways (10). In this study, it was observed that IL-17a is neutralizing antibody restrained partly by the JAK2 and STAT3 phosphorylation, which implied that IL-17a produced a partial contribution to the pro-tumor functions of CAFs on GC cells. We cannot exclude the participation of other growth factors or cytokines. However, this study of neutralizing IL-17a or blocking the JAK2/STAT3 pathway with AG490 clarified that IL-17a is a crucial regulator in pro-tumor functions of CAFs that facilitates EMT through the activating JAK2-STAT3 signaling pathway in GC.
Acknowledgments
Funding: This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS)—Spatial-Temporal Mapping Analysis on Chinese Cancer Burden (2018-I2M-3-003).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4843
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4843
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4843). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The work was undertaken within the Ethics Committee of The Affiliated Cancer Hospital of Zhengzhou University (approval ID: 2019209) and obtained informed consent from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Cunquero-Tomás AJ, Ortiz-Salvador JM, Iranzo V, et al. Sweet syndrome as the leading symptom in the diagnosis of gastric cancer. Chin Clin Oncol 2018;7:11. [Crossref] [PubMed]
- Zhang CD, Yamashita H, Seto Y. Gastric cancer surgery: historical background and perspective in Western countries versus Japan. Ann Transl Med 2019;7:493. [Crossref] [PubMed]
- Zhang W, Fang M, Dong D, et al. Development and validation of a CT-based radiomic nomogram for preoperative prediction of early recurrence in advanced gastric cancer. Radiother Oncol 2020;145:13-20. [Crossref] [PubMed]
- Iqbal S, McDonough S, Lenz HJ, et al. Randomized, Phase II Study Prospectively Evaluating Treatment of Metastatic Esophageal, Gastric, or Gastroesophageal Cancer by Gene Expression of ERCC1: SWOG S1201. J Clin Oncol 2020;38:472-9. [Crossref] [PubMed]
- Choi AH, Kim J, Chao J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World J Gastroenterol 2015;21:7343-8. [Crossref] [PubMed]
- Huang T, Song C, Zheng L, et al. The roles of extracellular vesicles in gastric cancer development, microenvironment, anti-cancer drug resistance, and therapy. Mol Cancer 2019;18:62. [Crossref] [PubMed]
- Yoshida GJ. Regulation of heterogeneous cancer-associated fibroblasts: the molecular pathology of activated signaling pathways. J Exp Clin Cancer Res 2020;39:112. [Crossref] [PubMed]
- Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett 2017;387:61-8. [Crossref] [PubMed]
- Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov 2019;18:99-115. [Crossref] [PubMed]
- Tu K, Li J, Verma VK, et al. Vasodilator-stimulated phosphoprotein promotes activation of hepatic stellate cells by regulating Rab11-dependent plasma membrane targeting of transforming growth factor beta receptors. Hepatology 2015;61:361-74. [Crossref] [PubMed]
- Dou C, Liu Z, Tu K, et al. P300 Acetyltransferase Mediates Stiffness-Induced Activation of Hepatic Stellate Cells Into Tumor-Promoting Myofibroblasts. Gastroenterology 2018;154:2209-2221.e14. [Crossref] [PubMed]
- Wang Y, Tu K, Liu D, et al. p300 Acetyltransferase Is a Cytoplasm-to-Nucleus Shuttle for SMAD2/3 and TAZ Nuclear Transport in Transforming Growth Factor beta-Stimulated Hepatic Stellate Cells. Hepatology 2019;70:1409-23. [Crossref] [PubMed]
- Cioni B, Nevedomskaya E, Melis MHM, et al. Loss of androgen receptor signaling in prostate cancer-associated fibroblasts (CAFs) promotes CCL2- and CXCL8-mediated cancer cell migration. Mol Oncol 2018;12:1308-23. [Crossref] [PubMed]
- Ben Baruch B, Mantsur E, Franco-Barraza J, et al. CD38 in cancer-associated fibroblasts promotes pro-tumoral activity. Lab Invest 2020. [Crossref] [PubMed]
- Wang X, Che X, Liu C, et al. Cancer-associated fibroblasts-stimulated interleukin-11 promotes metastasis of gastric cancer cells mediated by upregulation of MUC1. Exp Cell Res 2018;368:184-93. [Crossref] [PubMed]
- Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005;121:335-48. [Crossref] [PubMed]
- Mochizuki S, Ao T, Sugiura T, et al. Expression and Function of a Disintegrin and Metalloproteinases in Cancer-Associated Fibroblasts of Colorectal Cancer. Digestion 2020;101:18-24. [Crossref] [PubMed]
- Ock CY, Nam AR, Bang JH, et al. Signature of cytokines and angiogenic factors (CAFs) defines a clinically distinct subgroup of gastric cancer. Gastric Cancer 2017;20:164-74. [Crossref] [PubMed]
- Sun Y, Fan X, Zhang Q, et al. Cancer-associated fibroblasts secrete FGF-1 to promote ovarian proliferation, migration, and invasion through the activation of FGF-1/FGFR4 signaling. Tumour Biol 2017;39:1010428317712592. [Crossref] [PubMed]
- McGinley AM, Sutton CE, Edwards SC, et al. Interleukin-17A Serves a Priming Role in Autoimmunity by Recruiting IL-1beta-Producing Myeloid Cells that Promote Pathogenic T Cells. Immunity 2020;52:342-356.e6. [Crossref] [PubMed]
- Li TJ, Jiang YM, Hu YF, et al. Interleukin-17-Producing Neutrophils Link Inflammatory Stimuli to Disease Progression by Promoting Angiogenesis in Gastric Cancer. Clin Cancer Res 2017;23:1575-85. [Crossref] [PubMed]
- Li S, Cong X, Gao H, et al. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res 2019;38:6. [Crossref] [PubMed]
- Wang JT, Li H, Zhang H, et al. Intratumoral IL17-producing cells infiltration correlate with antitumor immune contexture and improved response to adjuvant chemotherapy in gastric cancer. Ann Oncol 2019;30:266-73. [Crossref] [PubMed]
- Jo S, Wang SE, Lee YL, et al. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther 2018;20:115. [Crossref] [PubMed]
- Gu FM, Li QL, Gao Q, et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer 2011;10:150. [Crossref] [PubMed]
- Pan YM, Wang CG, Zhu M, et al. STAT3 signaling drives EZH2 transcriptional activation and mediates poor prognosis in gastric cancer. Mol Cancer 2016;15:79. [Crossref] [PubMed]
- He S, Liao G, Liu Y, et al. Overexpression of STAT3/pSTAT3 was associated with poor prognosis in gastric cancer: a meta-analysis. Int J Clin Exp Med 2015;8:20014-23. [PubMed]
- Sun L, Wang Y, Wang L, et al. Resolvin D1 prevents epithelial-mesenchymal transition and reduces the stemness features of hepatocellular carcinoma by inhibiting paracrine of cancer-associated fibroblast-derived COMP. J Exp Clin Cancer Res 2019;38:170. [Crossref] [PubMed]
- Zhong F, Cui D, Tao H, et al. IL-17A-producing T cells and associated cytokines are involved in the progression of gastric cancer. Oncol Rep 2015;34:2365-74. [Crossref] [PubMed]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161-74. [Crossref] [PubMed]
- Folgueras AR, Pendas AM, Sanchez LM, et al. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol 2004;48:411-24. [Crossref] [PubMed]
- Kawamura T, Makuuchi R, Tokunaga M, et al. Long-Term Outcomes of Gastric Cancer Patients with Preoperative Sarcopenia. Ann Surg Oncol 2018;25:1625-32. [Crossref] [PubMed]
- Miyai Y, Esaki N, Takahashi M, et al. Cancer-associated fibroblasts that restrain cancer progression: Hypotheses and perspectives. Cancer Sci 2020;111:1047-57. [Crossref] [PubMed]
- Lotti F, Jarrar AM, Pai RK, et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J Exp Med 2013;210:2851-72. [Crossref] [PubMed]
- Winer A, Adams S, Mignatti P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol Cancer Ther 2018;17:1147-55. [Crossref] [PubMed]
- Łukaszewicz-Zając M, Szmitkowski M, Litman-Zawadzka A, et al. Matrix Metalloproteinases and Their Tissue Inhibitors in Comparison to Other Inflammatory Proteins in Gastric Cancer (GC). Cancer Invest 2016;34:305-12. [Crossref] [PubMed]
- Yuzhalin AE, Lim SY, Kutikhin AG, et al. Dynamic matrisome: ECM remodeling factors licensing cancer progression and metastasis. Biochim Biophys Acta Rev Cancer 2018;1870:207-28. [Crossref] [PubMed]
- Wu Z, He D, Zhao S, et al. IL-17A/IL-17RA promotes invasion and activates MMP-2 and MMP-9 expression via p38 MAPK signaling pathway in non-small cell lung cancer. Mol Cell Biochem 2019;455:195-206. [Crossref] [PubMed]
- Li K, Tay FR, Yiu CKY. The past, present and future perspectives of matrix metalloproteinase inhibitors. Pharmacol Ther 2020;207:107465. [Crossref] [PubMed]


