
Helicobacter pylori infection increases the risk of metabolic syndrome in pregnancy: a cohort study
Introduction
Metabolic syndrome (MetS), first identified as a syndrome in 1988, is increasingly affecting the health of many people around the world (1,2). It is identified as a series of metabolic disorders, including hypertension, impaired glucose tolerance, insulin resistance, high level of serum triglyceride (TG), low level of high-density lipoprotein (HDL), and abdominal obesity (3). There is no authoritative diagnostic criterion of MetS to date. Nowadays the most commonly used criterion defines MetS as at least three of the following characteristics: abdominal circumference ≥88 cm, TG ≥1.70 mmol/L, HDL cholesterol (HDL-c) <1.29 mmol/L, blood pressure (BP) ≥130/85 mmHg, and fasting blood glucose (BG) ≥5.6 mmol/L (4). The purpose of MetS treatment nowadays is to prevent the occurrence of complications through weight loss, reducing insulin resistance and improving blood lipid disorders.
The prevalence of MetS varies according to the age and gender of people and the development level of regions (5). As we know, normal pregnancy is a process consisting of pro-inflammatory, pro-thrombotic, insulin resistant, and hyperlipidemic states (6). All these states are like the metabolic disorders of MetS, potentially accelerating the development of MetS. It has been reported that the prevalence of MetS in pregnant and puerperal women were 12.4% and 29%, respectively (7,8). Moreover, MetS increase the risk of adverse pregnancy outcomes in pregnancy. More than half of pregnant women with MetS in early stages were found by Grieger et al. to develop at least one pregnancy complication, while only a third of women without MetS developed a pregnancy complication (9). There is an urgent need to figure out related risk factors of MetS in pregnancy to prevent MetS and improve pregnancy outcomes.
Several risk factors have been found for the development of MetS, including obesity. Obesity or overweight has been identified to be extremely associated with MetS by several studies (10-12), acting as one of the most important diagnostic criteria. Besides, some novel risk factors have been discovered in recent years, of which Helicobacter pylori (H. pylori) infection is one of the most concerned. H. pylori infection, one of the most common infections, affects over 50% population globally, as reported (13). It is initially regarded as an essential risk factor of chronic gastritis, peptic ulcers, and gastric cancer (14). In recent decades, the role of H. pylori as an accelerator of MetS is declared as well. In 2008, Gunji et al. performed a complete medical survey among a large Japanese population and found that H. pylori infection was significantly associated with MetS (15). Afterward, increased studies revealed the association between H. pylori infection and MetS in different groups of people (16-19). However, the effect of H. pylori infection on MetS in pregnant women is still unclear.
We here hypothesize that pregnant women with H. pylori infection are at a higher risk for MetS than those who have no H. pylori infection. The present study was performed to investigate the incidence of H. pylori infection in pregnancy, and to determine further whether H. pylori infection would increase the incidence of MetS and adverse pregnancy complications.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4863).
Methods
Study design
Our study was a prospective cohort study of pregnant women admitted to the Wuxi People’s Hospital between July 2017 and October 2018. All pregnant women who signed informed consent were enrolled in this study. Women were excluded from this study on the following criteria: pregnant women with multifetal pregnancy, pre-existing diabetes mellitus, pre-existing hypertension, high risk for preeclampsia, multiple terminations or miscarriages, already known fetal anomaly or abnormal karyotype, increased risk for depression, disability, malignant diseases, severe malnutrition, recent surgery or invasive procedure, and taking supplements or drugs which may modify the results of this study. For the exclusion criterion, 320 pregnant women were enrolled in this study. The study was performed in compliance with the ethical principles of the Declaration of Helsinki (as revised in 2013) and had been approved by the ethics committee of Wuxi People’s Hospital.
Data collection
Baseline data of enrolled pregnant women were firstly collected at 12±1 weeks’ gestation. The recorded data included demographic data, H. pylori infection, body mass index (BMI), history of smoking, reproductive history, BP, and biochemical indexes, including the levels of serum TG, HDL-c, and fasting BG. The C13 urea breath test was used to measure H. pylori infection in this study. Skilled operators performed the measurements, and 2 mL blood samples were collected when they were enrolled in this study.
Follow up
All enrolled patients were followed up until the last baby was born in August 2019. Each pregnant woman received at least 10 antepartum examinations every 2–4 weeks during the follow-up period. Waist circumference (WC), BP, and the levels of serum TG, HDL-c, and fasting BG were measured and recorded during the antepartum examination. MetS are defined according to an earlier study (9). WC >80 cm is a prerequisite for MetS, plus any 2 of the following variables: TG ≥1.70 mmol/L, HDL-C <1.29 mmol/L, systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg, and fasting BG ≥5.6 mmol/L.
Some other pregnancy outcomes were recorded, including gestational diabetes mellitus (GDM), preeclampsia, spontaneous preterm birth (SPB), fetal growth restriction (FGR), and uncomplicated pregnancy. GDM was diagnosed as fasting BG ≥5.1 mmol/L or a 2-hour oral glucose tolerance test ≥8.5 mmol/L. Preeclampsia is diagnosed as systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg on at least 2 occasions before the onset of labor, with either proteinuria or any multisystem complication of preeclampsia. SPB is defined as spontaneous delivery before 37+0 weeks’ gestation. FGR defined as newborns with a birth weight under 10th centiles. Uncomplicated pregnancy is defined as a normal pregnancy, delivered at ≥37 weeks and ≤42 weeks, with an appropriate for gestational age baby (neonates with birth weight between 10th and 90th centiles).
Statistical analysis
Stata (version 15.0) was used for all data analyses. All enrolled women in this study were divided into two groups on whether they had H. pylori infection or not (78 women in infection group and 242 women in non-infection group). Besides, the two groups were then divided into two subgroups on whether their BMI was more than 24 or not (211 women in BMI <24 group and 109 women in BMI ≥24 group). Continuous variables were reported as the means with standard deviations and analyzed with use 2-tailed T-tests for the comparisons between pregnant women of two groups or subgroups. Besides, categorical variables were reported as percentages and analyzed using χ2 tests. Univariate and multivariate logistic regression analysis was then performed to explore the relationship between H. pylori infection and MetS or other adverse pregnancy outcomes. The level of significance was set at the conventional level of α=0.05. All P values were two-sided, and P<0.05 was considered statistically significant.
Results
Patient characteristics
Their baseline data were shown in Table 1. As we can see, the mean age was 27.1±4.0 years old, and the BMI was 22.7±4.7. Only 14 women (4.4%) had a history of smoking in this study. Seventy-eight women were diagnosed as H. pylori infection using the C13 urea breath test, accounting for 24.4% of the total, and they did not receive any specific treatment before. There were 88 multiparas and 232 primiparas as well. WC, BP, fasting BG, TG, and HDL-c of all enrolled women were measured at 12±1 weeks’ gestation. The mean of WC was 73.7±9.8 cm, as shown. Systolic and diastolic BP are 114.0±8.3 and 71.1±6.9 mmHg, respectively. The mean of fasting BG was 4.4±1.4 mmol/L. The mean levels of TG and HDL-c were 0.72±0.12 and 1.5±0.4 mmol/L, respectively.
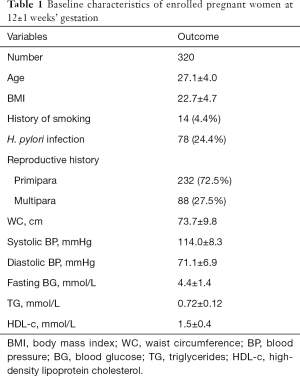
Full table
Follow up
Some critical metabolic disorders of MetS were recorded during follow up, as showed in Table 2. Total women were divided into two groups according to H. pylori infection, and it was revealed more women with H. pylori infection underwent metabolic disorders, including elevated BP, BG, TG, and HDL-c, all of which showed significant difference compared with women without H. pylori infection, (P=0.005, 0.015, 0.023 and 0.001, respectively). Moreover, more MetSs were found in women with H. pylori infection than those without H. pylori infection (P=0.001).
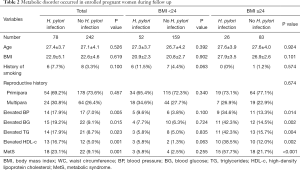
Full table
Subgroup analysis
Considering BMI is the most vital risk factor of metabolic disorders, we divided all women into two subgroups of BMI ≥24 or BMI <24 and compared the incidence of metabolic disorders between women with or without H. pylori infection as shown in Table 2. Expectedly, women with low BMI underwent fewer metabolic disorders than those with high BMI. There were 3 women with H. pylori infection and 4 women without H. pylori infection undergoing MetS in low BMI subgroups, respectively, which showed no significant difference. Furthermore, other metabolic parameters showed no significant difference between the two subgroups as well. However, H. pylori infection dramatically increased the incidence of MetS (15 women with H. pylori infection versus 18 women without H. pylori infection, P<0.001) as well as other metabolic disorders in high BMI subgroups.
Risk factors of metabolic disorders
Multivariable logistic regression analysis was performed to figure out the risk factors of MetS and elevated metabolic parameters, as shown in Table 3. After adjusting age, history of smoking and reproductive history, H. pylori infection, and high BMI were revealed to be the most critical risk factors of all studied metabolic disorders.
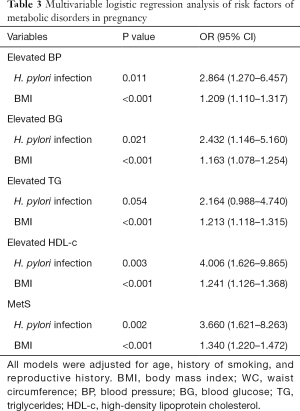
Full table
Some other pregnancy outcomes, including GDM, Preeclampsia, SPB, FGR, and uncomplicated pregnancy, were then recorded in this study and showed in Table 4. It was revealed that H. pylori infection increased the incidence of GDM and preeclampsia and decreased the incidence of an uncomplicated pregnancy, especially in the high BMI subgroup, like the results in Table 2. However, SPB and FGR in this study showed no significant relationship with H. pylori infection neither in high BMI subgroup nor in low BMI subgroup.

Full table
Risk factors of adverse pregnancy outcomes
In the results of multivariable logistic regression analysis, H. pylori infection and high BMI were considered risk factors of GDM and preeclampsia (Table 5). However, no variable in this study was found to be related to SPB. High BMI, rather than H. pylori infection, is an independent risk factor of both FGR and uncomplicated pregnancy. Interestingly, the results here demonstrated that H. pylori infection and age may be associated with the decrease of an uncomplicated pregnancy, though P value indicated no significant relationship between them (P=0.076 and 0.069, respectively).
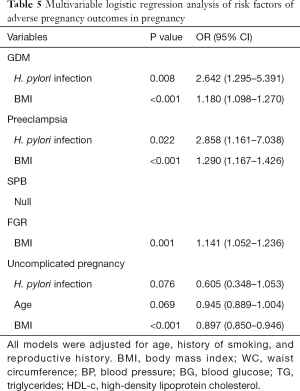
Full table
Discussion
We here performed a prospective cohort study to investigate the effects of H. pylori infection on MetS in pregnant women for the first time to our knowledge. A total of 320 pregnant women were enrolled in this study. H. pylori infection was verified to be associated with the incidence of MetS and other metabolic disorders following the results of the follow-up period. High BMI was the most crucial risk factor of MetS, while H. pylori infection could further increase the incidence of MetS in pregnant women with high BMI compared with those with low BMI. Additionally, H. pylori infection would increase several adverse pregnancy outcomes as well.
H. pylori infection has become an increasingly severe clinical hotpot nowadays. It was reported that 31.9–52.3% of Chinese people were positive for H. pylori infection (20-23). Although some studies indicated a striking decrease in the prevalence of H. pylori infection in urban China recently (23), the effects of H. pylori infection on the health of people cannot be ignored. The incidence of H. pylori infection in pregnant women, according to our study, was 24.4%, a low level. However, pregnant women with H. pylori infection were more likely to develop MetS and other metabolic disorders, which further increased the incidence of adverse pregnancy outcomes.
The relationship between H. pylori infection and MetS has been studied by many studies. Yu et al. found that H. pylori infection increased the incidence of MetS in the aged female population from the Zhejiang province of eastern China (13). Lim et al. also revealed that H. pylori infection played a vital role in MetS in-Koreans-under 65 years old, on a multicenter nationwide study (24). However, the mechanism of how H. pylori infection affects metabolic pathways in the body is still unclear. A study exploring the effects of H. pylori infection on the severity of nonalcoholic fatty liver disease (NAFLD) demonstrated patients with H. pylori infection had a more severe NAFLD compared with those without H. pylori infection (25). They found that H. pylori infection was related to promoted glycometabolism and lipid metabolism (25), which may explain the elevated TG, HDL-c, and BG in our study.
Moreover, H. pylori infection also activates inflammatory pathways (25). The inflammatory response is known to induce inflammatory cells to release plenty of cytokines, including interleukin 1 and 6, and tumor necrosis factor-alpha. The release of inflammatory cytokines could inactivate lipoprotein lipase, improve the synthesis of fatty acids in the liver, and activate HMG-CoA reductase (26). Also, some inflammatory cytokines could trigger the release of vascular contracting substances (27), inducing elevated BP, and even preeclampsia in pregnancy.
MetS were common in pregnancy, and the incidence of MetS in this study was 12.5%. Being obese or overweight has been recognized as a METS independent risk factor (28-30), which was like this study. What is more, we found for the first time that H. pylori infection could increase the incidence of MetS in pregnant women with high BMI and have no significant effect on those with low BMI. It can be explained that H. pylori infection exacerbates the uncertain status of obese or overweight pregnant women, and it may play a role as a trigger in MetS among them. However, the incidence of MetS in the low BMI subgroup was too small to analyze the effects of H. pylori infection on it, and the sample size of this study was too small as well.
Several previous studies have indicated that H, pylori infection increased the incidence of pregnancy-related diseases, including GDM, spontaneous abortion, and congenital disability (31-33). Our study also found H. pylori infection was the risk factor of GDM and preeclampsia. GDM and preeclampsia are the advanced stages of elevated BP and BG, which can be explained by the mechanism mentioned above. However, other adverse pregnancy outcomes, including SPB, FGR, and complicated pregnancy, showed no significant relationship with H. pylori infection. According to the P value of 0.076, H. pylori infection may play a role in the uncomplicated pregnancy. It may be beneficial to analyze the relationship between H. pylori infection and adverse pregnancy outcomes using a larger sample size of participants.
The limitations of our study should be noted. First, there are still some confounding factors that may affect the results existing in this study, but we did not get them, including physical activity and socioeconomic index. Second, the interval of follow up in this study is 2 to 4 weeks so that we cannot record metabolic disorders or adverse pregnancy outcomes if they occur during the interval. Third, insulin resistance is a crucial marker of MetS, yet we did not enroll it in this study, which may affect the results.
In this study, H. pylori infection existed in around a quarter of pregnant women and had a significant relationship with metabolic disorders, including MetS during pregnancy, especially in pregnant women with high BMI. Besides, H. pylori infection also increases the incidence of GDM and preeclampsia in obese or overweight pregnant women.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4863
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4863
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4863). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was performed in compliance with the ethical principles of the Declaration of Helsinki (as revised in 2013) and had been approved by the ethics committee of Wuxi People’s Hospital. All pregnant women signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am 2004;33:351-75. table of contents. [Crossref] [PubMed]
- Ji X, Leng XY, Dong Y, et al. Modifiable risk factors for carotid atherosclerosis: a meta-analysis and systematic review. Ann Transl Med 2019;7:632. [Crossref] [PubMed]
- Diabetes Canada Clinical Practice Guidelines Expert C, Punthakee Z, Goldenberg R, et al. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can J Diabetes 2018;42 Suppl 1:S10-5. [Crossref] [PubMed]
- Carson MP. Society for maternal and fetal medicine workshop on pregnancy as a window to future health: Clinical utility of classifying women with metabolic syndrome. Semin Perinatol 2015;39:284-9. [Crossref] [PubMed]
- Dos Prazeres Tavares H, Dos Santos DC, Abbade JF, et al. Prevalence of metabolic syndrome in non-diabetic, pregnant Angolan women according to four diagnostic criteria and its effects on adverse perinatal outcomes. Diabetol Metab Syndr 2016;8:27. [Crossref] [PubMed]
- Wiznitzer A, Mayer A, Novack V, et al. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol 2009;201:482.e1-8. [Crossref] [PubMed]
- Grieger JA, Grzeskowiak LE, Smithers LG, Bianco-Miotto T, Leemaqz SY, Andraweera P, et al. Metabolic syndrome and time to pregnancy: a retrospective study of nulliparous women. BJOG 2019;126:852-62. [Crossref] [PubMed]
- Rice MM, Landon MB, Varner MW, et al. Pregnancy-Associated Hypertension in Glucose-Intolerant Pregnancy and Subsequent Metabolic Syndrome. Obstet Gynecol 2016;127:771-9. [Crossref] [PubMed]
- Grieger JA, Bianco-Miotto T, Grzeskowiak LE, et al. Metabolic syndrome in pregnancy and risk for adverse pregnancy outcomes: A prospective cohort of nulliparous women. PLoS Med 2018;15:e1002710. [Crossref] [PubMed]
- Balgoon MJ, Al-Zahrani MH, Alkhattabi NA, et al. The correlation between obesity and metabolic syndrome in young female university students in the Kingdom of Saudi Arabia. Diabetes Metab Syndr 2019;13:2399-402. [Crossref] [PubMed]
- Simon S, Rahat H, Carreau AM, et al. Poor Sleep Is Related to Metabolic Syndrome Severity in Adolescents With PCOS and Obesity. J Clin Endocrinol Metab 2020;105:e1827-34. [Crossref] [PubMed]
- Bagasrawala SI, Sheth H, Shah H, et al. Metabolic Syndrome Rather than Obesity Alone Is More Significant for Kidney Disease. Obes Surg 2019;29:3478-83. [Crossref] [PubMed]
- Yu Y, Cai J, Song Z, et al. Association of Helicobacter pylori infection with metabolic syndrome in aged Chinese females. Exp Ther Med 2019;17:4403-8. [PubMed]
- Qadri Q, Rasool R, Gulzar GM, et al. H. pylori infection, inflammation and gastric cancer. J Gastrointest Cancer 2014;45:126-32. [Crossref] [PubMed]
- Gunji T, Matsuhashi N, Sato H, et al. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Am J Gastroenterol 2008;103:3005-10. [Crossref] [PubMed]
- Yang W, Xuan C. Influence of Helicobacter pylori Infection on Metabolic Syndrome in Old Chinese People. Gastroenterol Res Pract 2016;2016:6951264. [Crossref] [PubMed]
- Chen TP, Hung HF, Chen MK, et al. Helicobacter Pylori Infection is Positively Associated with Metabolic Syndrome in Taiwanese Adults: a Cross-Sectional Study. Helicobacter 2015;20:184-91. [Crossref] [PubMed]
- Refaeli R, Chodick G, Haj S, et al. Relationships of H. pylori infection and its related gastroduodenal morbidity with metabolic syndrome: a large cross-sectional study. Sci Rep 2018;8:4088. [Crossref] [PubMed]
- Chen YY, Fang WH, Wang CC, et al. Helicobacter pylori infection increases risk of incident metabolic syndrome and diabetes: A cohort study. PLoS One 2019;14:e0208913. [Crossref] [PubMed]
- Wang W, Jiang W, Zhu S, et al. Assessment of prevalence and risk factors of helicobacter pylori infection in an oilfield Community in Hebei, China. BMC Gastroenterol 2019;19:186. [Crossref] [PubMed]
- Xie S, Wang S, Xue L, et al. Helicobacter pylori Is Associated With Precancerous and Cancerous Lesions of the Gastric Cardia Mucosa: Results of a Large Population-Based Study in China. Front Oncol 2020;10:205. [Crossref] [PubMed]
- Jiang JX, Liu Q, Mao XY, et al. Downward trend in the prevalence of Helicobacter pylori infections and corresponding frequent upper gastrointestinal diseases profile changes in Southeastern China between 2003 and 2012. Springerplus 2016;5:1601. [Crossref] [PubMed]
- Yu X, Yang X, Yang T, et al. Decreasing prevalence of Helicobacter pylori according to birth cohorts in urban China. Turk J Gastroenterol 2017;28:94-7. [Crossref] [PubMed]
- Lim SH, Kim N, Kwon JW, et al. Positive Association Between Helicobacter pylori Infection and Metabolic Syndrome in a Korean Population: A Multicenter Nationwide Study. Dig Dis Sci 2019;64:2219-30. [Crossref] [PubMed]
- Chen C, Zhang C, Wang X, et al. Helicobacter pylori infection may increase the severity of nonalcoholic fatty liver disease via promoting liver function damage, glycometabolism, lipid metabolism, inflammatory reaction and metabolic syndrome. Eur J Gastroenterol Hepatol 2020;32:857-66. [Crossref] [PubMed]
- Memon RA, Grunfeld C, Moser AH, et al. Tumor necrosis factor mediates the effects of endotoxin on cholesterol and triglyceride metabolism in mice. Endocrinology 1993;132:2246-53. [Crossref] [PubMed]
- Migneco A, Ojetti V, Specchia L, et al. Eradication of Helicobacter pylori infection improves blood pressure values in patients affected by hypertension. Helicobacter 2003;8:585-9. [Crossref] [PubMed]
- Neri C, Di Cesare C, Labianca A, et al. Obesity in pregnancy as a model to identify women at risk for later metabolic syndrome. Gynecol Endocrinol 2018;34:28-31. [Crossref] [PubMed]
- Feresu SA, Wang Y, Dickinson S. Relationship between maternal obesity and prenatal, metabolic syndrome, obstetrical and perinatal complications of pregnancy in Indiana, 2008-2010. BMC Pregnancy Childbirth 2015;15:266. [Crossref] [PubMed]
- Zhang CM, Zhao Y, Li R, et al. Metabolic heterogeneity of follicular amino acids in polycystic ovary syndrome is affected by obesity and related to pregnancy outcome. BMC Pregnancy Childbirth 2014;14:11. [Crossref] [PubMed]
- Cardaropoli S, Giuffrida D, Piazzese A, et al. Helicobacter pylori seropositivity and pregnancy-related diseases: a prospective cohort study. J Reprod Immunol 2015;109:41-7. [Crossref] [PubMed]
- Grooten IJ, Den Hollander WJ, Roseboom TJ, et al. Helicobacter pylori infection: a predictor of vomiting severity in pregnancy and adverse birth outcome. Am J Obstet Gynecol 2017;216:512.e1-9. [Crossref] [PubMed]
- Zhan Y, Si M, Li M, et al. The risk of Helicobacter pylori infection for adverse pregnancy outcomes: A systematic review and meta-analysis. Helicobacter 2019;24:e12562. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


