
Optical coherence tomography angiography in glaucoma
Introduction
Glaucoma is a progressive optic neuropathy, characterized by both structural and functional visual defects. While functional damage can be assessed by visual field testing, structural damage can be detected by thinning of the retinal ganglion cell layer and retinal nerve fiber layer (RNFL) using optical coherence tomography (OCT) (1). In developed countries, glaucoma remains undiagnosed in 50% of those affected, while in developing countries, this number can reach as high as 90% (2). Therefore, early detection of glaucoma and improved glaucoma diagnosis are critical. However, early diagnosis of glaucoma is not straightforward. Disagreement between structural and functional tests have often been reported, as in the Ocular Hypertension Treatment Study (3) and European Glaucoma Prevention Study (4)] and both structural and functional assessments need to be considered (5).
OCT measurements of the optic disc and macula are important structural tests for glaucoma diagnosis. Regional macular ganglion cell (MGC)/inner plexiform layer thickness decreases in glaucoma (6) and performs as well as regional RNFL outcomes for detection of early glaucoma. Combining the measures from the two algorithms improves glaucoma detection (7,8). Since both mechanical and vascular theories have been proposed in glaucoma pathogenesis, vascular assessment is an important area to study in addition to evaluating structural and functional defects. Disturbance of vascular autoregulation and decreased optic nerve head (ONH) perfusion play important roles in glaucoma (9-12). Several studies have reported an association between reduced ocular perfusion pressure (the difference between IOP and mean arterial pressure) and the development and progression of glaucoma. A three-fold increased risk of glaucoma development between low ocular perfusion pressure and prevalence of glaucoma has been reported, with a linear relationship between the two (10,13-16). Therefore, evaluating optic nerve vascular supply could help assess glaucoma development and progression.
Blood supply to the ONH
Branches of the ophthalmic artery supply the ONH. The short posterior ciliary arteries arise from the ophthalmic artery and surround the optic nerve. The prelaminar ONH region is supplied by the peripapillary choroid, which provides blood through the scleral short posterior ciliary arteries and the recurrent choroidal arteries. The circle of Zinn and Haller, a rich vascular circle, supplies the lamina cribrosa (LC), which is also supplied by branches from the short posterior ciliary arteries either directly or through the circle of Zinn and Haller. The vascular supply of the retrolaminar region arises from the pial network of vessels and from the central retinal artery (17,18).
Vessel density measurements
Vessel density, defined as the percentage area occupied by the vessels inside the ONH can be evaluated noninvasively using optical coherence tomography angiography (OCTA) (19), as well as the peripapillary retina (20), peripapillary choroid (21) and the macula (22) (Figure 1). OCTA enhances our understanding of the role of ocular blood flow in glaucoma. Different OCTA devices employ different methods of vessel density measurement. While AngioVue (Optovue Inc., Fremont, CA), Spectralis angiography module (Heidelberg Engineering, Germany), and Swept-Source OCT Angio (Topcon Corporation, Tokyo, Japan) are based on split-spectrum amplitude-decorrelation angiography techniques (19), a combination of amplitude and phase-variance algorithms (Optical microangiography, OMAG) is used by AngioPlex (Zeiss Meditec Inc., Dublin, CA) (23).
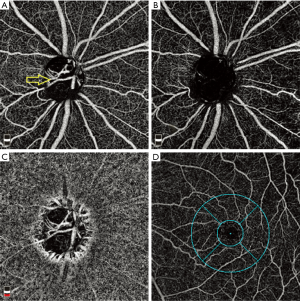
ONH OCTA
OCTA studies have qualitatively assessed the microcirculation within the optic disc and attempted to quantify the vascular density. The initial report on OCTA imaging in glaucoma used a split-spectrum amplitude-decorrelation algorithm and reported that preperimetric glaucoma patients had significantly reduced ONH perfusion compared to normal eyes (19). A dense microvascular network of the ONH had dropped out in subjects with glaucoma (24). Using an OCT-based OMAG prototype system, glaucomatous eyes also had significantly lower ONH perfusion in the pre-laminar layer. Significant correlations between ONH perfusion and disease severity as well as structural changes were detected in glaucomatous eyes (25).
Microvasculature dropout in the optic disc was further evaluated based on the visualization of the anterior LC in horizontal B-scans. Both eyes with true microvasculature dropout and pseudo-microvasculature dropout had severe OCTA signal loss on the temporal side of the optic disc in en face images. If the anterior LC portion was not well visualized in that area, it was defined as pseudo-microvasculature dropout and if it was well visualized in the OCTA signal loss area, it was defined as true microvasculature dropout. Obviously, true microvasculature dropout was associated with a larger cup-to-disc ratio, worse visual field mean deviation, and higher prevalence of focal LC defects (26). There was no difference in optic disc perfusion defects detected with OMAG comparing high tension and normal tension glaucoma eyes with similar levels of visual field damage (27).
Peripapillary vessel density
The peripapillary retina was defined variably from the circular region or whole square area centered on the ONH, or more commonly, the circumpapillary area of a 700-mm-wide elliptical annulus centered on the ONH, when the area of the ONH was excluded (Figure 2). In addition, segmentation of the peripapillary slabs differs from total peripapillary retinal slabs to superficial slabs within the RNFL thickness (radial peripapillary capillaries, RPC) which are the superficial capillary bed suppling the retinal ganglion cell axons (28). Peripapillary vessel density and flow index in glaucomatous eyes were lower than those in normal eyes and both were highly correlated with the visual field index (20,29,30) (Figure 2). In another study, sectoral association between visual field loss and peripapillary vessel density showed a strong correlation in the superotemporal and inferotemporal sectors. Of note, vessel density correlations were similar to the correlation between RNFL thickness and visual field loss (31).
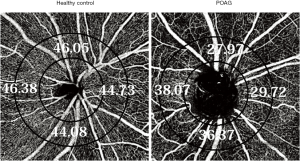
Peripapillary vessel density for glaucoma detection
The ability of peripapillary vessel density to detect glaucoma progression has been compared to that of RNFL thickness. While in one study, the areas under the receiver operating characteristic (ROC) curve of the peripapillary vessel density for discriminating between glaucomatous and normal eyes was 0.93 (better than peripapillary RNFL thickness) (29), Chen et al. (32) reported that RNFL thickness and macula ganglion cell complex (mGCC) thickness had higher areas under the ROC curve in normal vs. glaucomatous eyes (0.95 for both) compared to peripapillary vessel density (0.89). Yarmohammadi et al. (33) compared RNFL thickness and peripapillary retinal OCTA vessel density in healthy, glaucoma suspects, and glaucoma patients and found that the whole peripapillary image vessel density performed as well as RNFL thickness for discriminating these three categories. The whole image vessel density also had significantly better diagnostic accuracy than circumpapillary vessel density for differentiating between glaucoma patients and healthy groups (33). Peripapillary vessel density also demonstrated a progressive stepwise decrease from control eyes throughout worsening glaucoma stages (34). Vessel density was higher in normal eyes and decreased successively in glaucoma suspects, mild glaucoma, and moderate to severe glaucoma eyes; the association between visual field mean deviation with peripapillary vessel density was stronger than that between visual field mean deviation with RNFL and optic nerve rim area (35). Flow density and disappearance angle of the RPCs were significantly and independently correlated with RNFL and visual field values in glaucoma eyes suggesting that angle of RPC dropout is another index in evaluating the optic disc (36). Similarly, significant reductions in radial peripapillary vessel density which were correlated with sites of RNFL decrease and visual field loss in glaucoma were found using custom built speckle variance OCT-A (37).
Peripapillary vessel dropout in intact visual field
While several previously noted OCTA studies investigated vessel density values in the affected visual field, some studies evaluated vessel density measurements in the unaffected hemisphere or unaffected contralateral eyes of glaucoma eyes with the aim of exploring whether vascular defects occur before or after structural defects in early stages of glaucoma. Akagi et al. found reduced peripapillary vessel density of eyes with glaucoma at the corresponding area of the inferior field defects in both high myopia and low myopia. Reduced vessel density was not observed in the intact field of eyes with glaucoma. A decreased “inside disc” vessel density of abnormal visual field was also seen only in glaucomatous eyes without high myopia, but not in unaffected field of those eyes (38). On the other hand, other studies have shown vessel density dropout in the retinal and peripapillary regions corresponding to unaffected hemifields of glaucoma eyes with single-hemifield visual field defects. Yarmohammadi et al. showed that mean peripapillary vessel densities in the intact hemiretinas were lower than in healthy eyes. In this study, both the RNFL and peripapillary and macular vessel densities had lower values in the affected hemiretinas compared with perimetrically unaffected hemiretinas in glaucomatous eyes. Of note, vessel densities of both peripapillary and macular locations in the intact hemiretinas of glaucomatous eyes were lower compared with healthy eyes (39). Although the extent of peripapillary vessel dropout and RNFL thinning in perimetrically intact regions of glaucomatous eyes varied in different sectors in another study, they reach the same conclusion that vascular changes precede functional decline in intact visual field (40). Likewise, Chen et al. showed significantly lower peripapillary vessel density in the apparently intact hemiretina of glaucomatous eyes compared to normal eyes using OMAG algorithm despite the fact that there was no difference in RNFL thickness (41). Not only the vessel density but optic nerve rim area and mean RNFL thickness in unaffected eyes of patients with glaucoma were also lower than in healthy controls (42). Another study investigated inter-eye peripapillary vessel density asymmetry in healthy, glaucoma suspect, and glaucoma eyes for early detection of glaucomatous damage. Glaucoma suspect eyes had significantly higher asymmetry of peripapillary vessel density compared to normal eyes despite a lack of significance between asymmetric structural parameters of glaucoma suspects and controls (43). While the above mentioned studies suggested vascular changes before detectable visual field damage, debate remains whether impairment in blood flow is a result or a cause of glaucoma.
Peripapillary vessel involvement in different types of glaucoma
Several studies have studied the peripapillary vasculature measurements in different glaucomas. Area under the ROC curves of peripapillary vessel density of temporal and inferotemporal sectors were similar in open angle glaucoma (OAG) and in primary angle closure glaucoma (PACG). Diagnostic accuracy of vessel density measurements were comparable to RNFL thicknesses in both OAG and PACG (44). Zhu et al. (45) found lower vascular density in the parafoveal and peripapillary regions with more vessel dropout in the latter than in the former area in eyes with PACG than normal eyes. In PACG eyes, the vessel density in the peripapillary area correlated closely with the IOP, suggesting superiority of peripapillary vessel density compared to parafoveal vessel density in the management of PACG. Vessel densities in primary angle closure (PAC) without glaucoma were similar to those of controls; however, the superotemporal RNFL was significantly thinner in the PAC group, suggesting earlier reduction of structural markers in PACG compared to retinal vessel dropout (46). We previously found lower vessel density and higher RNFL thickness at one week after acute PAC compared to contralateral unaffected eyes. After 6 weeks, vessel density in the affected eyes was significantly lower than values at 1 week which showing decreasing peripapillary vessel density over 6 weeks after the PAC attack compared with controls. On the other hand, the initial increase in RNFL thickness was followed by a subsequent decrease (47). Normal-tension glaucoma eyes also showed a decreased perfused peripapillary capillary density compared to normal eyes. It appears that vessel density declines as glaucoma progresses; open-angle glaucoma patients had lower vessel density than normal-tension glaucoma patients (48).
Exfoliation syndrome (XFS) is associated with vascular pathology and leads to obliterative vasculopathy. Some studies have recently compared the peripapillary superficial vasculature in exfoliation, exfoliation glaucoma (XFG), and POAG with various results. We previously demonstrated a decreased superficial capillary density in exfoliation compared with controls (49). We (49) and another study (50) also showed XFG eyes had lower vessel values compared with POAG eyes after adjusting for age and stage. Park et al. (51) found reduced superficial peripapillary vessel density in the inferonasal and nasal sectors of eyes with XFG compared to POAG. Two recent studies showed that XFG eyes had a similar level of peripapillary and superficial macular vessel densities compared with open angle glaucoma eyes (52,53).
Peripapillary vessel evaluation in high myopia
Evaluation of the optic disc is difficult in highly myopic eyes for detection of glaucoma. We found a progressive decrease in vessel density from controls to myopia without glaucoma to glaucoma without myopia (54). In addition, vessel density correlated well with visual fields in both highly and non-highly myopic glaucoma eyes (38). Lee et al. also (55) showed reduction in peripapillary vessel density measurements in the outer area (3–6 mm around optic nerve) in highly myopic eyes than in non-highly myopic eyes and in glaucomatous eyes compared to unaffected eyes, suggesting that myopic changes might affect the outer area around the optic disc, but not the inner region (<3 mm). While the average outer macular vessel density was different between normal and glaucomatous eyes in both non-highly and highly myopic eyes, inner macular vessel density was not. Therefore, using a vessel density ratio defined as average outer macular vessel density divided by average inner macular vessel density, the inferior vessel density ratio had the highest discriminating performance for glaucoma in both highly and non-highly myopic eyes in contrast to the superior vessel density ratio. Another study showed a stronger correlation of peripapillary vessel density than RNFL thickness with visual fields in patients with highly myopic glaucoma, but not in the eyes without high myopia suggesting usefulness of peripapillary vessel density for following glaucoma progression in eyes with high myopia (56).
Peripapillary vasculature with and without excluding large vessels
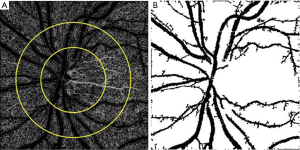
Finally, peripapillary vessel density measurement varies in different studies from including large and small vessels to only small vessels for vessel density calculation (Figure 3). In one study, we excluded large vessels and measured only small peripapillary vessel densities in glaucomatous eyes (57). Holló (58) prospectively compared all peripapillary vessels (large and small vessels) and RNFL thickness measurements over two year period in normal, ocular hypertensive, and glaucoma eyes and did not show progressive vessel density dropout, whereas about one third of glaucoma eyes had progressive RNFL loss. Then, they re-measured small vessels after excluding the large peripapillary vessels and progressive capillary dropout was demonstrated in a small percent of glaucoma eyes (59). Not only does small vessel density measurement assist to detect glaucoma progression, the association between visual field mean deviation and vessel density was highest for measures of ONH when large vessels were removed. Peripapillary small vessel density was also associated with the severity of visual field damage in severe OAG (60).
Macular OCTA
Since loss of retinal ganglion cells has been documented in glaucoma (61), MGC thickness measurements could enhance diagnostic performance for glaucoma (62). Furthermore, ganglion cells are supplied by the superficial macular vascular complex, the vessel density of which can be measured by OCTA. Other macular vascular structures visible on OCTA are the intermediate capillary plexus and deep capillary plexus (63).
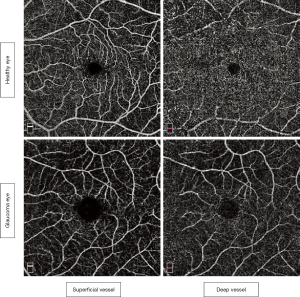
Superficial and deep macular vessel involvements
Reduced superficial macular vessel density was not demonstrated in pre-perimetric glaucoma (64), in contrast to glaucomatous eyes, in which decreased macular vessel density has been well documented (32,63,65-75). While localized capillary loss was found in the superficial macular vessels in glaucomatous eyes without involvement of deep macular vessels (63), another study found lower macular density in both superficial and deep macular layers in glaucomatous eyes than in healthy eyes (65) (Figure 4). It seems that different glaucoma severity in various studies affect different macular vasculatures.
Macular OCTA for glaucoma diagnosis
Comparing the diagnostic performance of macular and peripapillary vessel densities for glaucoma detection has shown that among the vessel density parameters, the average peripapillary vessel density has higher diagnostic performance than the macular region (66). In addition, diagnostic value of MGC thickness compared to macular vessel density has been widely studied and results differ among studies and remain inconclusive. Some studies showed no differences between vessel density and macula thickness to differentiate eyes with glaucoma from unaffected eyes (32,63). Others suggested that macular vessel density has better diagnostic accuracy for glaucoma detection compared with MGC measurements. This higher value of macular vasculature evaluation was also found for discriminating pre-perimetric glaucoma and healthy eyes compared with ganglion cell thickness (67). A strong correlation of macular vessel density with visual field mean deviation compared with MGC thickness and a progressive decline in macula vessel density without ganglion cell thinning in a longitudinal study of moderate glaucoma eyes highlight higher diagnostic performance of macular vessel measurement (35,68). Comparing the rate of change of MGC thickness and macular vessel density also showed that the reduction rate of macular vessel density in glaucoma eyes was significantly greater than ganglion cell thinning. More rapid macular vasculature density decline was observed in over two thirds of the eyes with glaucoma. In contrast to lack of association between ganglion cell thinning rate and glaucoma severity, the faster macular vessel density decrease was associated with severe glaucoma (69). A significant difference in macular vessel density inter-eye asymmetries between glaucoma suspect and healthy eyes also indicates early involvement of the macular vessel without MGC thickness asymmetry (43).
By contrast, Rao et al. found a higher diagnostic value of MGC thickness compared with vessel density of the macula for glaucoma detection. Specifically, the diagnostic abilities of peripapillary RNFL thickness and the MGC thickness in OAG were significantly greater than the corresponding vessel density (70,71). In another study, the same group explored diagnostic abilities of RNFL, MGC, and macular and peripapillary vessel density in glaucoma eyes with and without disc hemorrhage and found similar diagnostic ability of those measures (72). The diagnostic ability of MGC thickness for glaucoma detection was better than that of macular vessel density of inner retina in another study and the strength of the structure-function correlation was more for inner macular thickness than inner macular vessel density indicating the limited role of macular OCTA in the diagnostic evaluation of glaucoma (22). Diagnostic value of asymmetric loss of macular perfusion index was also worse than MGC thickness for glaucoma detection (73).
Discrepancies in the selection of retinal scan areas for vessel density and inclusion of different stages of glaucoma may account for different findings regarding the value of macular vessel vs. MGC. To assess the diagnostic accuracy of 6×6 (outer) and 3×3 (inner) mm macular scans, the authors found vessel density of the outer area had greater diagnostic performance compared with the inner area vessel density to discriminate control and mild glaucoma eyes. Outer region vessel density had similar diagnostic performance compared with the inner area when discriminating between control and moderate to severe glaucoma eyes, suggesting the difference in diagnostic ability of various macular scans (74). Furthermore, it appears that rate of MGC loss and vessel density dropout also differ across various stages of glaucoma. A study observed that despite the fact that those with early glaucoma had higher MGC loss compared to pre-perimetric glaucoma, vessel density was not different between them. In addition, the extent of the percent loss for the vessels and MGC thickness were similar in pre-perimetric glaucoma. However, in early OAG, percent loss for vessel density was lower than that of MGC thickness, which shows possible faster MGC degeneration than vascular damage in early glaucoma. Macula vessel density decreased more over time than MGC thinning, despite higher baseline MGC loss in early glaucoma (75). Comparing early and advanced glaucoma macular damage, advanced glaucomatous eyes showed the slowest rate of MGC thinning, whereas early glaucoma expressed the slowest decrease in vessel density of macula (69). In a similar study, the percent reduction of macular vessel density was lower than that of MGC thickness in early glaucoma eyes, while they were similar in pre-perimetric eyes. The diagnostic performance of macular vessel density is comparable to that of MGC thickness in detecting pre-perimetric glaucoma and early glaucoma (76).
Macular vessel density and glaucoma progression
Regarding the value of macular vessel density in predicting glaucoma progression, one recent cohort study explored the association of peripapillary and macular vessel densities at baseline with rate of RNFL loss and glaucoma progression. Glaucomatous eyes with lower macular vessel density at baseline have a tendency to faster progression than those with higher values. A more rapid loss of 0.11 µm per year in RNFL thickness was seen for each 1% lower baseline macular and ONH vessel densities, indicating that OCTA vascular density values are a risk factor for progression of glaucoma (77). Therefore, measurement of vessel density of ONH and macula provides important information for the assessment of the risk of glaucoma progression.
Parapapillary choroidal vasculature
Short posterior ciliary arteries, which supply the parapapillary choroid, course through the border tissue of Elschnig and sclera to perfuse the prelaminar ONH tissue as well (17,18). The prelaminar region of the ONH might be supplied by the parapapillary choroid in addition to the short posterior ciliary arteries based on in vivo imaging of three eyes (78) suggesting importance of the parapapillary choroidal microvasculature in glaucoma evaluation.
The accuracy of OCTA imaging of the parapillary choroid was compared to indocyanine green angiography and investigated as to whether OCTA accurately images impaired parapapillary choroidal circulation. The microvascular dropout as a sectoral capillary dropout in OCTA was almost equal to the filling defect in indocyanine green angiography (79).
Parapapillary choroidal microvasculature for glaucoma diagnosis
Several studies have evaluated parapapillary deep-layer microvascular dropout using OCTA in POAG eyes (Figure 5) and topographically correlated the findings with glaucomatous RNFL loss. Approximately half of the POAG patients (53.9%) showed microvascular dropout in OCTA in the parapapillary deep layer with a strong correlation with RNFL defect proposing primary involvement of deep circulation in the pathogenesis of glaucoma (80). Localized microvasculature dropouts in the peripapillary choroid have also identified in glaucoma patients adjacent to the optic disc with a topographic focal LC defect in addition to RNFL defects (21).
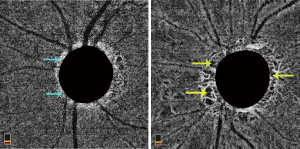
Parapapillary choroidal microvasculature in β-zone and γ-zone parapaillary atrophy
The relationship between peripapillary deep choroidal vessel dropout and β-zone parapaillary atrophy and γ-zone parapapillary was further investigated (81,82). Parapapillary atrophy is divided into a β-zone, which is a region that contains Bruch’s membrane and the underlying choroid, and a γ-zone, which is an area without Bruch’s membrane and choroid (82). While the primary results showed that vessel dropout location involved either the γ-zone, β-zone, or combination of both (81), other studies explored the proportion of vessel dropout in different zones. In eyes containing only a γ-zone, microvascular loss involved the γ-zone, while in eyes with both β- and γ-zones, microvascular dropout was confined within both zones. OAG eyes with microvascular dropout had larger areas of β- and γ-zones than eyes without microvascular dropout, indicating that γ-zone is associated with more severe functional and structural glaucomatous optic nerve damage (82). Pre-perimetric OAG eyes with microvasculature dropout in β-zones was also associated with a thinner RNFL, worse visual field, and higher prevalence of focal LC defect (83). Another study found that glaucomatous eyes with γ-parapapillary atrophy had a significantly higher percentage of parapapillary deep vessel dropout than those with β-parapapillary atrophy with Bruch’s membrane (75.7% versus 40.8%) and microvasculature dropout was correlated with the presence of and wider γ-zone atrophy (84). Furthermore, the correlation between parapapillary deep vasculature dropout and decreased peripapillary choroidal thickness was present only in the POAG eyes with a γ-zone (85). Eyes with focal γ-parapapillary atrophy localized to the inferior or superior hemi-retina had shorter axial lengths, smaller Bruch’s membrane opening areas and higher prevalence of focal LC defects (86).
Clinical implications of parapapillary choroidal microvasculature
Choroidal microvasculature dropout may present a potential vascular factor for both central visual field defect and glaucoma progression.
Because of high prevalence of vessel dropout in eyes with γ-parapapillary atrophy, it is assumed that the vessel dropout is associated with central visual field defects. It is asserted that the location of microvascular dropout is topographically associated with visual field defects in glaucomatous eyes (87,88) and that this vessel dropout correlates with worse visual field mean deviation even after adjusting for focal LC defects and extent of axonal loss (88). Therefore, more than ninety percent of eyes with choroidal vessel dropout show parafoveal visual field defects as opposed to only 39% of eyes without deep-layer vessel dropout (89).
Choroidal microcirculation focal loss was also linked with progressive RNFL loss (90). In fact, deep vessel dropout is associated with prior visual field progression after controlling for known risk factors related with visual field progression, including disc hemorrhage, focal LC defects, width of the β-parapapillary atrophy with Bruch’s membrane, and IOP. Faster progression of visual field defects in eyes with deep-layer vessel dropout indicates the clinical importance of parapapillary deep vessel measurement as a significant structural marker for progressive glaucomatous damage. It is recommended that absence of deep vessel loss is highly predictive of stable disease, whereas the existence of dropout does not assure visual field defect progression (91). In other words, given the association of baseline deep vessel dropout within the parapapillary β-zone with glaucoma progression as measured by visual fields, careful follow up of these glaucoma eyes is reasonable (92).
In conclusion, OCTA can provide an assessment of the vasculature within the optic nerve, peripapillary superficial retina, macula, and parapapillary choroid in glaucoma. The peripapillary superficial retinal vessel density allows diagnosis and detection of glaucoma progression as well as the peripapillary RNFL thickness. Decreased peripapillary vessel density of the intact field or unaffected eyes of glaucomatous eyes indicates that vascular changes may occur prior to detectable visual field damage. Vessel density measurement at the macula is reduced in glaucomatous eyes and can be used for detecting progression with MGC analysis. The higher frequency of choroidal microvasculature dropout in glaucomatous eyes with a parapapillary gamma zone is also associated with central visual field defects or progression of glaucoma.
Acknowledgments
Funding: Supported in part by the Michael and Francesca Freedman Glaucoma Research Fund of the New York Eye and Ear Infirmary of Mount Sinai, New York, NY (RR).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dr. Gabor Hollo) for the series “OCT Angiography in Glaucoma” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2828). The series “OCT Angiography in Glaucoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Quigley HA. Glaucoma. Lancet 2011;377:1367-77. [Crossref] [PubMed]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262-7. [Crossref] [PubMed]
- Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:701-13. [Crossref] [PubMed]
- Miglior S, Zeyen T, Pfeiffer N, et al. Results of the European Glaucoma Prevention Study. Ophthalmology 2005;112:366-75. [Crossref] [PubMed]
- Tatham AJ, Weinreb RN, Medeiros FA. Strategies for improving early detection of glaucoma: the combined structure-function index. Clin Ophthalmol 2014;8:611-21. [PubMed]
- Fard MA, Afzali M, Abdi P, et al. Comparison of the pattern of macular ganglion cell-inner plexiform layer defect between ischemic optic neuropathy and open-angle glaucoma. Invest Ophthalmol Vis Sci 2016;57:1011-6. [Crossref] [PubMed]
- Nouri-Mahdavi K, Nowroozizadeh S, Nassiri N, et al. Macular ganglion cell/inner plexiform layer measurements by spectral domain optical coherence tomography for detection of early glaucoma and comparison to retinal nerve fiber layer measurements. Am J Ophthalmol 2013;156:1297-1307.e2. [Crossref] [PubMed]
- Rolle T, Dallorto L, Tavassoli M, et al. Diagnostic ability and discriminant values of OCT-Angiography parameters in early glaucoma diagnosis. Ophthalmic Res 2019;61:143-52. [Crossref] [PubMed]
- Tobe LA, Harris A, Hussain RM, et al. The role of retrobulbar and retinal circulation on optic nerve head and retinal nerve fibre layer structure in patients with open-angle glaucoma over an 18-month period. Br J Ophthalmol 2015;99:609-12. [Crossref] [PubMed]
- Leske MC, Connell AM, Wu SY, et al. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch Ophthalmol 1995;113:918-24. [Crossref] [PubMed]
- De Moraes CG, Liebmann JM, Greenfield DS, et al. Risk factors for visual field progression in the low-pressure glaucoma treatment study. Am J Ophthalmol 2012;154:702-11. [Crossref] [PubMed]
- Harris A, Rechtman E, Siesky B, et al. The role of optic nerve blood flow in the pathogenesis of glaucoma. Ophthalmol Clin North Am 2005;18:345-53. [PubMed]
- Topouzis F, Wilson MR, Harris A, et al. Association of open-angle glaucoma with perfusion pressure status in the Thessaloniki eye study. Am J Ophthalmol 2013;155:843-51. [Crossref] [PubMed]
- Sommer A. Glaucoma risk factors observed in the Baltimore eye survey. Curr Opin Ophthalmol 1996;7:93-8. [Crossref] [PubMed]
- Tielsch JM, Katz J, Sommer A, et al. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch Ophthalmol 1995;113:216-21. [Crossref] [PubMed]
- Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: The Egna-Neumarkt study. Ophthalmology 2000;107:1287-93. [Crossref] [PubMed]
- Mackenzie PJ, Cioffi GA. Vascular anatomy of the optic nerve head. Can J Ophthalmol 2008;43:308-12. [Crossref] [PubMed]
- Hayreh SS. The blood supply of the optic nerve head and the evaluation of myth and reality. Prog Retin Eye Res 2001;20:563-93. [Crossref] [PubMed]
- Jia Y, Morrison JC, Tokayer J, et al. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express 2012;3:3127-37. [Crossref] [PubMed]
- Chen CL, Zhang A, Bojikian KD, et al. Peripapillary retinal nerve fiber layer vascular microcirculation in glaucoma using optical coherence tomography based microangiography. Invest Ophthalmol Vis Sci 2016;57:475-85. [Crossref] [PubMed]
- Suh MH, Zangwill LM, Manalastas PI, et al. Deep retinal layer microvasculature dropout detected by the optical coherence tomography angiography in glaucoma. Ophthalmology 2016;123:2509-18. [Crossref] [PubMed]
- Wan KH, Lam AKN, Leung CK. Optical coherence tomography angiography compared with optical coherence tomography macular measurements for detection of glaucoma. JAMA Ophthalmol 2018;136:866-74. [Crossref] [PubMed]
- Zhang A, Wang RK. Feature space optical coherence tomography based micro-angiography. Biomed Opt Express 2015;6:1919-28. [Crossref] [PubMed]
- Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014;121:1322-32. [Crossref] [PubMed]
- Chen CL, Bojikian KD, Gupta D, et al. Optic nerve head perfusion in normal eyes and eyes with glaucoma using optical coherence tomography-based microangiography. Quant Imaging Med Surg 2016;6:125-33. [Crossref] [PubMed]
- Akagi T, Zangwill LM, Shoji T, et al. Optic disc microvasculature dropout in primary open-angle glaucoma measured with optical coherence tomography angiography. PLoS One 2018;13:e0201729. [Crossref] [PubMed]
- Bojikian KD, Chen C, Wen JC, et al. Optic disc perfusion in primary open angle and normal tension glaucoma eyes using optical coherence tomography-based microangiography. PLoS One 2016;11:e0154691. [Crossref] [PubMed]
- Yu PK, Balaratnasingam C, Xu J, et al. Label-free density measurements of radial peripapillary capillaries in the human retina. PLoS One 2015;10:e0135151. [Crossref] [PubMed]
- Liu L, Jia Y, Takusagawa HL, et al. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol 2015;133:1045-52. [Crossref] [PubMed]
- Alnawaiseh M, Lahme L, Müller V, et al. Correlation of flow density, as measured using optical coherence tomography angiography, with structural and functional parameters in glaucoma patients. Graefes Arch Clin Exp Ophthalmol 2018;256:589-97. [Crossref] [PubMed]
- Holló G. Relationship between optical coherence tomography sector peripapillary angioflow-density and octopus visual field cluster mean defect values. PLoS One 2017;12:e0171541. [Crossref] [PubMed]
- Chen HS, Liu CH, Wu WC, et al. Optical coherence tomography angiography of the superficial microvasculature in the macular and peripapillary areas in glaucomatous and healthy eyes. Invest Ophthalmol Vis Sci 2017;58:3637-45. [Crossref] [PubMed]
- Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci 2016;57:OCT451-OCT459. [Crossref] [PubMed]
- Geyman LS, Garg RA, Suwan Y, et al. Peripapillary perfused capillary density in primary open-angle glaucoma across disease stage: an optical coherence tomography angiography study. Br J Ophthalmol 2017;101:1261-8. [Crossref] [PubMed]
- Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology 2016;123:2498-508. [Crossref] [PubMed]
- Igarashi R, Ochiai S, Sakaue Y, et al. Optical coherence tomography angiography of the peripapillary capillaries in primary open-angle and normal-tension glaucoma. PLoS ONE 2017;12:e0184301. [Crossref] [PubMed]
- Mammo Z, Heisler M, Balaratnasingam C, et al. Quantitative optical coherence tomography angiography of radial peripapillary capillaries in glaucoma, glaucoma suspect, and normal eyes. Am J Ophthalmol 2016;170:41-9. [Crossref] [PubMed]
- Akagi T, Iida Y, Nakanishi H, et al. Microvascular density in glaucomatous eyes with hemifield visual field defects: An optical coherence tomography angiography study. Am J Ophthalmol 2016;168:237-49. [Crossref] [PubMed]
- Yarmohammadi A, Zangwill L, Diniz-Filho A, et al. Peripapillary and macular vessel density in glaucoma patients with single-hemifield visual field defect. Ophthalmology 2017;124:709-19. [Crossref] [PubMed]
- Pradhan ZS, Dixit S, Sreenivasaiah S, et al. A sectoral analysis of vessel density measurements in perimetrically intact regions of glaucomatous eyes: an optical coherence tomography angiography study. J Glaucoma 2018;27:525-31. [Crossref] [PubMed]
- Chen CL, Bojikian KD, Wen JC, et al. Peripapillary retinal nerve fiber layer vascular microcirculation in eyes with glaucoma and single-hemifield visual field loss. JAMA Ophthalmol 2017;135:461-8. [Crossref] [PubMed]
- Yarmohammadi A, Zangwill LM, Manalastas PIC, et al. Peripapillary and macular vessel density in patients with primary open-angle glaucoma and unilateral visual field loss. Ophthalmology 2018;125:578-87. [Crossref] [PubMed]
- Hou H, Moghimi S, Zangwill LM, et al. Inter-eye asymmetry of optical coherence tomography angiography vessel density in bilateral glaucoma, glaucoma suspect, and healthy eyes. Am J Ophthalmol 2018;190:69-77. [Crossref] [PubMed]
- Rao HL, Kadambi SV, Weinreb RN, et al. Diagnostic ability of peripapillary vessel density measurements of optical coherence tomography angiography in primary open-angle and angle-closure glaucoma. Br J Ophthalmol 2017;101:1066-70. [Crossref] [PubMed]
- Zhu L, Zong Y, Yu J, et al. Reduced retinal vessel density in primary angle closure glaucoma: a quantitative study using optical coherence tomography angiography. J Glaucoma 2018;27:322-7. [Crossref] [PubMed]
- Rao HL, Pradhan ZS, Weinreb RN, et al. Vessel density and structural measurements of optical coherence tomography in primary angle closure and primary angle closure glaucoma. Am J Ophthalmol 2017;177:106-15. [Crossref] [PubMed]
- Moghimi S. Changes in optic nerve head vessel density after acute primary angle closure episode. Invest Ophthalmol Vis Sci 2019;60:552-8. [Crossref] [PubMed]
- Scripsema NK, Garcia PM, Bavier RD, et al. Optical coherence tomography angiography analysis of perfused peripapillary capillaries in primary open-angle glaucoma and normal-tension glaucoma. Invest Ophthalmol Vis Sci 2016;57:OCT611-OCT620. [Crossref] [PubMed]
- Suwan Y, Geyman LS, Fard MA, et al. Peripapillary perfused capillary density in exfoliation syndrome and exfoliation glaucoma versus POAG and healthy controls: an OCTA study. Asia Pac J Ophthalmol (Phila) 2018;7:84-9. [PubMed]
- Rebolleda G, Pérez-Sarriegui A, De Juan V, et al. A comparison of two optical coherence tomography-angiography devices in pseudoexfoliation glaucoma versus primary open-angle glaucoma and healthy subjects. Eur J Ophthalmol 2019;29:636-44. [Crossref] [PubMed]
- Park JH, Yoo C, Girard MJA, et al. Peripapillary vessel density in glaucomatous eyes: comparison between pseudoexfoliation glaucoma and primary open-angle glaucoma. J Glaucoma 2018;27:1009-16. [Crossref] [PubMed]
- Jo YH, Sung KR, Shin JW. Peripapillary and Macular Vessel Density Measurement by Optical Coherence Tomography Angiography in Pseudoexfoliation and Primary Open-angle Glaucoma. J Glaucoma 2020;29:381-5. [Crossref] [PubMed]
- Pradhan ZS, Rao HL, Dixit S, et al. Choroidal microvascular dropout in pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci 2019;60:2146-51. [Crossref] [PubMed]
- Suwan Y, Fard MA, Geyman LS, et al. Association of myopia with peripapillary perfused capillary density in patients with glaucoma: an optical coherence tomography angiography study. JAMA Ophthalmol 2018;136:507-13. [Crossref] [PubMed]
- Lee K, Maeng KJ, Kim JY, et al. Diagnostic ability of vessel density measured by spectral-domain optical coherence tomography angiography for glaucoma in patients with high myopia. Sci Rep 2020;10:3027. [Crossref] [PubMed]
- Shin JW, Kwon J, Lee J. at al. Relationship between vessel density and visual field sensitivity in glaucomatous eyes with high myopia. Br J Ophthalmol 2019;103:585-91. [Crossref]
- Fard MA, Suwan Y, Moghimi S, et al. Pattern of peripapillary capillary density loss in ischemic optic neuropathy compared to that in primary open-angle glaucoma. PLoS One 2018;13:e0189237. [Crossref] [PubMed]
- Holló G. Comparison of peripapillary OCT angiography vessel density and retinal nerve fiber layer thickness measurements for their ability to detect progression in glaucoma. J Glaucoma 2018;27:302-5. [Crossref] [PubMed]
- Holló G. Influence of removing the large retinal vessels-related effect on peripapillary vessel density progression analysis in glaucoma. J Glaucoma 2018;27:e137-e139. [Crossref] [PubMed]
- Ghahari E, Bowd C, Zangwill LM, et al. Association of macular and circumpapillary microvasculature with visual field sensitivity in advanced glaucoma. Am J Ophthalmol 2019;204:51-61. [Crossref] [PubMed]
- Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol 1989;107:453-64. [Crossref] [PubMed]
- Mwanza JC, Durbin MK, Budenz DL, et al. Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology 2012;119:1151-8. [Crossref] [PubMed]
- Takusagawa HL, Liu L, Ma KN, et al. Projection-resolved optical coherence tomography angiography of macular retinal circulation in glaucoma. Ophthalmology 2017;124:1589-99. [Crossref] [PubMed]
- Triolo G, Rabiolo A, Shemonski ND, et al. Optical coherence tomography angiography macular and peripapillary vessel perfusion density in healthy subjects, glaucoma suspects, and glaucoma Ppatients. Invest Ophthalmol Vis Sci 2017;58:5713-22. [Crossref] [PubMed]
- Fard MA, Fakhraee G, Ghahvechian H, Sahraian A, Moghimi S, Ritch R. Macular Vascularity in Ischemic Optic Neuropathy Compared to Glaucoma by Projection-Resolved Optical Coherence Tomography Angiography. Am J Ophthalmol 2020;209:27-34. [Crossref] [PubMed]
- Chung JK, Hwang YH, Wi JM, et al. Glaucoma diagnostic ability of the optical coherence tomography angiography vessel density parameters. Curr Eye Res 2017;42:1458-67. [Crossref] [PubMed]
- Penteado RC, Zangwill LM, Daga FB, et al. Optical coherence tomography angiography macular vascular density measurements and the central 10-2 visual field in glaucoma. J Glaucoma 2018;27:481-9. [Crossref] [PubMed]
- Shoji T, Zangwill LM, Akagi T, et al. Progressive macula vessel density loss in primary open-angle glaucoma: a longitudinal study. Am J Ophthalmol 2017;182:107-17. [Crossref] [PubMed]
- Hou H, Moghimi S, Proudfoot JA, et al. Ganglion cell complex thickness and macular vessel density loss in primary open-angle glaucoma. Ophthalmology 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Rao HL, Riyazuddin M, Dasari S, et al. Diagnostic abilities of the optical microangiography parameters of the 3 × 3 mm and 6 × 6 mm macular scans in glaucoma. J Glaucoma 2018;27:496-503. [Crossref] [PubMed]
- Rao HL, Pradhan ZS, Weinreb RN, et al. A comparison of the diagnostic ability of vessel density and structural measurements of optical coherence tomography in primary open angle glaucoma. PLoS One 2017;12:e0173930. [Crossref] [PubMed]
- Rao HL, Pradhan ZS, Weinreb RN, et al. Optical coherence tomography angiography vessel density measurements in eyes with primary open-angle glaucoma and disc hemorrhage. J Glaucoma 2017;26:888-95. [Crossref] [PubMed]
- Smith CA, West ME, Sharpe GP, et al. Asymmetry analysis of macular optical coherence tomography angiography in patients with glaucoma and healthy subjects. Br J Ophthalmol 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Penteado RC, Bowd C, Proudfoot J, et al. Diagnostic ability of optical coherence tomography angiography macula vessel density for the diagnosis of glaucoma using difference scan sizes. J Glaucoma 2020;29:245-51. [Crossref] [PubMed]
- Hou H, Moghimi S, Zangwill LM, et al. Macula vessel density and thickness in early primary open-angle Glaucoma. Am J Ophthalmol 2019;199:120-32. [Crossref] [PubMed]
- Wang Y, Xin C, Li M, et al. Macular vessel density versus ganglion cell complex thickness for detection of early primary open-angle glaucoma. BMC Ophthalmol 2020;20:17. [Crossref] [PubMed]
- Moghimi S, Zangwill LM, Penteado RC, et al. Macular and optic nerve head vessel density and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology 2018;125:1720-8. [Crossref] [PubMed]
- Na KI, Lee WJ, Kim YK, et al. Evaluation of optic nerve head and peripapillary choroidal vasculature using swept-source optical coherence tomography angiography. J Glaucoma 2017;26:665-8. [Crossref] [PubMed]
- Lee EJ, Lee KM, Lee SH, et al. Parapapillary choroidal microvasculature dropout in glaucoma: a comparison between optical coherence tomography angiography and indocyanine green angiography. Ophthalmology 2017;124:1209-17. [Crossref] [PubMed]
- Lee EJ, Lee SH, Kim JA, et al. Parapapillary deep-layer microvasculature dropout in glaucoma: topographic association with glaucomatous damage. Invest Ophthalmol Vis Sci 2017;58:3004-10. [Crossref] [PubMed]
- Lee EJ, Kim TW, Lee SH, et al. Underlying microstructure of parapapillary deep-layer capillary dropout identified by optical coherence tomography angiography. Invest Ophthalmol Vis Sci 2017;58:1621-7. [Crossref] [PubMed]
- Lee EJ, Kim TW, Kim JA, et al. Parapapillary deep-layer microvasculature dropout in primary open-angle glaucoma eyes with a parapapillary gamma-zone. Invest Ophthalmol Vis Sci 2017;58:5673-80. [Crossref] [PubMed]
- Suh MH, Na JH, Zangwill LM, et al. Deep-layer microvasculature dropout in pre-perimetric glaucoma patients. J Glaucoma 2020;29:423-8. [Crossref] [PubMed]
- Suh MH, Zangwill LM, Manalastas PIC, et al. Deep-layer microvasculature dropout by optical coherence tomography angiography and microstructure of parapapillary atrophy. Invest Ophthalmol Vis Sci 2018;59:1995-2004. [PubMed]
- Lee SH, Lee EJ, Kim TW. Topographic correlation between juxtapapillary choroidal thickness and parapapillary deep layer microvasculature dropout in primary open-angle glaucoma. Br J Ophthalmol 2018;102:1134-40. [Crossref] [PubMed]
- Kim HR, Weinreb RN, Zangwill LM, et al. Characteristics of focal gamma zone parapapillary atrophy. Invest Ophthalmol Vis Sci 2020;61:17. [Crossref] [PubMed]
- Lee EJ, Kim TW, Kim JA, et al. Central visual field damage and parapapillary choroidal microvasculature dropout in primary open-angle glaucoma. Ophthalmology 2018;125:588-96. [Crossref] [PubMed]
- Suh MH, Park JW, Kim HR. Association between the deep layer microvasculature dropout and the visual field damage in glaucoma. J Glaucoma 2018;27:543-51. [Crossref] [PubMed]
- Kwon J, Shin JW, Lee J, et al. Choroidal microvasculature dropout is associated with parafoveal visual field defects in glaucoma. Am J Ophthalmol 2018;188:141-54. [Crossref] [PubMed]
- Park HL, Kim JW, Park CK. Choroidal microvasculature dropout is associated with progressive retinal nerve fiber layer thinning in glaucoma with disc hemorrhage. Ophthalmology 2018;125:1003-13. [Crossref] [PubMed]
- Kwon JM, Weinreb RN, Zangwill LM, et al. Parapapillary deep-layer microvasculature dropout and visual field progression in glaucoma. Am J Ophthalmol 2019;200:65-75. [Crossref] [PubMed]
- Park HY, Shin DY, Jeon SJ, et al. Association between parapapillary choroidal vessel density measured with optical coherence tomography angiography and future visual field progression in patients with glaucoma. JAMA Ophthalmol 2019;137:681-8. [Crossref] [PubMed]


