
Natural compound Tan-I enhances the efficacy of Paclitaxel chemotherapy in ovarian cancer
Introduction
Ovarian cancer is the 10th most common type of cancer among women worldwide and the 5th most common cancer in China (1,2). Despite recent advances in clinical diagnosis, 75% of patients are still diagnosed at the late stage of the disease. The 5-year survival rate ranging from 15% to 25% is lower than that for many other leading cancers (1,3,4).
Chemotherapy is one of the methods of clinical therapy for ovarian cancer and most patients will attain a remission with initial treatment. Although the majority of ovarian cancer patients will respond to initial chemotherapy, most will ultimately develop disease recurrence (5). Paclitaxel is a first-line preventive chemotherapy drug for ovarian carcinoma, which is widely used. Paclitaxel can kill tumor cells due to promoting the polymerization of tubulin by disrupting the healthy standard microtubule dynamics required for cell division and vital interphase processes and chromosome missegregation on multipolar spindles (6). The Paclitaxel alone or combination therapy achieves a CR ranging from 50% to 81% and median PFS range of 13.6 to 19.3 months in advanced ovarian cancer (7-9). Nevertheless, several clinical studies confirmed that the occurrence of Paclitaxel resistance and toxicities contributed to the failure of ovarian carcinoma treatment (10,11).
Tanshinone is the main pharmacologically active component of Salvia miltiorrhiza Bunge, Chinese herbal medicine with many pharmacological activities, such as cardiovascular safety, anti-inflammatory, anti-hepatic fibrosis and antitumor characteristics (12-15). The reported studies demonstrated that Tanshinone markedly inhibited the growth of leukemia, colorectal cancer, lung cancer, Glioma, and breast cancer in vitro and vivo by induction of apoptosis and anti-angiogenesis activity (16-21). Our first study showed that Tanshinone I (Tan-I) is a soluble component that can inhibit the growth of ovarian cancer in vitro and vivo by promoting apoptosis and inducing autophagic cell death (3). Besides, Tan-I exhibit less toxicity in healthy cells and tissues (22).
In this study, Tan-I and Paclitaxel have been administered simultaneously in vivo to the ovarian cancer cell lines in vitro and xenograft ovarian cancer model to evaluate the potential as a combination drug therapy in this research.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4072).
Methods
Mice
Experiments were performed under a project license (license number KS2020038) granted by the Animal Care Committee of Sichuan Agriculture University.
Female 4- to 6-week-old BALB/c nude mice were obtained from Nanjing model animal research centre, and mouse research procedures in vivo were performed according to the Animal Care Committee of Sichuan agriculture university. All mice were littermates and were maintained under specific pathogen-free (SPF) conditions in the Animal Center of Sichuan agriculture university (Sichuan, China). Experimental groups were n=10 in tumor xenograft experiments and n=6 in immunohistochemistry assay. Numbers of mice used in experimental groups in the survival studies are shown in the respective figures.
Cell culture
The human A2780 and mouse ID-8 cell lines were bought from American Type Culture Collection (ATCC, USA). A2780 and ID-8 cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (10% fetal bovine serum 10% FBS and 100 U/mL penicillin and streptomycin) in a cell incubator with 5% CO2 at 37 °C.
CCK8 assay
Following the manufacture’s instructions, the cell viability was analyzed by the CCK8 kit (Beyotime, Shanghai, China). Cells were seeded in 96-well microplates at a density of 3×103/well in 100 µL of the medium. The cells were treated with Tan-I (4.8 µg/mL), Paclitaxel (0.1 µg/mL), or Tan-I combined with Paclitaxel for 24 hours. Then 10 µL of CCK-8 reagent was added to each well and then incubating for 2 hours. All experiments were performed three times. Using wells without cells as blanks, the absorbance was analyzed at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
EDU staining assay
EdU staining was used to analyze cell proliferation by the following protocol. Briefly, A2780 and ID-8 cell lines were incubated with Tan-I (4.8 µg/mL), Paclitaxel (0.1 µg/mL), or Tan-I combined with Paclitaxel for 24 hours. The cells were incubated with EdU After treatment for 24 hours (20 mM) with 5% CO2 at 37 °C for 2 hours and then cultured with Hoechest33342 to visualize the nuclei for 30 minutes. The cells were subsequently fixed with 4% paraformaldehyde for 20 minutes at room temperature. Proliferation was analyzed using the percentage of EdU positive cells in ten fields for each sample.
Flow cytometry assay
A2780 and ID-8 cells (1×105 cells/mL) were cultured in 10% FBS high glucose DMEM complete medium for 24 hours in 6-well plates. Then the cells were co-cultured with Tan-I (4.8 µg/mL), Paclitaxel (0.1 µg/mL), or Tan-I combined with Paclitaxel. Next, the treated cells were digested by 0.25% Trypsin-no EDTA after treatment for 24 hours and washed with cold PBS buffer. At 4 °C for 30 minutes in the dark, the cells were resuspended in 400 µL binding buffer and stained with 5 µL Annexin V-FITC combined with 5 µL propidium iodide (Beyotime, Shanghai, China). The stained cells were washed by binding buffer three times and then resuspended in 500 µL binding buffer. The percentages of apoptosis were recorded by flow cytometry (BD, FACSCalibur, USA). The experiment was repeated in triplicate.
TUNEL assay
The TUNEL assay in cells and tissues was performed according to the manufacturer’s instructions (Vazyme Biotech Co., Ltd, China). For cells, A2780 and ID-8 cells were treated with Tan-I (4.8 µg/mL), Paclitaxel (0.1 µg/mL), or Tan-I combined with Paclitaxel for 24 hours. The cells were first incubated in a bright red labeling mix for 45 minutes at 37 °C, followed by treatment with Click-iT reaction cocktail. The nucleus was stained with DAPI. For tissues, the sections of tumor tissue were deparaffinized in xylene and rehydrated in PBS buffer. The sections were incubated with Biotin-dUTP Labeling Mix for 1 hour at 37 °C in the dark and then were covered in Streptavidin-HRP for 30 minutes. The slides were visualized by the DAB substrate and looked at using a microscope (OLYMPUS, Japan). TUNEL-positive cells and the apoptotic index was calculated as a ratio of (apoptotic cell number)/(total cell number) in each field.
Transwell assay
Holding 10% FBS for 24 hours on 6-well plates A2780 and ID-8 cells (1×105 cells/mL) were grown in DMEM. Then the cells were treated with Tan-I (4.8 µg/mL), Paclitaxel (0.1 µg/mL), or Tan-I combined with Paclitaxel for 24 hours. These administrated cells were digested and suspended with a serum-free medium at a density of 1×106 cells/mL for cell migration. Cells were seeded into the upper chamber of a 24-well plate (Corning, Corning, NY, USA) with a volume of 100 µL, before which 500 µL of medium with 10% FBS was added into the lower chamber. The cells were washed with PBS three times after incubation for 24 hours, fixed 100% ice-cold methanol for 10 minutes, and stained with 0.4% crystal violet (Sigma-Aldrich) for 5 minutes. The non-migrated cells were cleared through using a wet cotton swab. For the assessment of cell migration, ten fields were randomly chosen and calculated the number of migrations under a Nikon microscope. Each experiment was repeated at least three times in triplicate.
Wound healing
A2780 and ID-8 cells (2×106 cells/mL) in a 6-well plate were treated with Tan-I (4.8 µg/mL), Paclitaxel (0.1 µg/mL) or Tan-I combined with Paclitaxel. After the treatment for 24 hours, a straight scratch was made in individual wells with a 200 mL pipette tip. This point was considered 0 hours. The width of the wound was photographed at 0 and 48 hours. Wound healing was measured by calculating the reduction of the width of the wound after incubation and comparing it to 0 hours, which were set at 100%.
Senescence assay
Holding 10% FBS for 24 hours at a density of 5×104 cells/well in 6-well plates, A2780, and ID-8 cells were grown in DMEM. Then the cells were treated with Tan-I (4.8 µg/mL), Paclitaxel (0.1 µg/mL), or Tan-I combined with Paclitaxel. The treated cells were fixed with 4% formaldehyde in PBS for 15 minutes at room temperature and incubated with SA b-gal staining solution at 37 °C overnight after the treatment for 24 hours. The SA b-gal positive cells were evaluated by counting 100 cells per dish. Data were expressed as the percentage of cells on each dish that was SA b-gal positive.
Tumor xenograft assay
Mouse research was performed according to the Animal Care Committee of Sichuan agriculture university. Female 4–6 weeks old BALB/c nude mice were obtained from the Nanjing model animal research center. The A2780 cells were injected bilaterally and subcutaneously into the flanks of the nude mice (100 µL, 2×107 cells). The mice were randomly divided into control groups (DMSO), Tan-I group (30 mg/kg), and Tan-I (30 mg/kg) combined with Paclitaxel (10 mg/kg) group. Three groups were administered by i.p. an injection every three days. Tumor weight was measured after treatment for 30 days.
Western blotting assay
The RIPA buffer (Beyotime, Shanghai, China) extracted the total protein used for western blot, and BCA Protein Assay Kit (Beyotime, Shanghai, China) quantified them. The protein bands were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a Nitrocellulose (NC) membrane (Millipore). After blocking with 5% bovine serum albumin (BSA) for 1 hour, the NC membrane was incubated with primary antibodies for 2 hours at room temperature. After washing by PBST for three times. Next, the NC membrane was incubated with horseradish peroxidase conjugated secondary antibodies for 1 hour at room temperature. Finally, enhanced chemiluminescence was visualized the immunoblot by Bio-Imaging System. β-actin was used as an internal control, and protein expression was determined by ImageJ software.
Immunohistochemistry assay
After mice were sacrificed, the tumor tissues were isolated. The tissues were at once fixed in 4% paraformaldehyde for 24 hours and embedded in paraffin. The embedded sections were sliced into 5 µm sections. Caspase-3 and Ki67 staining were performed as previously described (22).
Statistical analysis
The data from these experiments are presented as mean ± SD. The statistical analyses were assessed using the SPSS software version19.0. The statistical significance for the comparisons among these groups of either two or three was analyzed using the Student t-test or one-way ANOVA, respectively. A P value of <0.05 showed a statistically significant result.
Results
Tan-I combined with Paclitaxel inhibit proliferation of ovarian cancer cell lines
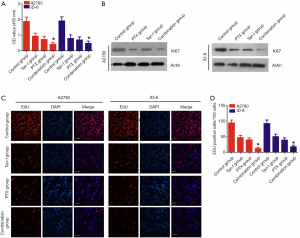
CCK-8 assays and EDU staining were used to detect the viability of ovarian cancer cells (A2780 and ID-8) after treatment with alone Tan-I, Paclitaxel, or Tan-I combined with Paclitaxel for 24 hours to explore the activity of Tan-I Combination with Paclitaxel in the proliferation of ovarian cancer cells. As showed in Figure 1A, Tan-I and Paclitaxel significantly inhibited the growth of ovarian cancer cells than that of the control group. Compared with Tan-I and Paclitaxel, Tan-I combined with Paclitaxel further induced about 50% growth inhibition in A2780 and ID-8 ovarian cancer cells. Western blot assay indicated that the treatment of Tan-I combined with Paclitaxel markedly reduced the Ki67 protein expression in A2780 cells and ID-8 cells than that of alone Tan-I or Paclitaxel (Figure 1B). Furthermore, the EdU positive cells markedly inhibited by alone Tan-I or Paclitaxel treatment than that of the control group (P<0.05) (Figure 1C,D). Compared with alone Tan-I or Paclitaxel treatment, the EdU positive cells significantly reduced by treatment of Tan-I combined with Paclitaxel. These results suggested that Tan-I combined with Paclitaxel could significantly suppress cell proliferation in A2780 cells and ID-8 cells.
Tan-I combined with Paclitaxel promote apoptosis of cancer cell
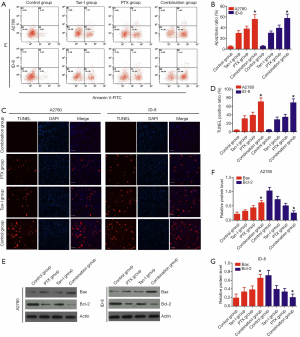
Flow cytometry and TUNEL staining were performed in A2780 and ID-8 cells to analyze the apoptosis level of Tan-I combined with Paclitaxel. After being treated with alone Tan-I or Paclitaxel for 24 hours, the apoptotic level significantly increased in A2780 and ID-8 cells compared with the control group. Tan-I combined with Paclitaxel markedly induce apoptosis of A2780 and ID-8 cells than that of alone Tan-I or Paclitaxel treatment (P<0.05) (Figure 2A,B). TUNEL staining showed that the number of apoptotic cells significantly increased with treatment of Tan-I or Paclitaxel than that of the control group (P<0.05), and then Tan-I combined with Paclitaxel further promote the apoptosis of A2780 and ID-8 cells compared to the treatment of Tan-I or Paclitaxel alone (Figure 2C,D). Besides, Tan-I combined with Paclitaxel significantly promoted Bax expression and reduced Bcl-2 expression in A2780 and ID-8 cells (Figure 2E,F,G). These data suggested that combined with Paclitaxel promoted apoptosis of cancer cells by inducing apoptosis-associated protein expression and cleavage.
Tan-I combined with Paclitaxel inhibits migration of cancer cells
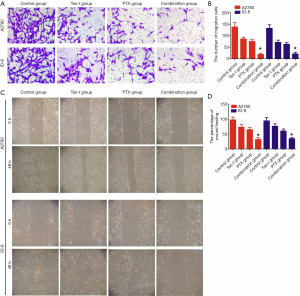
We performed wound-healing assay and Transwell assay to investigate whether Tan-I combined with Paclitaxel had an impact on the migration of cancer cells. The distance of cell migration significantly shorted in the Tan-I combined with Paclitaxel group than that of either alone group after 24 hours of treatment with Tan-I or Paclitaxel alone or Tan-I combined with Paclitaxel (P<0.05) (Figure 3A,B). The wound-healing assay showed that the number of cells passing through the membrane significantly decreased in the Tan-I combined with the Paclitaxel group compared to that in either alone group (P<0.05) (Figure 3C,D). These data illustrated that Tan-I could inhibit the migration of ovarian cancer cells when combined with Paclitaxel.
Tan-I combined with Paclitaxel accelerate senescence of cancer cells
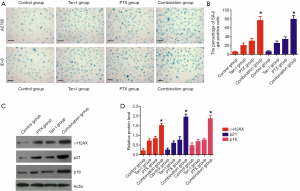
Cell senescence was evaluated by SA-β-Gal staining to evaluate the effect of Tan-I combined with Paclitaxel on the senescence of cancer cells. The results showed that Tan-I combined with Paclitaxel treatments caused evident cellular senescence than that of Tan-I or Paclitaxel alone (P<0.05) (Figure 4A,B). To further insights into the molecular mechanism of Tan-I combined with Paclitaxel during the senescence development, we evaluated DNA damage response (DDR) signals and senescence-associated proteins by western blot. We found that Tan-I combined with Paclitaxel treatment almost simultaneously with activation of the DDR machinery (phosphorylated histone H2AX (γ-H2AX)) and senescence-associated proteins (p21 and p16) (P<0.05) (Figure 4C,D).
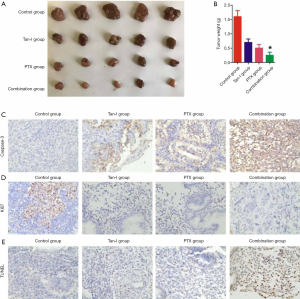
Tan-I combination with Paclitaxel inhibits tumor growth in vivo
To analysis, the antitumor effect of Tan-I combined with Paclitaxel in vivo, A2780 tumor-bearing xenograft was constructed and treated. The results showed that tumor weight in Tan-I combined with Paclitaxel group markedly decreased than that of Tan-I group and Paclitaxel group (P<0.05) (Figure 5A,B). Caspase-3 positive cells in Tan-I combined with Paclitaxel group significantly enhanced than that of Tan-I group and Paclitaxel group (P<0.05) (Figure 5C). Nevertheless, the Ki67 positive ratio in Tan-I combined with the Paclitaxel group significantly inhibited compared to the Tan-I group and Paclitaxel group (P<0.05) (Figure 5D). TUNEL positive cells in Tan-I combined with Paclitaxel group significantly enhanced than that of Tan-I group and Paclitaxel group (P<0.05) (Figure 5E). The dates showed that Tan-I combination with Paclitaxel inhibits tumor growth in vivo.
Discussion
At a five-year survival rate, ovarian carcinoma has become the most aggressive genital system tumor in women for the resistance of chemotherapy (23,24). It is an essential value to chemotherapy that can enhance efficacy and alleviate side effects. We, in our study, analyzed the efficacy of Tan-I combination with Paclitaxel on ovarian carcinoma A2780 and ID-8 cells. Inducing cell apoptosis and promoting cell senescence, the results showed that Tan-I enhanced the efficacy of ovarian cancer to Paclitaxel chemotherapy by inhibiting cell proliferation and cell migration.
Cell apoptosis is an essential manifestation of cell death, which is implicated with the development of tumors (25). The studies had shown that Paclitaxel causes cell death by stabilization of microtubule dynamics resulting in activation of the spindle assembly checkpoint and apoptosis (26-28). The first study had shown that Tan-1 could inhibit the growth of ovarian cancer by promoting cell apoptosis (22). We postulated that the combination therapy of Tan-1 and Paclitaxel further promoted the apoptosis of tumor cells. The results manifested that Tan-1 can significantly promote cell apoptosis and inhibit cell proliferation further compared with Tan-1 or Paclitaxel alone combined with Paclitaxel. This result is consistent with our hypothesis.
Tumor cell migration is a crucial process for cancer cell dissemination and metastasis that is controlled by extracellular matrix (ECM) remodeling, and dynamic reorganization of cell adhesions with neighboring cells and with the underlying connective tissue (29,30). Paclitaxel inhibited numbers of tumor cell migration by suppressing microtubule dynamics (31,32). Our study first showed that Tan-1 could inhibit the migration of ovarian cancer cells. Then, the combination therapy of Tan-1 and Paclitaxel could block cell migration. Migration-associated proteins such as FAK, ROCK1, p-AKT, and uPA were more inhibited to determine the probable mechanisms of combining Tan-1 and Paclitaxel and treatment and Tan-1 or Paclitaxel alone.
Cellular senescence is elicited in response to endogenous or exogenous stress signals, which is an irreversible cell-cycle arrest (33,34). The senescence of cancer cells is widely recognized as a potent tumor-suppressive mechanism. The reports showed that mice with tumors capable of TIS had a much better prognosis following chemotherapy (35). Another study further showed that sunitinib treatment of OS-RC-2 RCC xenografts inhibited tumor formation by promoting SA-β-Gal activity (36). The study reported that senescence was induced in various cancer cell lines following treatment with doxorubicin, irinotecan, methotrexate, 5-fluorouracil, oxaliplatin, or Paclitaxel (37). Our study showed that Paclitaxel could significantly induce senescence of ovarian carcinoma. Furthermore, Tan-1, combined with Paclitaxel, promoted senescence of ovarian carcinoma cells compared with Tan-1 or Paclitaxel alone.
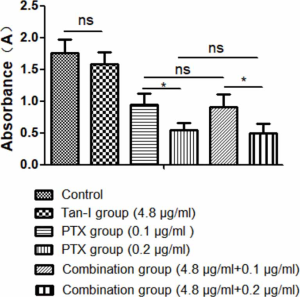
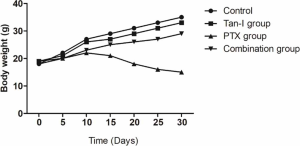
Also, the toxicity of antitumor therapy not only brings great pain to patients; however, it reduces compliance with patients. Anemia, nausea, vomiting, fatigue, diarrhea, cardiotoxicity, and leukopenia for myeloid inhibition are the main side effect of chemotherapy. Paclitaxel showed a significant side effect in healthy ovarian cells, rather than Tan-I. The treatment of Tan-I combination with Paclitaxel did not enhance cell cytotoxicity of Paclitaxel in healthy ovarian cells (Figure S1). In the tumor model, Paclitaxel treatment caused the reduction of body weight in bearing tumor mice; however, the treatment of Tan-I combination with Paclitaxel significantly alleviate cell cytotoxicity of Paclitaxel in vivo (Figure S2).
Base on the above mechanism, we found that Tan-I combination with Paclitaxel inhibits tumor growth in vivo. In summary, Tan-I enhances the efficacy of ovarian cancer to Paclitaxel chemotherapy by inhibiting cell proliferation, migration, and senescence. Therefore, Tan-I is a potential antitumor compound in ovarian cancer for low toxicity, and it may supply the potential clinical use to ovarian carcinoma.
Acknowledgments
Funding: This study was supported by the Sichuan Science and Technology Program (20ZDYF1286, 2019YFS0021), Sichuan Crops, and Animals Breeding Special Project (2016NYZ0036) and Sichuan Agricultural University funding (03572198, 03572098, 03571541).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4072
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4072
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4072). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (license number KS2020038) granted by the Animal Care Committee of Sichuan Agriculture University. Mouse research procedures in vivo were performed according to the guideline of the Animal Care Committee of Sichuan Agriculture University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gorodnova TV, Sokolenko AP, Kuligina E, et al. Principles of clinical management of ovarian cancer. Chin Clin Oncol 2018;7:56. [Crossref] [PubMed]
- Shetty M. Imaging and Differential Diagnosis of Ovarian Cancer. Semin Ultrasound CT MR 2019;40:302-18. [Crossref] [PubMed]
- Cao W, Wang L. Common and specific genes in ovarian clear cell carcinoma and serous carcinoma by gene expression analysis. Transl Cancer Res 2018;7:1501-9. [Crossref]
- Salani R, Backes FJ, Fung MF, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol 2011;204:466-78. [Crossref] [PubMed]
- Marchetti C, Rosati A, Scambia G, Fagotti A. Secondary cytoreduction in platinum-sensitive recurrent ovarian cancer: are we missing something? Ann Transl Med 2019;7:S372. [Crossref] [PubMed]
- Mekhail TM, Markman M. Paclitaxel in cancer therapy. Expert Opin Pharmacother 2002;3:755-66. [Crossref] [PubMed]
- Tropé C, Kaern J, Kristensen G, et al. Paclitaxel in untreated FIGO stage III suboptimally resected ovarian cancer. Ann Oncol 1997;8:803-6. [Crossref] [PubMed]
- Meier W, du Bois A, Rau J, et al. Randomized phase II trial of carboplatin and Paclitaxel with or without lonafarnib in first-line treatment of epithelial ovarian cancer stage IIB-IV. Gynecol Oncol 2012;126:236-40. [Crossref] [PubMed]
- Hainsworth JD, Thompson DS, Bismayer JA, et al. Paclitaxel/carboplatin with or without sorafenib in the first-line treatment of patients with stage III/IV epithelial ovarian cancer: a randomized phase II study of the Sarah Cannon Research Institute. Cancer Med 2015;4:673-81. [Crossref] [PubMed]
- Richardson DL, Sill MW, Coleman RL, et al. Paclitaxel With and Without Pazopanib for Persistent or Recurrent Ovarian Cancer: A Randomized Clinical Trial. JAMA Oncol 2018;4:196-202. [Crossref] [PubMed]
- Pignata S, Lorusso D, Scambia G, et al. Pazopanib plus weekly Paclitaxel versus weekly Paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomised, open-label, phase 2 trial. Lancet Oncol 2015;16:561-8. [Crossref] [PubMed]
- Jiao J, Wen F. Tanshinone IIA Acts via p38 MAPK to Induce Apoptosis and the Down-Regulation of ERCC1 and Lung-Resistance Protein in Cisplatin-Resistant Ovarian Cancer Cells. Oncol Rep 2011;25:781-8. [PubMed]
- Cai Y, Zhang W, Chen Z, et al. Recent insights into the biological activities and drug delivery systems of tanshinones. Int J Nanomedicine 2016;11:121-30. [PubMed]
- Zhang Y, Jiang P, Ye M, et al. Tanshinones: sources, pharmacokinetics and anti-cancer activities. Int J Mol Sci 2012;13:13621-66. [Crossref] [PubMed]
- Tang Y, Chen Y, Chu Z, et al. Protective effect of cryptotanshinone on lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol 2014;723:494-500. [Crossref] [PubMed]
- Jing X, Xu Y, Cheng W, et al. Tanshinone I induces apoptosis and pro-survival autophagy in gastric cancers. Cancer Chemother Pharmacol 2016;77:1171-81. [Crossref] [PubMed]
- Wang F, Ma J, Wang KS, et al. Blockade of TNF-alpha-induced NF-kappaB signaling pathway and anti-cancer therapeutic response of dihydrotanshinone I. Int Immunopharmacol 2015;28:764-72. [Crossref] [PubMed]
- Chen W, Luo Y, Liu L, et al. Cryptotanshinone inhibits cancer cell proliferation by suppressing Mammalian target of rapamycin-mediated cyclin D1 expression and Rb phosphorylation. Cancer Prev Res (Phila) 2010;3:1015-25. [Crossref] [PubMed]
- Xie J, Liu J, Liu H, et al. The antitumor effect of tanshinone IIA on anti-proliferation and decreasing VEGF/VEGFR2 expression on the human non-small cell lung cancer A549 cell line. Acta Pharm Sin B 2015;5:554-63. [Crossref] [PubMed]
- Wang X, Wei Y, Yuan S, et al. Potential anticancer activity of tanshinone IIA against human breast cancer. Int J Cancer 2005;116:799-807. [Crossref] [PubMed]
- Yang L, Guo H, Dong L, et al. Tanshinone IIA inhibits the growth, attenuates the stemness and induces the apoptosis of human glioma stem cells. Oncol Rep 2014;32:1303-11. [Crossref] [PubMed]
- Zhou J, Jiang YY, Chen H, et al. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif 2020;53:e12739. [Crossref] [PubMed]
- Lee RFS, Riedel T, Escrig S, et al. Differences in cisplatin distribution in sensitive and resistant ovarian cancer cells: a TEM/NanoSIMS study. Metallomics 2017;9:1413-20. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature 2001;411:342-8. [Crossref] [PubMed]
- Ling YH, Yang Y, Tornos C, et al. Paclitaxel-induced apoptosis is associated with expression and activation of c-Mos gene product in human ovarian carcinoma SKOV3 cells. Cancer Res 1998;58:3633-40. [PubMed]
- Jones NA, Turner J, McIlwrath AJ, et al. Cisplatin- and paclitaxel-induced apoptosis of ovarian carcinoma cells and the relationship between bax and bak up-regulation and the functional status of p53. Mol Pharmacol 1998;53:819-26. [PubMed]
- Orr GA, Verdier-Pinard P, McDaid H, et al. Mechanisms of Taxol resistance related to microtubules. Oncogene 2003;22:7280-95. [Crossref] [PubMed]
- van Roosmalen W, Le Devedec SE, Golani O, et al. Tumor cell migration screen identifies SRPK1 as breast cancer metastasis determinant. J Clin Invest 2015;125:1648-64. [Crossref] [PubMed]
- Kovaříková P, Michalova E, Knopfova L, et al. Methods for studying tumor cell migration and invasiveness. Klin Onkol 2014;27 Suppl 1:S22-27. [Crossref] [PubMed]
- Fu S, Chen X, Lo HW, et al. Combined bazedoxifene and paclitaxel treatments inhibit cell viability, cell migration, colony formation, and tumor growth and induce apoptosis in breast cancer. Cancer Lett 2019;448:11-9. [Crossref] [PubMed]
- Ganguly A, Yang H, Cabral F. Class III beta-tubulin counteracts the ability of Paclitaxel to inhibit cell migration. Oncotarget 2011;2:368-77. [Crossref] [PubMed]
- Schmitt CA. Cellular senescence and cancer treatment. Biochim Biophys Acta 2007;1775:5-20.
- Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest 2018;128:1238-46. [Crossref] [PubMed]
- Schmitt CA, Fridman JS, Yang M, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 2002;109:335-46. [Crossref] [PubMed]
- Zhu Y, Xu L, Zhang J, et al. Sunitinib induces cellular senescence via p53/Dec1 activation in renal cell carcinoma cells. Cancer Sci 2013;104:1052-61. [Crossref] [PubMed]
- Bojko A, Czarnecka-Herok J, Charzynska A, et al. Diversity of the Senescence Phenotype of Cancer Cells Treated with Chemotherapeutic Agents. Cells 2019. [Crossref] [PubMed]


