
The relationship between estrogen-induced phenotypic transformation and proliferation of vascular smooth muscle and hypertensive intracerebral hemorrhage
Introduction
The risk factors of hypertensive cerebral hemorrhage include hypertension, diabetes, smoking, and other factors, with hypertension being the main risk factor of hypertensive cerebral hemorrhage (1). At present, most of the research on hypertensive cerebral hemorrhage has been focused on the repair of nerve damage after cerebral hemorrhage, while the etiology of hypertensive cerebral hemorrhage, especially the pathological mechanism of cerebrovascular disease under hypertensive conditions, has garnered little attention. In clinical autopsy, the cerebral vascular smooth muscle layer of patients with hypertensive intracerebral hemorrhage is degenerated, with the smooth muscle cells being atrophied or having disappeared altogether. However, clinical and animal experiments show an increased apoptosis rate of vascular smooth muscle cells (VSMCs) in the hypertensive cerebral hemorrhage area. Apoptosis also exists in human vascular endothelium and smooth muscle under the induction of various stimulating factors. These apoptotic cells participate in the pathological changes of the disease and are the cytological basis for the occurrence and evolution of various vascular diseases. It is currently believed that apoptosis is an independent risk factor for vascular disease (2-4), and peripheral nerve cells at the site of cerebral hemorrhage have been associated with apoptosis (5-7).
Estrogen represents a class of hormones comprising estrone (E1), estriol (E3), 17-proxie-estradiol (E2) and 17-β-estradiol (8). It is generally accepted that estrogen induces the apoptosis of VSMCs. In one study, when the VSMCs were treated with 17-β-estradiol, the number of apoptotic VSMCs increased significantly, and the ratio of Bax/Bcl-2 increased in the G2-M apoptotic stage (9). In human aortic VSMCs, 17-β-estradiol also promotes a rapid and temporary increase in the apoptosis of VSMCs and the phosphorylation of p38 through activating mitogen-activated protein kinase (MAPK) (10). Overall, these studies suggest a protective effect of estrogen on the cardiovascular system. However, there is no clinical evidence to prove that these hormones can replace drugs with cardiovascular protective effects and eliminate potential hazards in the cardiovascular system.
G protein-coupled estrogen receptor 1 (GPER) is a newly discovered estrogen-related receptor, belonging to the G protein-coupled receptor family called G PR30. GPER is different from classical estrogen receptors (ESRs), and its cellular localization and specific mechanism remains controversial (11). As a factor in CVD, myocardin (MYOCD) has attracted intense research focus since it was discovered in 2001. Some studies have shown that cardiomyosin is expressed in some cells, such as smooth muscle precursor A404 cell line, which do not express known smooth muscle marker genes; thus, cardiomyosin can be used as a new marker for the identification of smooth muscle cells (12). The complex formed by cardiomyosin and serum response factor (SRF) is an important cofactor in cardiomyocytes and smooth muscle cells, as it can regulate their proliferation, differentiation, and migration (13). In this study, the estrogen and ESR blocker effect on the proliferation of VSMCs and the expression of estrogen-related receptor genes ESR (ESR1, ESR2, GPER), MYOCD, SRF, and apoptosis gene caspase-3 were explored, and the protective mechanism of estrogen on human cerebrovascular system was clarified. Our results may provide an important reference basis for the clinical treatment of hypertensive intracerebral hemorrhage.
Mature VSMCs have high cellular specificity, mainly for vasoconstriction, blood pressure regulation, and blood flow distribution. Damaged VSMCs can undergo phenotypic transformation due to their strong phenotypic plasticity. In normal adult arteries, VSMCs mainly exist in their contractile form. The pathological vascular phenotype is primarily the synthetic type and plays an important role in CVDs, including atherosclerosis (AS) and hypertension (14-16). The proliferation and migration of VSMCs are the common pathological features of the occurrence and development of vascular diseases such as hypertension and pulmonary hypertension, and the phenotypic transformation of VSMCs plays an important role in the proliferation and migration of VSMCs. Therefore, the study of VSMC phenotypic transformation is of great significance for the prevention and treatment of these diseases. α-smooth muscle actin (α-SMA) is a ubiquitous and abundant protein in VSMCs, which marks the completion of VSMC differentiation (17). It is predominantly expressed in contractile cells, but not in synthetic cells. Also, it is the main marker for the contractile VSMC type and the most widely used marker in the literature, followed by SM22α. Angiotensin II (Ang II) is an effector molecule of the renin-angiotensin system (RAS), and can induce VSMCs to change from the contractile type to the synthetic type. The abnormal increase of Ang II has also been implicated in the pathological process of hypertension, atherosclerosis, and vascular fibrosis (18). Zheng et al. found that Ang II could reduce the expression of SM-α-actin, SM-MHC, and SM22α in VSMCs and promote VSMC proliferation and hypertrophy. This leads to vascular wall hardening and lumen stenosis, suggesting that Ang II induces the phenotypic transformation of VSMCs (19). Mori-Abe et al. (20) found that physiological dose of 17 β-estradiol could induce the apoptosis of synthetic VSMCs. Therefore, it is speculated that estrogen may inhibit the phenotypic transformation of VSMCs induced by Ang II. To confirm this, we observed the effects of estradiol on human cerebral VSMCs treated with Ang II and evaluated the effect of estrogen on the phenotypic transformation and apoptosis of VSMCs by measuring the expression of vascular smooth muscle markers α-SMA, SM22α, FLN, MCP-1, and TLR4. In addition, in order to mimic the pathophysiological process of human cerebral hemorrhage in the experimental study, an animal model of hypertensive intracerebral hemorrhage was established to better study the relationship between estrogen and hypertensive intracerebral hemorrhage.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4567).
Methods
Culture and treatment of human cerebral VSMCs
Human cerebral VSMCs were purchased from the American Type Culture Collection (ATCC) and were then cultured in Dulbecco’s Modified Eagle Medium (DMEM)-high glucose medium (Hyclone; cat. no. SH30022.01B) containing 10% fetal bovine serum (FBS) (Hyclone; cat. no. SH30087.01) and 1% penicillin streptomycin (Hyclone; cat. no. SH30010) and incubated in a constant-temperature incubator at 37 °C with 5% CO2. Human brain smooth muscle cells were divided into seven groups: the first experimental group was estradiol (Sigma-Aldrich, Cat.No BP729) at concentrations of 10−9, 10−8, and 10−7 mM; the second experimental group was tamoxifen (Supelco, Cat.no. 06734) at concentrations of 10−8, 10−7, and 10−6 mM; the control group did not undergo any intervention; the Ang II group was stimulated by 10−7 mmol/L Ang II for 72 hours; the Ang II-low estradiol concentration group was treated with estradiol at a concentration of 10−9 mmol/L for 24 hours after 72 hours of Ang II treatment; the Ang II-medium estradiol concentration group was stimulated with Ang II for 72 hours, and then treated with 10−8 mmol/L estradiol for 24 hours; the Ang II-high estradiol concentration group was treated with Ang II for 72 hours, and then treated with 10−7 mmol/L estradiol for 24 hours.
Grouping and establishment of the animal model
In all, 24 eight-week-old SD rats, weighing 200–250 g, were randomly divided into six groups, the low estrogen group (n=3), the high estrogen group (n=6), the ESR agonist group (n=3), the ESR antagonist group (n=3), the normal estrogen group (n=6), and the sham operation group (n=6). The rat model of renal hypertension was established by unilateral coarctation of the renal artery in the low estrogen group, the high estrogen group, the ESR activation group, the ESR antagonist group, and the normal estrogen group. In the sham operation group, only the left renal artery was dissociated, with the abdominal cavity being sutured. Then treat the model group as follows: (I) low estrogen group: ovarian removal surgery on rats; (II) high estrogen group: Continuous feeding of estradiol (100 µg/kg/d) to rats; (III) ESR agonist group: After ovary removal surgery, rats are given hormone hormone agonist estradiol (100 µg/kg/d); (IV) ESR antagonist group: Normal Tamoxifen (3 mg/kg/d), an ESR antagonist in the estrogen group. This study was approved by the ethics committee of the First Affiliated Hospital of Nanchang University (No. 2014-72). All procedures are performed in compliance with the guidelines of the Institutional Animal Care and Use Committee.
The proliferation of human brain smooth muscle cells was detected by CCK8 method
Cells with a density of 1×105/mL were inoculated on a 96-well plate, and 100 µL cell suspension was added to each well. Blank wells were set up by adding 100 µL of culture medium with no cells, and then the cells were incubated in 5% CO2 at 37 °C for 24 hours. After that, the cells were further incubated in 5% CO2 incubator at 37 °C at concentrations of 10−9, 10−8, and 10−7 mM estradiol, and 10−8, 10−7, and 10−6 mM tamoxifen. Then, CCK-8 reagent (10 µL/well; Biyuntian; cat. no. C0037) was added to incubate for 4 hours, and Thermo Fisher Scientific Multiskan MK3 was used to determine the absorbance of optical density (OD)450 at the wavelength of 450 nm. The cell inhibition rate was expressed as a percentage and calculated by the following equation: inhibition rate = (1-average OD value of the experimental group/average OD value of the control group) × 100% (at the same time).
Flow cytometry (FCM) detection of apoptosis of human brain smooth muscle cells
For FCM, a 0.5 mL suspension of the above-mentioned treated cells (density = 5×105/mL) was transferred into a clean centrifuge tube, and 1.25 µL Annexin V-FITC (Keygen; cat. no. KGA106) was added. This tube was kept at room temperature (18–24 °C), with light avoidance reaction for 15 minutes and then centrifuged at 1,000 ×g for 5 minutes to remove the supernatant. The cells were resuspended lightly with 0.5 mL precooled 1×binding buffer, and then 10 µL propidium iodide was added. The tubes were stored on ice to avoid light. FCM (BD FACSCalibur) was immediately used for detection and analysis.
RNA extraction and real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted from the treated cells in each group (Trizol method), the OD260 and OD280 of each RNA sample were determined, and the concentration was calculated. The reverse transcription steps were carried out strictly according to the instructions of TaKaRa PrimeScript II 1st Strand cDNA Synthesis Kit (D6210A). The mRNA of ESR1, ESR2, GPER, MYOCD, SPF, caspase-3, α-SMA, SM22α, and FLN was detected by a real time polymerase chain reaction (RT-PCR) instrument (the sequence of each gene amplification product is shown in Table 1). Reaction system and reaction parameters were prepared according to the instructions of TaKaRa SYBR® Premix Ex Taq II (Perfect Real Time). The amplification curve and melting curve of (RT-PCR) were carried out following the operation method of ABI PRISM® 7500 Sequence Detection System Real-Time PCR System and the 2−ΔΔCt method was used for any calculations.

Full table
Western blot
After centrifugation, the precipitates of cells in each group were collected and lysed with 1× RIPA Buffer (CST # 9806S). After full lysis, the lysed cells were centrifuged at 10,000–14,000 g for 3 to 5 minutes and the supernatant was taken. The concentration of total protein in the supernatant was determined according to the method provided by bicinchonic acid (BCA) protein concentration determination reagent (Pierce # 23225). The same amount of total protein (30 µg) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membrane. After being sealed by 5% skimmed milk powder, the membrane reacted with anti-ESR1, ESR2, GPER, caspase-3, MYOCD, SRF, α-SMA, SM22α, FLN, MCP-1, and TLR4 antibodies overnight at 4 degrees, was washed, and then reacted with the second antibody, horseradish peroxidase (HRP) goat anti-mouse IgG (Bioworld), at room temperature for 1 hour. Then, 350 µL of enhanced chemiluminescence (ECL) luminous substrate was added, and images were developed in the darkroom. β-actin antibody and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-HRP (Shanghai Kangcheng, KC5G5) antibody were used as the internal controls of the experiment.
Hematoxylin-eosin staining
Take 2 and 6 weeks of low estrogen group, high estrogen group and normal estrogen group, one in each group, fixed in 4% paraformaldehyde for 24h, and then gradient alcohol dehydration. After the tissues were embedded and sliced, dewaxing and rehydrating were carried out, and then the sections were treated with hematoxylin for 3–8 minutes and stained with eosin for 1–3 minutes. After that, the sections were gradient dehydrated sequentially and sealed for fluorescence imaging to observe the pathological changes of arteries and blood vessels in the bleeding area.
Immunofluorescence staining to detect the expression of cerebrovascular smooth muscle- and cerebrovascular-related proteins before and after modeling
Human cerebral VSMCs from the control group, Ang II group, Ang II estradiol-low-concentration group, Ang II estradiol-medium-concentration group, Ang II estradiol-high-concentration group were grown on a glass slide and were treated with 4% neutral paraformaldehyde for 15 minutes for fixing, and then 0.25% Triton XMel 100 was applied for 5 minutes. The paraffin sections of the normal estrogen group, ESR agonist group, and ESR antagonist group were grilled, dewaxed, antigen-repaired, and had endogenous enzymes removed. The slides of above-mentioned groups were sealed with 10% normal sheep serum for 30 minutes, and then, anti-α-SMA, SM22α, FLN, ERs, α-SMA, Desmin antibodies were added and incubated overnight at 4 °C. Phosphate-buffered saline (PBS) was used to wash the slides 3 times, and then the slides reacted with HRP-labeled secondary antibody for 1 hour. The slides were then washed with PBS three times and mixed with anti-glyceraldehyde-3-phosphate dehydrogenase (DAPI). After incubating for 5 minutes in the dark to restain cell nuclei, the sealed slides were photographed by fluorescence microscope (Leica, DMI6000B) and plotted with software.
Statistical analysis
The experimental results were expressed by mean ± standard deviation (), and SPSS 20.0 statistical software was used for statistical analysis to compare the mean between groups using analysis of variance, with P<0.05 as the test level of statisitical significance.
Results
Change of blood pressure detected before and after modeling
The comparison of blood pressure in each group before and after modeling is shown in Table 2. Rat systolic blood pressure in the 11.3–17.95 kPa range was considered normal blood pressure. Blood pressure after modeling surgery was higher than that before operation by more than 2.66 kPa which was greater than three times the standard deviation of normal blood pressure. Blood pressure after surgery higher than 15.30 kPa indicated a surgery success, whereas no significant increase in blood pressure or death after operation indicated failure. Table 3 shows that the hypertension model operation in each experimental group was successful.
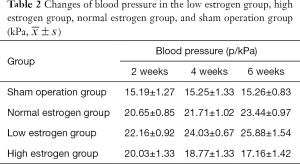
Full table
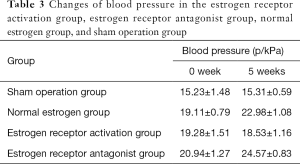
Full table
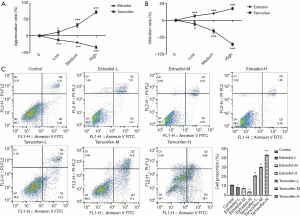
Effects of estrogen on proliferation and apoptosis of human cerebral VSMCs
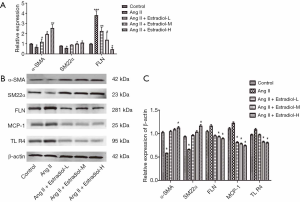
CCK-8 method and flow cytometry were used to detect the cell proliferation and apoptosis of human cerebral vascular smooth muscle in each group. It can be seen from Figure 1A,B that as the concentration of estradiol increases, the rate of proliferation of human cerebral vascular smooth muscle cells gradually decreases, and the rate of inhibition gradually increases, while the effect of tamoxifen is just in phase (P<0.05). From the results in Figure 1C, we can see that compared with the control group, the apoptosis rate of human cerebral vascular smooth muscle cells gradually decreased with the increase of estradiol concentration, and the effect of tamoxifen was just the opposite (P<0.05).
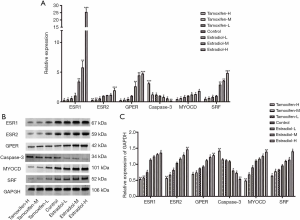
Effects of estrogen on the expression of ESR1, ESR2, GPER, caspase-3, MYOCD, SRF mRNA, and protein in human cerebral VSMCs
Compared with the control group, the expression of estrogen-related receptor genes ESR1, ESR2, GPER, MYOCD, and SRF mRNA in estradiol groups increased significantly (P<0.05), while the expression of caspase-3 mRNA decreased significantly (P<0.05) in a concentration-dependent manner. In the tamoxifen group, the expression of estrogen-related receptor genes ESR1, ESR2, GPER, MYOCD, and SRF mRNA decreased significantly (P<0.05), while the expression of caspase-3 mRNA increased significantly (P<0.05) in a concentration-dependent manner (Figure 2A). Meanwhile, compared with the control group, estrogen-related receptor genes, ESR1, ESR2, GPER, MYOCD, and SRF protein expression increased, while Caspase-3 protein expression decreased in estradiol groups in a concentration-dependent manner (Figure 2B). In the tamoxifen groups, the expression of estrogen-related receptor genes, ESR1, ESR2, GPER, MYOCD, and SRF increased, while the expression of Caspase-3 decreased in a concentration-dependent manner.
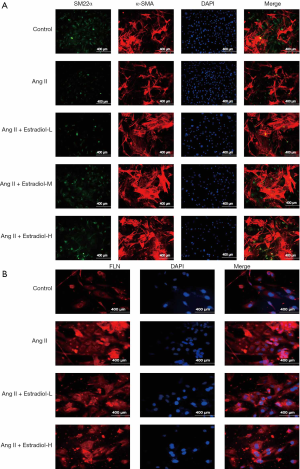
Immunofluorescence staining confirms that estrogen affects the expression of α-SMA, SM22α, and FLN markers in Ang II induced VSMC phenotype transformation.
The immunofluorescence staining results (Figure 3A,B) indicate that the expression of α-SMA and SM22α decreased while FLN expression increased in the Ang II groups; meanwhile, in the low-, medium-, and high-estradiol-concentration groups, the expression of α-SMA and SM22α increased while FLN expression decreased.
Estradiol effect on mRNA and protein expression of α-SMA, SM22α and FLN, along with the protein expression of MCP-1 and TLR4
As shown in Figure 4A,B, compared with the control group, the mRNA and protein expression of α-SMA and SM22α decreased, while the mRNA and protein expression of FLN increased; furthermore, the protein expression of phenotypic markers in the human cerebral VSMCs, MCP-1 and TLR4, increased in the Ang II group. In the low-, medium- and high-estradiol-concentration groups, the mRNA and protein expression of α-SMA and SM22α increased, the mRNA and protein expression of FLN decreased, and the protein expression of MCP-1 and TLR4 decreased, compared with the Ang II group.
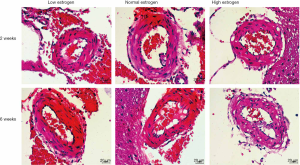
Hematoxylin-eosin staining to observe the effect of estrogen on cerebrovascular hemorrhage in hypertensive model group
As shown in Figure 5, compared with the normal estradiol group, the bleeding in the low estradiol group was obvious, the number of bleeding spots was higher, the amount of bleeding was greater, and the blood vessels at the bleeding site were ruptured. Moreover, with the increase of experimental time, the number of bleeding points increased and the amount of bleeding increased. Meanwhile, in the high estradiol group, there was less bleeding and fewer bleeding spots, and with the increase of experimental time, the bleeding was alleviated, the number of bleeding points decreased, and the amount of bleeding decreased, which indicates that estradiol can improve the cerebral hemorrhage caused by hypertension.
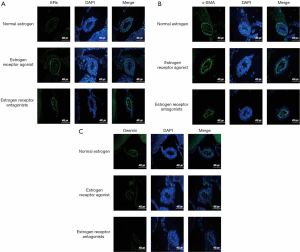
Immunofluorescence detection of the expression of ER, α-SMA, and Desmin in cerebral vessels of different treatment groups before and after modeling
Compared with the normal estrogen group, the expression of ERα, Erβ, α-SMA, and Desmin in the ESR antagonist group decreased, while the expression of Erα, Erβ, α-SMA, and Desmin increased in the ESR agonist group (Figure 6).
Discussion
Hypertensive intracerebral hemorrhage is one of the most serious complications of hypertension. At present, most of the research on hypertensive intracerebral hemorrhage focuses on the repair of nerve damage after intracerebral hemorrhage, but there are few studies that have examined the causes of hypertensive intracerebral hemorrhage, especially the pathological mechanism of cerebrovascular disease under hypertensive conditions. The pathological progression of hypertension often involves the process of cardiovascular remodeling. Research has shown that vascular remodeling is associated with the imbalance between VSMC proliferation and apoptosis (21). The imbalance between vascular VSMC proliferation and apoptosis—that is, the increase of cell proliferation and/or the decrease of apoptosis—contributes to the occurrence and development of vascular remodeling (22).
It can be speculated that regulating the balance between apoptosis and proliferation may be one of the important ways to prevent cardiovascular remodeling. It is widely accepted that the proliferation and migration of VSMCs are the result of a series of complex gene expression regulations (23). The characteristic marker genes of VSMCs, such as α-SMA, SM22α, and SRF, are only temporarily expressed during the development of VSMCs and are not expressed in other cells; thus, they can be used to identify VSMCs. These specific VSMC marker genes are regulated by a common upstream signal molecule, MYOCD, which can upregulate the expression of α-SMA, SM22α, and SRF, and promote the proliferation of smooth muscle cells (24). Some studies focusing on the relationship between MYOCD and CVD indicate that the upregulated expression of the MYOCD gene may increase the risk of CVDs such as atherosclerosis and myocardial hypertrophy (25,26). Therefore, suppressing the overexpression of MYOCD could inhibit the proliferation of smooth muscle cells, which is likely to become a new target for drugs that treat CVDs.
The effect of estrogen on CVD has been identified through in vitro and in vivo models and observational studies. To put simply, estrogen has a certain protective effect on the cardiovascular system, but the specific effect and mechanism are not clear. In the present study, the results of CCK-8 cytotoxicity test showed that estrogen could suppress the proliferation of VSMCs, which may be related to the expression of MYOCD. Compared with the control group, the expression of MYOCD mRNA and protein in the estradiol group gradually decreased with the increase of estradiol concentration, and SRF showed the same expression trend. On the other hand, tamoxifen, an ESR blocker, exhibited the opposite effects. Therefore, estradiol can inhibit the proliferation of VSMCs by suppressing the overexpression of MYOCD and SRF, which is one of the possible mechanisms through which estrogen protects blood vessels.
Previous studies have also shown that estrogen inhibits the proliferation and inward migration of VSMCs and delays the formation and development of arterial plaque, which is mediated by the classical intranuclear ESR ERα/ERβ and the activation of MAPK, PI3K/Akt, Erk1/Erk2, and other pathways. In this study, the experimental results indicated that estradiol could upregulate the expression of estrogen-related receptor genes, ERS1 and ERS2, in human brain VSMCs in a concentration-dependent manner which increased with the increase of drug concentration. From the model of hypertensive intracerebral hemorrhage, it was observed that estrogen can upregulate the expression of ERα/ERβ, in a concentration-dependent manner, increasing with the increase of drug concentration, and that ESR agonists can also upregulate ERα/ERβ, while ESR antagonists showed the opposite effect. Via the mediation of ERα/ERβ, estrogen can inhibit the proliferation of rat VSMCs and upregulate the expression of ESR-related genes, ERS1 and ERS2, in a concentration-dependent manner. In addition, GPER can specifically bind to estrogens, estrogen-like hormones, and estrogen antagonists, resulting in biological effects similar to those of ESR; GPER can also mediate the physiological effects of estrogen on tissues. It is also confirmed that in addition to the distribution of ERα/ERβ on human brain VSMCs, GPER is also present, and that estradiol can increase the expression of GPER in human brain VSMC, which is positively correlated with the drug concentration. Therefore, estrogen can inhibit the proliferation of rat VSMC, which is not only mediated by ERα/ERβ, but also related to GPER in a concentration-dependent manner. It is another possible mechanism through which estrogen can protect blood vessels.
The results of the experiment on the expression of VSMC apoptosis gene caspase-3 in the human brain showed that estrogen could inhibit the apoptosis of VSMCs by downregulating the expression of VSMC apoptosis gene caspase-3, possibly by inhibiting caspase-3 pathway to inhibit its apoptosis in turn. The imbalance between VSCM proliferation and apoptosis—that is, the increase of cell proliferation and/or the decrease of apoptosis—contributes to the occurrence and development of vascular remodeling. When local environmental factors change, such as when vascular injury occurs, VSMCs undergo a reversible phenotypic transformation, intracellular synthesis becomes active, and the pattern of gene expression changes accordingly (27). Significant changes are found in the expression of α-SMA, SM22α, FLN, MCP-1, and TLR4 genes switching from the “off” state to the “on” state, which regulates the proliferation and migration of VSMCs. Previous studies have shown that Ang is one of the growth and proliferation factors of VSMCs. After 72 hours of VSMC proliferation stimulated by Ang II (10−7 mM), the mRNA and protein expression of VSMC contractile phenotypic marker genesm α-SMA and SM22α, decreased significantly, while the mRNA and protein expression of FLN, a synthetic phenotypic marker gene, increased significantly, indicating that VSMC transformed from the contractile phenotype to synthetic phenotype. After estrogen treatment, the decrease of α-SMA and SM22α expression and the increase of FLN expression induced by Ang II were inhibited to varying degrees, as shown by increased α-SMA and SM22α expression and decreased FLN expression; that is, estrogen treatment could significantly inhibit the phenotypic transformation of VSMC in a concentration-dependent manner. Also, in the model of hypertensive intracerebral hemorrhage, estrogen and ESR agonists increased the expression of α-SMA and Desmin, while ESR antagonists decreased the expression of α-SMA and Desmin. In addition, it was observed that estrogen could improve the cerebral hemorrhage caused by hypertension in the model of hypertensive intracerebral hemorrhage with hematoxylin and eosin stain (HE staining).
Finally, in cardiovascular and cerebrovascular diseases, synthetic VSMCs induce the expression of various matrix metalloproteinases and inflammatory mediators, such as monocyte chemoattractant protein-1 (MCP)-1. In hypertension, the increased activation of DAMP-TLR may affect vascular reactivity and cause vasculitis, which is also important in the pathophysiology of hypertension (28,29). The experiment also proved that estrogen reduces the expression of MCP-1 and TLR in human brain VSMCs, and regulates perivascular inflammation.
Conclusions
These experimental results indicate that estrogen inhibits the proliferation of human cerebral VSMCs to inhibit their apoptosis, and inhibits the transformation of angiotensin II-induced VSMC from contractile to synthetic. It was also found that estrogen prevents vascular injury and regulates perivascular inflammatory reaction; thus, estrogen has a protective effect on the cardiovascular and cerebrovascular, and provides a new pathway for the treatment of hypertensive intracerebral hemorrhage. Ultimately, the regulation of estrogen to control the phenotypic transformation of VSMCs under different pathological conditions may be a new pathway for the treatment of vascular proliferative diseases such as hypertensive intracerebral hemorrhage. However, the specific molecular mechanism of estrogen involved in regulating the phenotypic transformation of VSMC is unknown. Furthermore, it is unclear whether cell-to-cell interactions are involved in regulating the phenotypic transformation of VSMC, and further studies are needed to elucidate these issues.
Acknowledgments
Acknowledgments: We thank Guangzhou Yujia Biotechnology Co., Ltd., in whose lab some of these experiments were performed.
Funding: 2014 medical research plan project of Chongqing Health and Family Planning Commission (No. 20142059).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4567
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4567
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4567). The authors have no conflicts of interest to declare.
(English Language Editor: J. Gray)
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of the First Affiliated Hospital of Nanchang University (No. 2014-72). All procedures are performed in compliance with the guidelines of the Institutional Animal Care and Use Committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ariesen MJ, Claus SP, Rinkel GJ, et al. Risk factors forintracerebral hemorrhage in the general population: a systematic review. Stroke 2003;34:2060-5. [Crossref] [PubMed]
- Lee HY, You HJ, Won JY, et al. Forkheed factor, Foxo3a, induces apoptosis of endothelial cells through activation of matrix metalloproteinases. Arterioscler Thromb Vasc Biol 2008;28:302. [Crossref] [PubMed]
- Zeini M. L Pez Fontal R, Través PG. Differential sensitivity to apoptosis among the cells that contribute to the atherosclerotic disease. Biochem Biophys Res Commun 2007;363:444. [Crossref] [PubMed]
- Trostdorf F, Landgraf C, Kablau M, et al. Increased endothelial cell apoptosis in symptomatic high-grade carotid artery stenosis: preliminary data. Eur J Vasc Endovasc Surg 2007;33:65. [Crossref] [PubMed]
- Shen J, Liu Y, Song Y, et al. CHMP4B, ESCRT-III associating protein, associated with neuronal apoptosis following intracerebral hemorrhage. Brain research 2015;1597:1-13. [Crossref] [PubMed]
- Sun H, Tang Y, Li L, et al. Effects of local hypothermia onneuronal cell apoptosis after intracerebral hemorrhage in rats. The journal of nutrition, health & aging 2015;19:291-8. [Crossref] [PubMed]
- Zhang XQ, Zhang ZM, Yin XL, et al. Exploring the optimal operation time for patients with hypertensive intracerebral hemorrhage: tracking the expression and progress of cell apoptosis of prehematomal brain tissues. Chinese medical journal. 2010;123:1246-50. [PubMed]
- Hall JE. Guyton and Hall Textbook of Medical Physiology, Elsevier, Inc., Philadelphia, PA, 2016.
- Yang Z, Cheng B, Song J, et al. Estrogen accelerates G1 to S phase transition and induces a G2/M phase-predominant apoptosis in synthetic vascular smooth muscle cells. Int J Cardiol 2007;118:381-8. [Crossref] [PubMed]
- Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 2007;1773:1358-75.
- Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 2011;7:715-26. [Crossref] [PubMed]
- Pérot G, Derré J, Coindre JM, et al. Strong smooth muscle differentiation is dependent on myocardin gene amplification in most human retroperitoneal leiomyosarcomas. Cancer Res 2009;69:2269-78. [Crossref] [PubMed]
- Parmacek MS. Myocardin: dominant driver of the smooth muscle cell contractile phenotype. Arterioscler Thromb Vasc Biol 2008;28:1416-7. [Crossref] [PubMed]
- Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 2012;74:13-40. [Crossref] [PubMed]
- Ewart MA, Kennedy S, Macmillan D, et al. Altered vascular smooth muscle function in the ApoE knockout mouse during the progression of atherosclerosis. Atherosclerosis 2014;234:154-61. [Crossref] [PubMed]
- Ng FL, Boedtkjer E, Witkowska K, et al. Increased NBCn1 expression, Na+/HCO3- co-transport and intracellular pH in human vascular smooth muscle cells with a risk allele for hypertension. Hum Mol Genet 2017;26:989-1002. [PubMed]
- Subramanian S V, Kelm RJ, Polikandriotis J A, et al. Reprogram-ming of vascular smooth muscleA-actin gene expression as an early indicator of dysfunctional remodeling following heart transplant. Cardiovasc Res 2002;54:539-48. [Crossref] [PubMed]
- Yi B, Cui J, Ning J N, et al. Over-expression of PKGIalpha inhibits hypoxia-induced proliferation, Akt activation, and phenotype modulation of human PASMCs: the role of phenotype modulation of PASMCs in pulmonary vascular remodeling. Gene 2012;492:354-60. [Crossref] [PubMed]
- Gosse P. A review of telmisartan in the treatment of hypertension: blood pressure control in the early morning hours. Vasc Health Risk Manag 2006;2:195-201. [Crossref] [PubMed]
- Mori-Abe A, Tsutsumi S, Takahashi K, et al. Estrogen and raloxifene induce apoptosis by activating p38 mitogen-activated protein kinase cascade in synthetic vascular smooth muscle cells. J Endocrinol 2003;178:417-26. [Crossref] [PubMed]
- Imanishi T, Hano T, Nishio I, Han DK, Schwartz SM, Karsan A. Apoptosis of vascular smooth muscle cells is induced by Fas ligand derived from endothelial cells. Jpn Circ J 2001;65:556-60. [Crossref] [PubMed]
- Jung F, Haendeler J, Goebel C, et al. Growth factor- indueed phosphoinositide 3-OH kinase/Akt phosphorylation in smooth muscle cells: induction of cell proliferation and inhibition of cell death. Cardiovasc Res 2000;48:148-57. [Crossref] [PubMed]
- Sinha S, Wamhoff BR, Hoofnagle MH, et al. Assessment of contractility of purified smooth muscle cells derived fromembryonic stem cells. Stem Cells 2006;24:1678-88. [Crossref] [PubMed]
- Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res 2005;96:280-91. [Crossref] [PubMed]
- Xing W, Zhang TC, Cao D, et al. Myocardin induces cardiomyocyte hypertrophy. Circ Res 2006;98:1089-97. [Crossref] [PubMed]
- Liao XH, Wang N, Liu QX, et al. Myocardin-related transcription factor-A induces cardiomyocyte hypertrophy. IUBMB Life 2011;63:54-61. [Crossref] [PubMed]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004;84:767-801. [Crossref] [PubMed]
- Bomfim GF, Dos Santos RA, Oliveira MA, et al. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci 2012;122:535-43. [Crossref] [PubMed]
- Guzik TJ, Skiba DS, Touyz RM, et al. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res 2017;113:1009-23. [Crossref] [PubMed]
(English Language Editor: J. Gray)


