
Polydatin relieves paraquat-induced human MRC-5 fibroblast injury through inhibiting the activation of the NLRP3 inflammasome
Introduction
Paraquat (1,1'-dimethyl 4,4'-bipyridine dichloride; PQ) is a quick-acting and non-selective herbicide that is widely used in agricultural production (1). It is highly toxic to humans and livestock, and acute poisoning caused by accidental or self-exposure to PQ has become a frequent cause of pesticide poisoning death (2). Despite many therapeutic methods being able to achieve some clinical effects, in the absence of a specific antidote, PQ poisoning has a mortality rate exceeding 90%, and the vast majority of surviving patients develop pulmonary interstitial fibrosis and have a poor prognosis (3). Previous studies have found that after entering the lung through energy-dependent polyamine intake, PQ accumulates and carries out an oxidation-reduction reaction, thus inducing the generation of a large number of reactive oxygen species (ROS) (4). Then, through lipid peroxidation, the relative balance between internal oxidation and antioxidant reaction is destroyed (5). Meanwhile, lung damage causes various inflammatory cells to accumulate and induces the secretion of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and other inflammatory factors. This sees an uncontrolled inflammatory response in the lung, leading to the aggravation of lipid peroxidation, inflammatory cell infiltration, and alveolar epithelial cell apoptosis. The subsequent pulmonary hemorrhage and destruction of alveolar epithelial cells, eventually cause acute lung injury (6-8). Many therapeutic strategies have been devised for the pathogenesis of PQ poisoning; however, their clinical efficacy has been unsatisfactory.
Polydatin, which is also known as resveratrol glycoside (resveratrol-3-O-beta-mono-D-glucoside; PD), is an active ingredient derived from the root of Polygonum cuspidatum (9,10). Previous reports have shown that resveratrol can play a vital role in preventing and treating hepatic fibrosis (11). Other studies confirmed that it demonstrated powerful antioxidant properties in different hepatitis models, thus reducing liver fibrosis (12,13). Compared with resveratrol, PD has higher water solubility and resistance to enzyme oxidation (14). Also, unlike resveratrol, which passively penetrates cells, PD enters cells through active mechanisms using glucose carriers (15). These characteristics mean that PD is more bioavailable than resveratrol. PD has been shown to play a biological role in oxidation and inflammation through the inhibition of ROS production and inflammatory injury (16,17). Recent reports have shown that PD has a protective effect on CCl4-induced liver injury in mice (18). The mechanism underlying PQ poisoning bears much similarity to the pathological characteristics exhibited during disease development in a hepatitis model (19). Therefore, we speculate that PD also has a protective effect on the disease. However, the effect of PQ in protecting human embryonic lung fibroblasts from damage has not been elucidated. In the current study, we aimed to examine the effect of PD on human embryonic lung fibroblasts induced by PQ poisoning to provide new ideas for the clinical treatment of patients with PQ poisoning.
We present the following article in accordance with the ARRVIE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4570).
Methods
Experimental drugs and main reagents
MiR-155 and 20% PQ, PD, glutathione peroxidase (GSH-Px), malondialdehyde (MDA), and superoxide dismutase (SOD) were all purchased by Beyotime company (Guangzhou, China). Tumor necrosis factor alpha (TNF-α), transforming growth factor beta (TGF-β), interleukin 1 beta (IL-1β), and interleukin 6 (IL-6) were provided by Wuhan EIAab Science Co., Ltd. (Wuhan, China).
Cell lines
Human embryonic lung fibroblasts [Medical Research Council cell strain 5 (MRC-5)] were purchased from the cell bank of Shanghai Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 25 µg/mL penicillin, and 25 µg/mL streptomycin (Invitrogen, Shanghai, China). All cells were incubated in a cell culture incubator with 5% CO2 at a constant temperature of 37 °C.
Determination of cell activity
MRC-5 cells were inoculated into 96-well culture plates at a density of 5×103 cells/well and cultured at 37 °C for 24 h. Select PQ and PD at concentrations of 12.5, 25, 50, 100, 200, and 400 µmol/L for 24 h. PQ and PD were not added as negative controls. Next, 50 µL of MTT (5 g/L) solution was added to each 96-well plate, and the plates were incubated at 37 °C for 4 h. After incubation, 150 µL of dimethyl sulfoxide (DMSO) solution was added to each well. Subsequently, an enzyme reader (Bio-Rad Laboratory) was used to detect the values at 490 nm. Cell activity (%) = [(A treatment group-A blank group)/(A control group-A blank group)] × 100 (%).
Detection of apoptosis by flow cytometry
Cell suspension was transferred from the cell culture plate (5×105 cells) to a clean centrifuge tube. Then, 1.25 µL Annexin V-FITC and propidium iodide (PI, Sigma, Beijing, China) was added. The cells were kept in the dark for 15 min, and then centrifuged at 1,000 ×g for 5 min to remove the supernatant. The cells were gently resuspended with 0.5 mL of precooled 1× binding buffer, and 10 µL propidiumiode was added. The sample was placed on ice and kept away from light. The stained cells were analyzed by flow cytometry (FAC Scan). The results were analyzed with Cell Quest software.
GSH-Px, MDA and SOD content detection
The levels of GSH-Px, SOD and MDA were determined according to the GSH-PX kit, SOD detection kit and MDA detection kit. (Beyotime, Shanghai, China).
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of TNF-α, TGF-β, IL-1β, and IL-6 were measured using concentration detection kits according to the manufacturer’s instructions (R&D Systems products). For the ELISA, the absorbance value was read at a wavelength of 540 nm, and the TNF-α, TGF-β, IL-1β, and IL-6 concentrations were calculated according to the standard curve.
Western blot
Total protein (Sigma, Beijing, China) was extracted from the cell line using RIPA lysate according to the manufacturer's instructions. To minimize protein degradation, each step was carried out on ice. The total protein was heated to 100 °C and incubated for 5 min. Then, SDS-polyacrylamide gel electrophoresis (120 V, 100 min) was used. The isolated protein was then transferred to a polyvinylidene fluoride (PVDF) membrane (300 mA, 80 min). After the transfer, the target band was sealed with 5% tris-buffered saline (TBS), and the membrane was incubated with anti-rabbit polyclonal antibody NLRP3 (ab214185, Abcam), rabbit monoclonal antibody caspase-1 (ab207802, Abcam), rabbit monoclonal antibody ASC (ab155970, Abcam), and rabbit polyclonal antibody GAPDH (ab9485, Abcam) at 4 °C overnight with a dilution of 1:1,000. After 48 hours of incubation, the polyclonal Goat anti rabbit IgG (Catalog No. ab6721) secondary antibody (1:10,000) coupled with horseradish peroxidase (HRP) was taken out, washed 3 times, and then placed at room temperature for 1 hour, then washed several times. The film had an enhanced chemiluminescence system and was imaged with X-ray film. The image was then quantized with Image J software (National Institutes of Health).
Statistical analysis
All data were expressed as mean ± standard deviation (SD) and analyzed with SPSS Statistics 20.0 (IBM, USA). A t-test was used to compare between the two groups; multiple comparisons between groups used one-way analysis of variance (ANOVA). A P value of <0.05 was considered to be statistically significant.
Results
PD enhanced cell activity after PQ-induced MRC-5 injury
The activity of MRC-5 cells was measured by MTT assay after treatment with PQ at different doses (12.5, 25, 50, 100, 200, and 400 µmol/L) for 24 h. The results of the MTT assay showed that compared with the 0 µmol/L group, the activity and survival rate of MRC-5 cells in the 12.5, 25, 50, 100, 200, and 400 µmol/L groups were both decreased significantly (P<0.05) in a dose-dependent manner. The higher the PQ concentration, the lower the activity and survival rate of the cells. Among the groups, the cell activity of the cells treated with 100 µmol/L PQ decreased by 47%, which was consistent with the model concentration of induced cytotoxicity; therefore, the subsequent experiments were performed using this dose (Figure 1A).
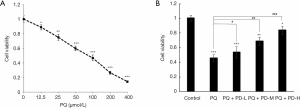
The cells were then randomly divided into five groups: (I) the control group, (II) the PQ group (100 µmol/L), (III) the PQ + PD-L group (100 µmol/L PQ and 60 µmol/L PD), (IV) the PQ + PD-M group (100 µmol/L PQ and 80 µmol/L PD), and (V) the PQ+PD-H group (100 µmol/L PQ and 100 µmol/L PD) and concurrently treated for 24 h. The activity of the MRC-5 cells was detected by MTT. The results showed that compared with the control group, the other groups had significantly lower cell activity and survival rates (P<0.05). Compared with the PQ group, the PQ + PD-L, PQ + PD-M, and PQ + PD-H groups had significantly increased cell activity and survival rates, which was dose dependent. The higher the concentration of PD, the higher the viability and survival rate of the cells (P<0.05) (Figure 1B).
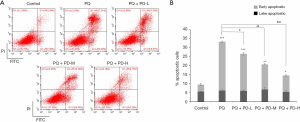
PD slowed down the PQ-induced apoptosis of MRC-5 cells
Flow cytometry was used to detect the apoptosis rate of MRC-5 cells after injury. The results showed that compared with the control group, the apoptosis rates of the PQ group, PQ + PD-L group, PQ + PD-M, group and PQ + PD-H group were significantly increased (P<0.05). Compared with the PQ group, the apoptosis rates of the PQ + PD-L group, PQ + PD-M group, and PQ + PD-H group were decreased significantly, which was dose dependent. The higher the PD concentration, the lower the apoptosis rate (P<0.05) (Figure 2A,B).
PD improved the level of antioxidant stress after PQ-induced MRC-5 injury
GSH-Px, MDA, and SOD kits were used to detect the corresponding contents in MRC-5 cells after PQ-induced injury. The results showed that compared with the control group, the GSH-Px and SOD levels in the cell supernatant of the PQ group, PQ + PD-L group, PQ + PD-M group, and PQ + PD-H group were significantly decreased, whereas the MDA level was significantly increased (P<0.05). Compared with the PQ group, the GSH-Px and SOD levels in the cell supernatant of PQ + PD-L group, PQ + PD-M group, and PQ + PD-H group were significantly increased, while the MDA level was significantly reduced (P<0.05) (Figure 3A,B,C).

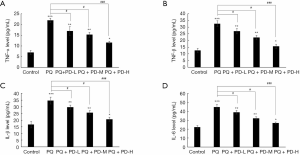
PD reduced the expression of inflammatory factors after PQ-induced MRC-5 injury
ELISA was performed to detect the expression of inflammatory factors in the MRC-5 cells. The results showed that the levels of TNF-α, TGF-β, IL-1β, and IL-6 in the PQ group, PQ + PD-L group, PQ + PD-M group, and PQ + PD-H group were significantly higher than those in the control group (P<0.05). Meanwhile, the levels of TNF-α, TGF-β, IL-1β, and IL-6 in the PQ + PD-L group, PQ + PD-M group, and PQ + PD-H group were significantly lower than those in the PQ group in a dose-dependent manner (P<0.05) (Figure 4A,B,C,D).
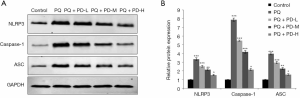
PD inhibited the activation of NLRP3 inflammasomes after PQ-induced MRC-5 injury
Western blot was used to detect the expression of activation-related proteins in the NLRP3 inflammasome after MRC-5 cell injury. The results showed that the protein levels of NLRP3, caspase-1, and apoptosis-related speck-like protein containing a CARD (ASC) in the PQ group, PQ + PD-L group, PQ + PD-M group, and PQ + PD-H group were significantly higher than those in the control group (P<0.05). Meanwhile, the protein levels of NLRP3, caspase-1, and ASC in the PQ + PD-L group, PQ + PD-M group, and PQ + PD-H group were significantly lower than those in the PQ group in a dose-dependent manner (P<0.05) (Figure 5A,B).
Discussion
Previous studies have shown that PQ enters the esophagus, before being actively transported into the lung by alveolar type II cells, making the lung the main organ affected by PQ poisoning (20,21). PQ poisoning causes ALI and acute respiratory distress syndrome, and ultimately leads to respiratory failure, which is the major cause of PQ-associated death (22). After PQ has invaded the body, it destroys the balance of oxidation and antioxidation. Firstly, it produces a large number of superoxide radicals (H2O2, O2−, -OH) (23), induces the accumulation of lipid peroxidation (24), generates peroxidation products, MDA and other substances and destroys the cell membrane, endoplasmic reticulum, mitochondria, and other parts of the cell. Secondly, it reduces the activity of reductases, such as SOD (25,26). The inflammatory response in the lung is exacerbated by the accumulation of positive cells such as neutrophils and the activation of the NLRP3 inflammasome, and finally leads to apoptosis of lung cells, fibrosis, a sharp reduction in energy synthesis, and the inability of surfactant production, which results in further deterioration of the PQ-induced lung injury (27,28).
The normal level of oxidative stress mainly refers to the state of relative balance of oxides and antioxidants in the body (29). A large number of studies on PQ poisoning have confirmed that GSH PX is an important peroxidase that widely exists in the body, the activity of which can reflect the body’s antioxidant capacity (30). MDA is one of the lipid peroxides produced by the peroxidation of polyunsaturated fatty acids and is a reliable index for reflecting the metabolism of ROS in vivo and the degree of free radical attack on tissues (31). Its level is greatly elevated after PQ exposure, which can indirectly reflect the degree of PQ-induced lung injury (32). SOD is a marker of the body's secondary antioxidant system, which has the functions of scavenging free radicals and anti-lipid peroxidation. The reduction of its activity reflects the decrease in the antioxidant defense ability of the body (33). Our study shows that the MDA level in the supernatant of PQ-induced MRC-5 cells was significantly higher than that in the supernatant of the normal control group, while the levels of GSH PX and SOD were significantly lower. The results showed that PQ caused an obvious oxidative stress response in cells in lung tissue, produced superoxide free radicals, and led to lipid peroxidation, which caused MDA to attack the biofilm and destroyed the antioxidant defense ability; these findings are consistent with the existing reports (34). At present, the pharmacological study of PD has been confirmed, and its pharmacological antioxidant effect has been confirmed. In this study, compared with the PD induced group, the MDA level in the cell supernatant of PD intervention group was significantly decreased, while the levels of GSH PX and SOD were significantly increased, suggesting that PD has a certain regulatory effect on the oxidative stress response induced by PQ poisoning in human lung fibroblasts and reduces the production of ROS and other superoxide free radicals.
In the mechanism of acute lung injury, the inflammatory response mediated by lung tissue injury has attracted an increasing amount of attention. There is large bank of evidence that shows that PQ can mediate the production of excessive interleukin and TNF-α by inflammatory cells, which is closely related to the level of inflammation in lung tissue (35). TNF-α has been confirmed to be the promoter of the cytokine regulatory network in vivo. An increase in TNF-α triggers inflammatory response further, which is called an early inflammatory response cytokine (36). IL-6 has a wide range of biological functions. In the inflammatory stage, it mediates the inflammatory response through regulating the activity of helper lymphocytes, chemotactic granulocytes, macrophages and lymphocyte accumulation, which leads to the increase of IL-6 level (37). In the process of PQ-induced acute lung injury, the NLRP3 inflammasome is activated, which results in an increase in the level of inflammatory apoptosis genes, thus aggravating the inflammatory response and generating a vicious cycle (38,39). In this study, the levels of TNF-α, TGF-β, IL-1β, and IL-6 in MRC-5 cell supernatants in the PQ induction group were higher than those in the control group, and the levels of NLRP3, caspase-1, and ASC activated by NLRP3 inflammatory corpuscles were significantly increased. This was the direct cause of the increase of the lung wet-to-dry ratio, which results in cell fibrosis, increased lung permeability, and pulmonary edema and hyaline membrane formation. Eventually, this leads to uncontrolled inflammatory response in the lung and is closely related to the occurrence of ALI (40). After PD intervention, TNF-α, TGF-β, IL-1β, and IL-6 levels in the supernatant of MRC-5 cells were significantly lower than those in PQ group, and the levels of NLRP3, caspase-1, and ASC were significantly lower. It is suggested that PD plays an anti-inflammatory and anti-apoptosis role in PQ-induced MRC-5 damage.
Conclusions
In conclusion, oxidative stress and inflammatory factors (including TNF-α, TGF-β, IL-1β, and IL-6, and proteins related to NLRP3 inflammasome activation-including NLRP3, caspase-1, and ASC) are all involved in the process of PQ-induced MRC-5 cell injury. PD intervention inhibited oxidative stress, significantly reduced the levels of inflammatory factors, and inhibited the activation of the NLRP3 inflammasome. However, since this study was conducted on MRC-5 cells in vitro, the effect of PD on PQ-induced lung injury needs to be further verified in an animal model, which is worth further study.
Acknowledgments
Funding: Foshan Science and Technology Innovation Project (2016AB002621), Science and Technology Program of Guangdong Province (2013B0218000038).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4570
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4570
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4570). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tyagi N, Singh R. Paraquat-Induced Oxidative Stress and Lung Inflammation. Oxidative Stress in Lung Diseases: Springer; 2020:245-70.
- Somu B, Shankar SH, Baitha U, et al. Paraquat Poisoning. QJM: monthly journal of the Association of Physicians. 2020.
- Chen J, Jian X, Yu G, et al. Fetal outcomes after intentional ingestion of paraquat: a case report. Medicine 2020;99:e18136. [Crossref] [PubMed]
- Liu H, Wu Q, Chu T, et al. High-dose acute exposure of paraquat induces injuries of swim bladder, gastrointestinal tract and liver via neutrophil-mediated ROS in zebrafish and their relevance for human health risk assessment. Chemosphere 2018;205:662-73. [Crossref] [PubMed]
- Hichor M, Sampathkumar NK, Montanaro J, et al. Paraquat Induces Peripheral Myelin Disruption and Locomotor Defects: Crosstalk with LXR and Wnt Pathways. Antioxid Redox Signal 2017;27:168-83. [Crossref] [PubMed]
- Chen H, Yang R, Tang Y, et al. Effects of curcumin on the lung collagen area and the expressions of TNF-α, IL-6 and NE in paraquat-poisoned rats. Chinese Journal of Emergency Medicine 2017;26:1252-6.
- Chen T, Wang R, Jiang W, et al. Protective effect of astragaloside IV against paraquat-induced lung injury in mice by suppressing Rho signaling. Inflammation 2016;39:483-92. [Crossref] [PubMed]
- Huang J, Ning N, Zhang W. Effects of paraquat on IL-6 and TNF-α in macrophages. Exp Ther Med 2019;17:1783-9. [PubMed]
- Zhao X, Li R, Liu Y, et al. Polydatin protects against carbon tetrachloride-induced liver fibrosis in mice. Arch Biochem Biophys 2017;629:1-7. [Crossref] [PubMed]
- Liao P, He Y, Yang F, et al. Polydatin effectively attenuates disease activity in lupus-prone mouse models by blocking ROS-mediated NET formation. Arthritis Res Ther 2018;20:254. [Crossref] [PubMed]
- Hessin AF, Hegazy RR, Hassan AA, et al. Resveratrol prevents liver fibrosis via two possible pathways: Modulation of alpha fetoprotein transcriptional levels and normalization of protein kinase C responses. Indian J Pharmacol 2017;49:282-9. [Crossref] [PubMed]
- Faghihzadeh F, Hekmatdoost A, Adibi P. Resveratrol and liver: A systematic review. J Res Med Sci 2015;20:797-810. [Crossref] [PubMed]
- Ma Z, Zhang Y, Li Q, et al. Resveratrol improves alcoholic fatty liver disease by downregulating HIF-1α expression and mitochondrial ROS production. Plos One 2017;12:e0183426. [Crossref] [PubMed]
- Liu YH, Huang QH, Wu X, et al. Polydatin protects against acetaminophen-induced hepatotoxicity in mice via anti-oxidative and anti-apoptotic activities. Food Funct 2018;9:5891-902. [Crossref] [PubMed]
- Coppa T, Lazzè MC, Cazzalini O, et al. Structure-activity relationship of resveratrol and its analogue, 4,4'-dihydroxy-trans-stilbene, toward the endothelin axis in human endothelial cells. J Med Food 2011;14:1173-80. [Crossref] [PubMed]
- Huang QH, Xu LQ, Liu YH, et al. Polydatin Protects Rat Liver against Ethanol-Induced Injury: Involvement of CYP2E1/ROS/Nrf2 and TLR4/NF-κB p65 Pathway. Evid Based Complement Alternat Med 2017;2017:7953850.
- Ye J, Piao H, Jiang J, et al. Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-κB and Nrf2/HO-1 pathways. Sci Rep 2017;7:11895. [Crossref] [PubMed]
- Zhang H, Yu CH, Jiang YP, et al. Protective effects of polydatin from Polygonum cuspidatum against carbon tetrachloride-induced liver injury in mice. PLoS One 2012;7:e46574. [Crossref] [PubMed]
- Chen JL, Dai L, Zhang P, et al. Methylene blue attenuates acute liver injury induced by paraquat in rats. Int Immunopharmacol 2015;28:808-12. [Crossref] [PubMed]
- Shen H, Wu N, Wang Y, et al. JNK Inhibitor SP600125 Attenuates Paraquat-Induced Acute Lung Injury: an In Vivo and In Vitro Study. Inflammation 2017;40:1319-30. [Crossref] [PubMed]
- Yang X, Zhang JH, Zhang JF, et al. Imbalance of Th17/Treg in the Pathogenesis of Mice with Paraquat-induced Acute Lung Injury. Iran J Allergy Asthma Immunol 2017;16:511-9. [PubMed]
- Hu X, Shen H, Wang Y, et al. Liver X Receptor Agonist TO901317 Attenuates Paraquat-Induced Acute Lung Injury through Inhibition of NF-κB and JNK/p38 MAPK Signal Pathways. Biomed Res Int 2017;2017:4652695.
- Wang Y, Wu H, Niu W, et al. Tanshinone IIA attenuates paraquat-induced acute lung injury by modulating angiotensin-converting enzyme 2/angiotensin-(1-7) in rats. Mol Med Rep 2018;18:2955-62. [PubMed]
- Fan H, Huang H, Hu L, et al. The activation of STIM1 mediates S-phase arrest and cell death in paraquat induced acute lung intoxication. Toxicol Lett 2018;292:123-35. [Crossref] [PubMed]
- Hosseini A, Rasaie D, Asl SS, et al. Evaluation of the protective effects of curcumin and nanocurcumin against lung injury induced by sub-acute exposure to paraquat in rats. Toxin Reviews 2019. [Crossref]
- Slater HE, Okoye OCA, Okperi O, et al. Acute kidney injury from Paraquat poisoning: a case report. Annals of Biomedical Sciences 2017;16:24-30.
- Hale RR, Bararpour T, Kaur G, et al. Sensitivity and Recovery of Grain Sorghum to Simulated Drift Rates of Glyphosate, Glufosinate, and Paraquat. Agriculture 2019;9:70. [Crossref]
- Wang ZW, Li XX, Jian XD, et al. The Study of Using Salvianolate Treat Acute Lung Injury in Rats Induced by Paraquat. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2017;35:342-7. [PubMed]
- Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem 2017;86:715-48. [Crossref] [PubMed]
- Maćczak A, Cyrkler M, Bukowska B, et al. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicol In Vitro 2017;41:143-9. [Crossref] [PubMed]
- Amin MM, Rafiei N, Poursafa P, et al. Association of benzene exposure with insulin resistance, SOD, and MDA as markers of oxidative stress in children and adolescents. Environ Sci Pollut Res Int 2018;25:34046-52. [Crossref] [PubMed]
- Senchuk MM, Dues DJ, Van Raamsdonk JM. Measuring Oxidative Stress in Caenorhabditis elegans: Paraquat and Juglone Sensitivity Assays. Bio Protoc 2017;7:e2086. [Crossref] [PubMed]
- Filograna R, Godena VK, Sanchez-Martinez A, et al. Superoxide Dismutase (SOD)-mimetic M40403 Is Protective in Cell and Fly Models of Paraquat Toxicity: IMPLICATIONS FOR PARKINSON DISEASE. J Biol Chem 2016;291:9257-67. [Crossref] [PubMed]
- Yuliati MEP, Aman IGM, Dewi NNA. Macassar fruit extract (Brucea javanica (l.) merr) increased the level of superoxide dismutase (SOD) but had no effect on the level of malondialdehyde (mda) in paraquat-treated male swiss Webster mice. IJAAM (Indonesian Journal of Anti-Aging Medicine) 2019;3:29-32.
- Toygar M, Aydin I, Agilli M, et al. The relation between oxidative stress, inflammation, and neopterin in the paraquat-induced lung toxicity. Hum Exp Toxicol 2015;34:198-204. [Crossref] [PubMed]
- Zhang H, Xu CN, Mine Y. Effects of a synthetic di-phosphoserine peptide (SS-2) on gene expression profiling against TNF-α induced inflammation. Int J Food Sci Technol 2019;54:2010-20. [Crossref]
- Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014;6:a016295. [Crossref] [PubMed]
- Liu Z, Sun M, Wang Y, et al. Silymarin attenuated paraquat-induced cytotoxicity in macrophage by regulating Trx/TXNIP complex, inhibiting NLRP3 inflammasome activation and apoptosis. Toxicol In Vitro 2018;46:265-72. [Crossref] [PubMed]
- Chen L, Na R, Boldt E, Ran Q. NLRP3 inflammasome activation by mitochondrial reactive oxygen species plays a key role in long-term cognitive impairment induced by paraquat exposure. Neurobiol Aging 2015;36:2533-43. [Crossref] [PubMed]
- Liu T, Xie Y, Xu M, et al. Protective Effect of Thalidomide on ALI Induced by Paraquat Poisoning in Rats and Its Mechanism. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017;29:977-81. [PubMed]
(English Language Editor: J. Reynolds)


