An evaluation of the feasibility of an expanded indication of endoscopic submucosal dissection for ulcer positive early gastric cancer: a case-control study from two medical centers
Introduction
In line with the treatment principle of advanced gastric cancer, the curability of early gastric cancer (EGC) patients is highly determined by the radical resection of the tumor and its metastatic lymph nodes (1-3). Surgery is the primary treatment option for patients with early gastric cancer. R0 resection has been widely considered as a definite goal, whereas the type of resection (subtotal/total gastrectomy or function preserving gastrectomy) along with extent of lymph node dissection remain a subject of controversy. Meanwhile, lymph node metastasis (LNM) is a decisive factor influencing the long-term survival of EGC patients. Although a substantial proportion of EGC patients are not presented for LNM, it has been increasingly observed in the clinical practice. Furthermore, there are absolute indications proposed by the Japanese Gastroenterological Endoscopy Society (JGES) in collaboration with the Japanese Gastric Cancer Association (JGCA) in 2016 for endoscopic submucosal dissection (ESD). These indications are too strict to include more EGC patients for ESD treatment and, therefore, lead to a limited benefit for EGC patients (4). In light of this, Chinese gastric cancer treatment guidelines proposed by the National Health Commission of China PR in 2018 expanded the absolute indication for ESD: a ulcer positive (UL+), differentiated-type adenocarcinoma with tumor invasion pathologically defined as T1a and tumor diameter ≤3 cm (used to be confirmed as the expanded indication in Japanese gastric cancer guidelines) (5).
Therefore, this study aimed to identify the predictive factors for LNM in a large cohort of EGC patients undergoing radical surgery performed in two clinical centers for gastric cancer treatment in China. We aim to assess the feasibility of applying ESD in UL (+) EGC patients who fail to meet all the conventional absolute criteria for ESD.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4303).
Methods
The study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Institutional Ethical Board of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. Because of the retrospective nature of the research, the requirement for informed consent was waived. The amount of EGC cases takes about 20% of the Chinese gastric cancer patients, and the prognosis of EGC patients is much better than that of advanced gastric cancer. Therefore, for a case-control study of Chinese EGC population, the sample size included in the retrospective analysis should be more than 2,000 cases.
From January 2012 and December 2018, 10,211 patients with gastric cancer underwent curative resection with D2 lymphadenectomy in the Department of Surgery, Ruijin Hospital, and the department of gastric surgery, Fudan University Shanghai Cancer Center. A total of 3248 EGC patients were enrolled in the study who were scrutinized and pathologically examined and diagnosed.
The inclusion criteria included (I) histologically diagnosed as EGC, (II) single primary lesion, (III) aged between 15 and 95 years old, (IV) no distant metastasis (cM0 or sM0) for an enhanced CT scan and a pre-operative laparoscopic examination, (V) R0 resection, (VI) total or subtotal gastrectomy, (VII) D2 lymphadenectomy, (VIII) no history of operation for peptic ulceration or any abdominal carcinoma, (IX) no history of other malignant types of carcinoma, (X) no history of neoadjuvant chemotherapy or radiation therapy for this or any other carcinoma, (XI) no history of severe sepsis or the pre-operative administration of therapeutic antibiotics. Exclusion criteria included (I) incomplete patient information, (II) less than 15 lymph nodes examined, (III) broken or fragmentary surgical specimens.
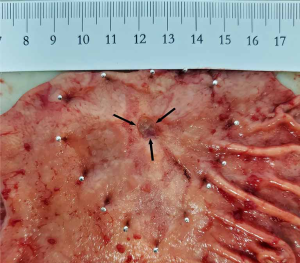
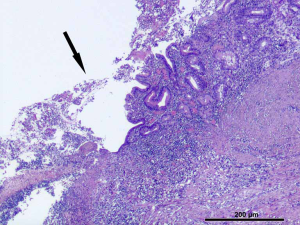
The pathological observation was performed as tumor size, tumor location, and gross types according to the rules published in the Japanese Classification of Gastric Carcinoma (3rd English Edition) (6). Pathological evaluations were performed as follows: (I) the resected stomach specimen was fixated on an flat board with the mucosal side up, pinned at the edges with stainless steel pins, and fixed in a 10% buffered formalin solution. The diagnosis of the tumor with ulcer [UL (+)] is determined with the macroscopic predominance of intratumoral ulcerative findings. A biopsy-induced scar is usually histologically observed as fibrosis restricted to limited areas just beneath the muscularis mucosae. In case the biopsy-induced scar was indiscriminative from the ulcer scar, such a case was classified as UL (+). Representative images showing the gross and pathologic UL (+) EGC lesions are shown in Figure 1 and Figure 2. (II) The representative sections, including the carcinoma, were microscopically examined along the lesser curvature as a reference line to assess background mucosal changes. In Type 0 superficial tumors, a set of sections parallel to the reference line were constructed at 2 to 3 mm intervals. (III) Furthermore, the fixed specimen was histologically classified into either the differentiated or undifferentiated type. The former type includes papillary adenocarcinoma (pap) and tubular adenocarcinoma (tub1, tub2), whereas the latter one includes poorly differentiated adenocarcinoma (por1, por2), mucinous adenocarcinoma (muc) and signet-ring cell carcinoma (sig). In the case of more than two histological types coexisting in the specimen, the predominant type was prioritized for the study. Other types (e.g., carcinoid tumor, endocrine carcinoma, adenosquamous carcinoma, squamous cell carcinoma, miscellaneous carcinoma) were excluded from the study.
According to the results of the pathological evaluation, EGC patients were subsequently divided into two groups by LNM or not: LNM positive and LNM negative groups. The clinicopathological factors such as age (the continuous variables of age were categorized by WHO), sex, ulcer positive or not, tumor size (the continuous variables of tumor size were categorized by ESD indications), depth of invasion, tumor location, differentiated type, lymphovascular infiltration, and perineural invasion) were compared between these two groups.
Statistical analysis
Samples that are subject to the normal distribution are described using mean value and standard deviation. Otherwise, the median and quartile values were used. The frequency and percentage are used to describe enumeration data. For samples that are subject to the normal distribution, the comparisons are conducted by using a t-test or analysis of variance (ANOVA). Otherwise, the rank-sum methods were used.
Comparisons between enumeration data were conducted by the chi-square or Fisher exact method. In order to minimize the influence of the common confounding bias in case control study, a stepwise multiple logistic regression analysis was used to explore whether each clinicopathological parameter could serve as an independent risk factor for gastric LNM. P<0.05 was considered statistically significant.
Statistical analysis was conducted using the statistical software SAS ‘PROCGLM’ 9.2 for Windows (SAS, Cary, NC, USA).
Results
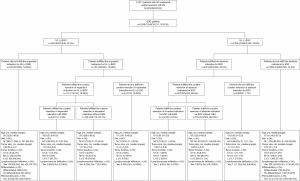
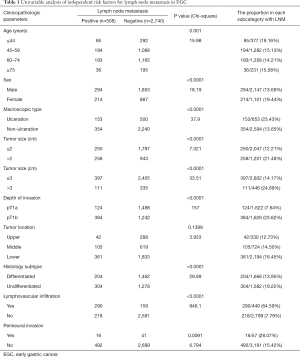
Between January 2012 and December 2018, many 3,248 EGC patients underwent radical surgery at the Department of Surgery, Ruijin Hospital (1,794 EGC patients), and the department of gastric surgery, Fudan University Shanghai Cancer Center (1,454 EGC patients). A brief schema for the study are presented in Figure 3. By using the derivation set, we performed a univariable analysis which demonstrated that age at diagnosis (P=0.001), larger tumor size (P<0.0001), UL (+) (P=0.001), submucosa invasion (P<0.0001), histological subtype (P<0.0001), lymphovascular infiltration (P<0.0001) and perineural invasion (P=0.0091) were significantly associated with LNM in EGC patients (Table 1).
Full table
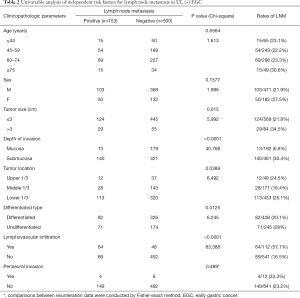
With strict evaluating, 653 UL (+) EGC patients (20.1%, 653/3248) were included in this study. LNM was pathologically confirmed in 23.4% (153/653) of the UL (+) EGC patients. Univariate analysis revealed that LNM in UL (+) EGC patients was significantly associated with tumor size (>3 cm) (P=0.0150), submucosal invasion (P<0.0001), lower tumor location (P=0.0389) and prevalence of lymphatic or venous infiltration (P<0.0001) (Table 2). Multivariate linear regression analysis further confirmed that submucosal tumor invasion (P<0.0001) and lymphovascular infiltration (P<0.0001) were independent risk factors for LNM (Table 3).
Full table

Full table
As shown in Table 4, the intergroup analysis of EGC patients revealed that 532 Chinese EGC patients met the absolute indication of ESD proposed by the JGES. Among them, 22 patients (4.14%, 22/532) were presented with LNM, and four patients (0.75%, 4/532) were positive for lymphovascular infiltration. However, many 120 UL (+) EGC patients fully met the expanded indication of ESD, with eight patients (6.67%, 8/120) diagnosed with positive LNM. No lymphovascular infiltration was found in any EGC patient with tumor size ≤3 cm, UL (+), T1a, histologically of differentiated type of tumor. Notably, there was a significant difference in LNM (P=0.000274) between EGC patients, which were subject to absolute and expanded indications for ESD.

Full table
Discussion
Our study is the first case-control study from the cooperation of two clinical centers for gastric cancer treatment in Shanghai; each of them provided medical services for more than 350 EGC patients yearly. This study aims to investigate the feasibility and safety of expanding the absolute indication of ESD for UL (+) EGC patients. The diagnosis of EGC proposed by the Japanese Classification of Gastric Carcinoma (JCGC) stresses the importance of tumor invasion depth rather than LNM (6). However, multiple studies repeatedly prove the significantly worse prognosis of EGC patients with LNM compared with EGC patients without LNM (7).
According to a mono-institutional study (8) of 1,663 patients in China, the 5-year survival rate of gastric cancer patients undergoing radical surgical resection was 94.5% at stage IA (pT1N0M0) and 88.4% at stage IB (pT1N1M0/pT2N0M0), respectively, demonstrating the notable survival difference between LNM positive and negative EGC patients as well as the significance of treating these two groups of EGC patients differently. To address LNM, in this study, we reviewed 3,248 EGC patients and then confirmed this ulceration was the independent risk factor for LNM. Among 653 UL (+) EGC patients, LNM was proven to be more common in tumors with diameter >3 cm. Compared with the patients meeting the absolute indication (4.14%), the incidence of LNM in UL (+) EGC patients were meeting the expanded indication (6.67%) of ESD was not significantly increased.
Regarding the expanded ESD indication, in 2018, Chinese gastric cancer treatment guidelines recommended the inclusion of (I) tumor size ≤3 cm, (II) cT1a, (III) UL (+), and (IV) differentiated-type adenocarcinoma into the absolute indication for ESD considering the results from JCOG0607 study (9). As a multi-institutional, single-arm, confirmatory clinical trial, the JCO0607 study demonstrated that the 5-year survival rate of EGC patients receiving ESD treatment was approximately 97% and, therefore, suggested that ESD meeting the criteria above should be considered the standard treatment instead of radical gastrectomy. However, we can still put forward some disputes. Tanabe et al. (10) conducted a multicenter descriptive study reviewing the management and follow-up of 10,658 EGC patients. Among all patients, no case of local/distant recurrence nor cancer-related death was observed from various 6,456 EGC patients who met the absolute indication. On the contrary, 5 cases of perigastric nodes metastases, 1 case of lung metastasis, and 3 cases of cancer-related death were found in the 4,202 EGC patients who met the expanded indication of ESD. It is still essential to find the patient population that fits the expanded ESD indication considering these events, which should be executed with extreme cautiousness.
Moreover, this study adopted a 5-year follow-up for the patients. Notably, however, the survival of EGC patients with local or even distant recurrence often exceeds five years. Therefore, we believe that a follow-up of 60 months for EGC patients might not be sufficient to reflect the truth and suggest a more extended period of follow-up for them.
As for the ESD-associated adverse events, the relatively elevated risk of perforation (11-14) and bleeding (15) are enlisted on the top for UL (+) patients proposed by the JCOG0607 study. Understandably, it stressed the significance of tumor size and suggested that postoperative hemorrhage tended to be less common with tumor diameter less than 3 cm. Another major concern regards the curability of endoscopic resection. It is easily confused with R0 resection, which indicates the negativity of the surgical margin, whereas the negative endoscopic margin does not secure curative resection.
It has been recommended recently that the expanded indication for ESD resection is determined as curative when all the following conditions are fulfilled to unify the criteria of prognosis evaluation, eCura system (16,17). An en bloc resection, tumor size, ≤3 cm, differentiated type, pT1a, UL (+), negative horizontal margin (HM0), negative vertical margin (VM0), and no lymphovascular infiltration [ly(−), v(−)].
Follow-up with annual or biannual endoscopy is recommended after curative ESD resection of tumors under the expanded indications (5). Our study found the lymphovascular infiltration as an independent risk factor for LNM in EGC. Unfortunately, due to the limitation of gastroscopic biopsy, the diagnosis of lymphovascular infiltration in EGC is not 100% secured. Fujimoto et al. (18) proposed that ESD could be an essential way to determine if the metastasis of perigastric lymph nodes exists or not. Therefore, patients may have to face the risk of remedial surgical resection if ESD identifies positive LNM is. In this study, many 532 EGC patients met the absolute indications of ESD, and 22 cases (4.15%, 22/532) demonstrated perigastric LNM validated by pathological examination. Among these 22 LNM positive EGC patients, 18 were positive for lymphovascular infiltration.
Notably, there were still 4 (4/532, 0.75%) patients who “escaped” the eCura system and developed LNM. Simultaneously, there were 120 cases of UL (+) EGC patients meeting the expanded indications of ESD following the Japanese gastric cancer treatment guidelines, 8 of which were positive for perigastric LNM (8/120, 6.67%). However, whereas with the high incidence of lymphovascular infiltration (81.8%, 18/22) in the group of absolute indication, none of these eight patients was detected with lymphovascular infiltration. Moreover, with the help of eCura system UL (+), EGC patients meeting expanded indications had a significantly higher rate of perigastric LNM when compared with patients meeting the absolute indication (P=0.000274).
Although the eCura system seemingly works, there is still a potential risk of recurrence in patients with curative resection by ESD (19,20). The local recurrence rate is 0.13–1.3%, whereas the incidence of synchronous cancer and metachronous cancer is 4.0–2.9% and 2.5–5.1% (20-23), respectively. Moreover, the cumulative risk rate of 5, 7 and 10 years is 9.5%, 13.1% and 22.7% (20). Recent large-scale Korean EGC patient studies reported that the frequency of EGC recurrence was 2.0% to 5.0% after curative resection (24-26). Although the reasons for local or distant recurrence remain unclear, it might be correlated with the synchronous micro-metastasis of perigastric lymph nodes, in particular of UL (+) EGC patients. They met the criteria of curative resection according to the eCura system. Moreover, according to the large-scale evidence-based medicine research and database analysis of clinicopathological data from the Chinese Association of Gastrointestinal Cancer Surgery (27), the perioperative mortality of Chinese gastric cancer patients in 2018 is only 0.23%.
However, there are also some limitations in this study. We use an accurate pathological assessment method to determine whether EGC lesions are associated with ulcer and the existense of gastric LNM, and the degree of selection bias and information bias are reduced with the gold standard to the greatest extent, but as an observational study, the confounding bias may still inevitably. For example, there are more ulcer positive EGC cases with gastric LNM than ulcer negative cases, and the LNM is also possiblely caused by poorer tumor differentiation. Besides, there is also a correlation between the degree of tumor differentiation and EGC with ulcers or not. In order to minimize confounding bias, the study used multivariate techniques to control the effects of multiple covariates to determine whether ulcer in EGC lesions was a independent risk factor for gastric LNM. Secondly, the external validity should be considered. Although the population reviewed in this study were from two Chinese large gastric treatment centers, the observation population was only Chinese. Therefore, the results of this study can only represente Chinese or Asian ethnicity, which cannot be easily extended to all people around the world.
Further studies should be designed to investigate these unsolved doubts, which pave the way for a better understanding of EGC recurrence and a rational selection of multiple treatment strategies like ESD and radical surgery for EGC patients.
Acknowledgments
Completed clinical and pathological data were collected from the large-scale Data Analysis Center of Cancer Precision Medicine-LinkDoc database and analyzed by using data technology support by LinkDoc.
Funding: Grants supported this study from the Cross Research Fund for Translational Medicine of Shanghai Jiao Tong University (ZH2018QNA55) and Shanghai Anticancer Association Eyas Project (SACA-CY1C04).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4303
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4303
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4303). The authors have no conflicts of interest to declare.
(English Language Editor: J. Chapnick)
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained from the Ruijin Hospital Ethics Committee, Shanghai Jiao Tong University School of Medicine, China (No. 2018-151). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jiang L, Yang KH, Guan QL, et al. Survival and recurrence free benefits with different lymphadenectomy for resectable gastric cancer: a meta-analysis. J Surg Oncol 2013;107:807-14. [Crossref] [PubMed]
- Cunquero-Tomás AJ, Ortiz-Salvador JM, Iranzo V, et al. Sweet syndrome as the leading symptom in the diagnosis of gastric cancer. Chin Clin Oncol 2018;7:11. [Crossref] [PubMed]
- Zhang CD, Yamashita H, Seto Y. Gastric cancer surgery: historical background and perspective in Western countries versus Japan. Ann Transl Med 2019;7:493. [Crossref] [PubMed]
- Gotoda T. Endoscopic resection for premalignant and malignant lesions of the gastrointestinal tract from the esophagus to the colon. Gastrointest Endosc Clin N Am 2008;18:435-50. viii. [Crossref] [PubMed]
- National Health Commission Of The People's Republic Of China. Chinese guidelines for diagnosis and treatment of gastric cancer 2018 (English version). Chin J Cancer Res 2019;31:707. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [Crossref] [PubMed]
- Kim JJ, Lee JH, Jung HY, et al. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc 2007;66:693-700. [Crossref] [PubMed]
- Ji X, Bu ZD, Yan Y, et al. The 8th edition of the American Joint Committee on Cancer tumor-node-metastasis staging system for gastric cancer is superior to the 7th edition: results from a Chinese mono-institutional study of 1663 patients. Gastric Cancer 2018;21:643-52.
- Hasuike N, Ono H, Boku N, et al. A non-randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal-type gastric cancer (cT1a): the Japan Clinical Oncology Group study (JCOG0607). Gastric Cancer 2018;21:114-23. [Crossref] [PubMed]
- Tanabe S, Ishido K, Matsumoto T, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a multicenter collaborative study. Gastric Cancer 2017;20:45-52. [Crossref] [PubMed]
- Oda I, Odagaki T, Suzuki H, et al. Learning curve for endoscopic submucosal dissection of early gastric cancer based on trainee experience. Dig Endosc 2012;24 Suppl 1:129-32. [Crossref] [PubMed]
- Kakushima N, Fujishiro M, Kodashima S, et al. A learning curve for endoscopic submucosal dissection of gastric epithelial neoplasms. Endoscopy 2006;38:991-5. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Nakamura M, et al. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc 2006;63:243-9. [Crossref] [PubMed]
- Nakamoto S, Sakai Y, Kasanuki J, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy 2009;41:746-50. [Crossref] [PubMed]
- Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 2009;58:331-6. [Crossref] [PubMed]
- Hatta W, Gotoda T, Oyama T, et al. A scoring system to stratify curability after endoscopic submucosal dissection for early gastric cancer:“eCura system American Journal of Gastroenterology 2017;112:874-81. [Crossref] [PubMed]
- Hatta W, Gotoda T, Oyama T, et al. Is the eCura system useful for selecting patients who require radical surgery after noncurative endoscopic submucosal dissection for early gastric cancer? A comparative study. Gastric Cancer 2018;21:481-9. [Crossref] [PubMed]
- Fujimoto A, Goto O, Nishizawa T, et al. Gastric ESD may be useful as accurate staging and decision of future therapeutic strategy. Endosc Int Open 2017;5:E90-5. [Crossref] [PubMed]
- Choi MK, Kim GH, Park DY, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc 2013;27:4250-8. [Crossref] [PubMed]
- Min BH, Kim ER, Kim KM, et al. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy 2015;47:784-93. [Crossref] [PubMed]
- Abe S, Oda I, Suzuki H, et al. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 2015;47:1113-8. [Crossref] [PubMed]
- Han JS, Jang JS, Choi SR, et al. A study of metachronous cancer after endoscopic resection of early gastric cancer. Scand J Gastroenterol 2011;46:1099-104. [Crossref] [PubMed]
- Jang MY, Cho JW, Oh WG, et al. Clinicopathological characteristics of synchronous and metachronous gastric neoplasms after endoscopic submucosal dissection. Korean J Intern Med 2013;28:687-93. [Crossref] [PubMed]
- Youn HG, An JY, Choi MG, Noh JH, Sohn TS, Kim S. Recurrence after curative resection of early gastric cancer. Ann Surg Oncol 2010;17:448-54. [Crossref] [PubMed]
- Lai JF, Kim S, Kim K, et al. Prediction of recurrence of early gastric cancer after curative resection. Annals of surgical oncology 2009;16:1896-902. [Crossref] [PubMed]
- Hyung WJ, Cheong JH, Kim JU, et al. Analyses of prognostic factors and gastric cancer specific survival rate in early gastric cancer patients and its clinical implication. Annals of Surgical Treatment and Research 2003;65:309-15.
- China Gastrointestinal Cancer Surgery Union. Data report of China Gastrointestinal Cancer Surgery Union (2014-2016). Chinese Journal of Practical Surgery 2018;38:90-3.
(English Language Editor: J. Chapnick)