A narrative review of heart rate and variability in sepsis
Introduction
Pediatric sepsis, a serious and potentially lethal condition, remains an important cause of morbidity and mortality (1). The World Health Organisation (WHO) estimates that 1.2 million children suffer from sepsis every year (2), and infections account for more than half of the deaths in children under 5 years old (3). While isolating the offending agent allows for definitive management, microbiological investigations usually take about 24–72 hours to return and are often influenced by factors including the adequacy of blood volume taken and the presence of low, transient bacteraemia (4,5). Therefore, early identification of patients with sepsis cannot rely on microbiology investigations alone (6); accurate prediction of which child has sepsis remains a clinical challenge.
The 2018 update to The Surviving Sepsis Campaign Bundle included an “hour-1 bundle” to be achieved within one hour from the time of triage in the Emergency Department (ED) (6). Clinicians need to be able to quickly identify a critically ill child and administer treatment protocols without definitive blood culture results. Many clinicians define pediatric sepsis based on the systemic inflammatory response syndrome (SIRS) criteria established by Goldstein in 2005 to identify a critically ill child with sepsis (7). However, the SIRS criteria has limited clinical applicability. A recent study showed that most children who fulfil the SIRS criteria do not require critical care, and many children who required resuscitation did not meet the SIRS criteria at the time of presentation (8). Moreover, identification of a septic child is often complicated by its heterogeneous clinical presentation, which can range from a non-specific presentation like fever, irritability, and poor feeding to fulminant sepsis with hemodynamic compromise (9).
The need for an objective assessment in pediatric critical illness has led to the use of vital signs. Vital signs have been and continue to be extensively studied as part of triage systems and warning scores (10-16). Scoring and triggering systems which include heart rate have been used to predict potential deterioration and the need for intensive care (17). Other investigators have attempted to define normal ranges for heart rate, built on the understanding that an abnormal heart rate is an important indicator of critical illness, including sepsis (18-20). As such, heart rate parameters are attractive to clinicians in that they can potentially add to the timely recognition of pediatric sepsis.
This review seeks to: (I) review the role of heart rate in pediatric warning scores, including its role as a predictor for critical illness including sepsis; and (II) introduce the concept of heart rate variability (HRV) analysis. In this review, we will highlight the current uses of HRV analysis (including in adult and neonatal sepsis) and explore how it can be utilized in pediatric sepsis. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/atm-20-148).
Methods
We conducted a literature search for papers published up to December 2019 on the utility of heart rate and HRV analysis in the diagnosis and management of sepsis, using four medical databases: PubMed, Google Scholar, EMBASE and Web of Science. Study selection was based on three medical databases, PubMed, EMBASE and Web of Science, with the following search strategy (including specific Medical Subject Headings, MeSH): “heart rate/physiology”, “sepsis/diagnosis”, “sepsis/mortality” and “sepsis/complications”. Other free text terms used were: “heart rate variability”, “emergency medical service”, “critical care”, “intensive care unit”, “neonate”, “infant” and “child”. Qualitative and quantitative data were extracted through interpretation of each article in cycles to avoid missing on data of potential value.
Discussion
Heart rate as a predictor for critical illness including sepsis
Heart rate is an easily obtained parameter that many clinicians use as an early marker of deterioration in children. Various hospitals have developed pediatric early warning systems (PEWS) (10-13). A large systematic review of 66 studies of various PEWS and its derivatives demonstrated limited evidence of PEWS in identifying children with impending clinical deterioration (16). Of note, there was marked heterogeneity of outcome metrics, interventions, populations and clinical settings in the various studies (16). Categorical examples with their respective heart rate reference ranges can be found in Table S1, where we compare age-related thresholds for abnormal heart rate in selected PEWS scores to currently published heart rate guidelines and threshold limits used in large cross-sectional studies.

Full table
Although heart rate is a convenient and easily accessed physiological parameter, there is a lack of consensus among warning scores on what constitutes significant, out-of-proportion tachycardia in a sick child (Table S1). For instance, the normal heart rate of a 8-year-old child is between 80 to 120 bpm according to APLS guidelines, and PALS places the normal heart rate of a 8-year-old child at between 60 to 140 bpm (21,22).
There have been multiple attempts to define a normal heart rate range for different age groups in large and robust study populations (18-20). When one considers the normal ranges of heart rate based on these population studies, the heart rate ranges in various PEWS scores may not necessarily be representative of a “sick” child. This is especially so for PEWS scores that incorporate wide age bands. We know that with increasing age comes a steady decrease in heart rate. Consequently, these PEWS scores may result in false positive triggers (18-20). If implemented into monitoring systems, it can result in unnecessary resource utilisation, poor performance of scoring and triggering systems, and ultimately alarm fatigue (23). This creates a conundrum fundamental to the importance of heart rate as a vital component of prediction scores.
Tachycardia out of proportion to age and height of fever has also been proven not to have good discriminatory value in the prediction for infants with sepsis (24). Multiple contributors to tachycardia including pain, anxiety, and fever limit the interpretation of this important vital sign in children. It is recognized that continuously measured physiologic variables and their trends may better inform monitoring strategies for critically ill children with different admission diagnoses (including sepsis) (25).
The clinical value of traditional heart rate as a predictive tool is thus limited, and this has led to the exploration of novel methods such as HRV analysis.
Heart rate variability (HRV)
The study on measuring heart rate and rhythm has progressed from cardiac auscultation, the advent of the galvanometer (used to detect and measure small electric currents), to the era of digital signal processing systems such as the electrocardiogram (ECG). It was through the 1996 report of the Task Force of the European Society of Cardiology and The North American Society of Pacing and Electrophysiology that a novel method of HRV analysis was definitively introduced (26). HRV analyzes the oscillation in the interval between consecutive heart beats (RR interval), and is a measure of autonomic nervous system (ANS) regulation (27). ANS dysfunction is a maladaptive response in injury and critical illness, including sepsis (27). In the past few decades, more studies have delved into the relationship between HRV as a measure of ANS dysfunction, and various critical illnesses (26,28).
HRV parameters
Data collected from an ECG over a continuous time-period forms the basis for HRV analysis. Figures 1 and 2 show the difference between a patient with good heart rate variability and one without.
The three domains employed in HRV are the time-domain; frequency-domain; and the non-linear domain. Time-domain parameters are derived from measuring the normal “RR intervals”, otherwise known as “NN intervals”, or from the differences between NN intervals. In practice, these three measurements are often used interchangeably. Frequency-domain parameters measure how often a signal recurs within a specific frequency band, and this is derived from spectral analysis. Non-linear methods are a means to explain the complex interactions of HRV involving the hemodynamic, electrophysiological and humoral variables, and our autonomic and central nervous system (26). Many of these parameters are well-described in the report of the Task Force (26). Some of the parameters discussed in this report are listed in Table 1.

Full table
Current applications of HRV
The clinical applications of HRV analysis are well documented in the practice of adult cardiology. Reduced HRV in adults is predictive for sudden cardiac death (29-31), increased mortality after an acute myocardial infarction (26,32,33), and is deemed an independent risk factor for developing cardiovascular diseases (34,35). In recent years, as a risk stratification tool in chest pain, HRV has been consistently demonstrated to outperform commonly used validated scoring systems [e.g., thrombolysis in myocardial infarction (TIMI) score, patient acuity category scale (PACS), and the modified early warning score (MEWS)] (36,37). In diabetes mellitus, patients with diabetic autonomic neuropathy demonstrated a reduced low frequency/high frequency (LF/HF) ratio (38) and this reduction in variability preceded clinical symptoms of diabetic neuropathy (39,40). In anesthesiology, reduced HRV is also associated with the risk for developing hypotension following general anesthesia induction (41). In sepsis, HRV analysis has been studied in the adult and neonatal setting with promising results when compared to traditional heart rate alone. In the next two subsections, we will describe the use of HRV analysis in these settings and explore how these methods can be potentially applied in pediatrics.
Adult sepsis
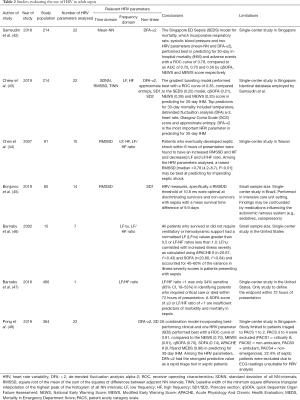
In adult sepsis, HRV analysis has paved the way for the development of new scoring systems, such as the Singapore Emergency Department Sepsis (SEDS) model (42). The SEDS model, which includes HRV-derived parameters (mean NN and DFA alpha-2) and other parameters such as age, respiratory rate and systolic blood pressure, was shown to outperform the qSOFA score, MEWS and NEWS in predicting 30-day in-hospital mortality for adults with sepsis (42). This was followed by a subsequent study which outlined the high performance of a HRV-based machine learning model in predicting 30-day in-hospital mortality among suspected sepsis patient in the ED (43).
Specific HRV parameters to predict sepsis in the ED have also been explored. An increased square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMSSD) was found in patients who developed septic shock within 6 hours of presentation at the ED (44). Reduced RMSSD has also been shown to predict for 28-day mortality in septic patients presenting at the ED (45). Some prior studies demonstrated that a decreased LF/HF ratio of less than 1.0 may predict severity of illness in septic patients (46,47). When integrated with various components of other warning scores, HRV parameters have been found to be superior in risk-stratifying septic patients at the ED (48). In the adult intensive care units (ICU), the use of artificial intelligence has allowed HRV parameters to be incorporated into systems and scores that detect sepsis and severe sepsis (49-51). Continuous HRV monitoring has reduced the gap between the onset of sepsis and its clinical recognition by up to five hours, allowing clinicians to direct early intervention efforts in sepsis treatment (52-54).
Given current research efforts, scoring systems incorporating HRV parameters may outperform those with traditional heart rate alone (Table 2). Short-term HRV analysis is a potential game-changer in better diagnostic accuracy for adult sepsis in the ED and ICUs and should be integrated with other vital signs obtained at triage for better performance (55,56). HRV remains a promising research field and there are ongoing studies that evaluate its utility as part of scoring systems (57).
Full table
Neonatal sepsis
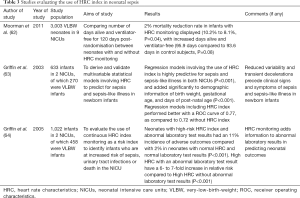
Unlike pediatric and adult sepsis, there is still no commonly agreed definition for neonatal sepsis (58-60). Since continuous non-invasive cardiac monitoring is often standard procedure in the neonatal intensive care unit (NICU), studies have explored the use of HRV analysis as a diagnostic and prognostic tool for sepsis. Heart rate characteristic (HRC) index measures the degree of reduced variability and decelerations to determine the fold-increase in the risk of developing neonatal sepsis (61,62). The detection of sepsis by HRC index precedes clinician suspicion of sepsis (62-64). Studies have shown that HRC index monitoring in the NICU resulted in a reduction in mortality in very-low-birth-weight (VLBW) neonates, and its use as an independent tool in predicting for neonatal sepsis has been validated (62-65) (Table 3).
Full table
Looking at specific HRV parameters, time-domain indices such as NN50 count divided by the total number of all NN intervals (pNN50) was significantly decreased in neonates with sepsis as compared to healthy controls, while changes in frequency-domain indices such as very low frequency (VLF), LF, HF, and LF/HF ratio were statistically insignificant (66). However, another study showed that specific HRV parameters were not significantly modified following sepsis (67). As such, further studies are needed to determine the added value of HRV, and if time domain indices are truly more sensitive than frequency domain indices in predicting for neonatal sepsis.
Pediatric sepsis
HRV prediction in sepsis has been explored in the adults and neonates, but data on infants and children beyond the first month of life is very limited. An old PICU study of children with critical illness and injury demonstrated that HRV trends correlate with severity of illness and may have important clinical implications, but did not focus specifically on sepsis (28). ANS dysfunction in pediatric septic shock has been successfully demonstrated with HRV, but this has not translated into the use of HRV to guide clinical practice (68). In this small study of 7 children with septic shock, 6 children showed changes in the low/high frequency ratio and the authors postulate added value in using loss of HRV and complexity in monitoring these ill children (68). In another study of 22 children with known cardiovascular diseases, HRV changes may precede clinical diagnosis of sepsis by up to 24 hours (69).
Given the potential application demonstrated in adult and neonatal patients with sepsis, we postulate that HRV use can be expanded in the pediatric population. For example, HRV analysis can be investigated as a potential tool to identify septic children in the pediatric ED. Higher frequency data collection would allow the patient’s evolving clinical status to be more accurately captured (70). If proven to discriminate between children with sepsis and those without, it has the potential to add to the ED physician’s armamentarium on which child should have early cultures and urgent antibiotic administration, thus guiding resource utilisation.
Continuous HRV analysis, similar to those performed in the NICU, could also be explored in the PICU. Similar to the NICU septic population, a reduction in HRV could potentially precede clinical symptoms and signs of sepsis. New applications of HRV including predicting and prognosticating nosocomial and line-related sepsis in the critically ill PICU population should also be explored. The responsiveness of abnormal HRV parameters in successful treatment and resolving sepsis deserves further study. If promising, it may prove useful to guide clinicians in their monitoring and treatment strategy (28).
Limitations of HRV
Despite ongoing research efforts for the past century, HRV remains a relatively new concept to many. Before considering its use for pediatric sepsis, several limitations of HRV need to be taken into account. The current availability of ECG machines does not equate to the commercial availability of HRV analysis, and this may explain why HRV analysis has not been integrated into everyday use. There is often proprietary software and hardware requirements, with upfront cost barriers that need to be overcome. Training of personnel in data interpretation remains an issue, and healthcare professionals including doctors and nurses have to understand the principles of HRV analysis and how it may impact sepsis diagnosis and prognostication. In children specifically, artefacts need to be dealt with and post-processing capabilities are needed for meaningful analysis of HRV signals. In neonates, abnormal HRV can be affected by factors other than sepsis, such as gestational age and underlying medical conditions (71), and as such needs to be interpreted accordingly. Many prior studies have also excluded patients with cardiovascular diseases such as arrhythmias due to the lack of satisfactory RR intervals for analysis (36,37,42,43). This may potentially limit the generalisability to the wider population with a history of congenital and acquired cardiac disorders. The use of HRV in different settings can also result in undesirable effects on health services with an overall increase in interventions, hence impact on the wider healthcare setting must be evaluated before translation into practice (72). Most importantly, we acknowledge that the use of HRV has not been documented in pediatric sepsis, and the findings relating HRV to adult sepsis and neonatal sepsis may not apply similarly in this population. To establish definitive evidence, it is necessary to conduct similar studies in this setting.
Conclusions
When compared to traditional heart rate, HRV has been shown to value-add in the identification and prognostication of adults and neonates with sepsis. This exciting non-invasive tool could guide the recognition and management of pediatric sepsis and impact clinical practice.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-148
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-148
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-148). JHL reports personal fees from KK Women’s and Children’s Hospital, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tan B, Wong JJ, Sultana R, et al. Global case-fatality rates in pediatric severe sepsis and septic shock: A systematic review and meta-analysis. JAMA Pediatr 2019;173:352-62. [Crossref] [PubMed]
- World Health Organisation: Sepsis. Available online: https://www.who.int/news-room/fact-sheets/detail/sepsis. Accessed April 3 2019.
- World Health Organisation: Causes of child mortality. Available online: https://www.who.int/gho/child_health/mortality/causes/en/. Accessed April 29, 2019.
- Connell TG, Rele M, Cowley D, et al. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children’s hospital. Pediatrics 2007;119:891-6. [Crossref] [PubMed]
- Tam PY, Bendel CM. Diagnostics for neonatal sepsis: current approaches and future directions. Pediatr Res 2017;82:574. [Crossref] [PubMed]
- Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med 2018;44:925-8. [Crossref] [PubMed]
- Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005;6:2-8. [Crossref] [PubMed]
- Scott HF, Deakyne SJ, Woods JM, et al. The prevalence and diagnostic utility of systemic inflammatory response syndrome vital signs in a pediatric emergency department. Acad Emerg Med 2015;22:381-9. [Crossref] [PubMed]
- Plunkett A, Tong J. Sepsis in children. BMJ 2015;350:h3017. [Crossref] [PubMed]
- Sandell JM, Maconochie IK. Pediatric early warning systems (PEWS) in the ED. Emerg Med J 2016;33:754-5. [Crossref] [PubMed]
- Cotterill S, Rowland AG, Kelly J, et al. Diagnostic accuracy of PAT-POPS and ManChEWS for admissions of children from the emergency department. Emerg Med J 2016;33:756-62. [Crossref] [PubMed]
- Haines C, Perrott M, Weir P. Promoting care for acutely ill children—development and evaluation of a pediatric early warning tool. Intensive Crit Care Nurs 2006;22:73-81. [Crossref] [PubMed]
- Edwards ED, Mason BW, Oliver A, et al. Cohort study to test the predictability of the Melbourne criteria for activation of the medical emergency team. Arch Dis Child 2011;96:174-9. [Crossref] [PubMed]
- Duncan H, Hutchison J, Parshuram CS. The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care 2006;21:271-8. [Crossref] [PubMed]
- Skaletzky SM, Raszynski A, Totapally BR. Validation of a modified pediatric early warning system score: a retrospective case–control study. Clin Pediatr (Phila) 2012;51:431-5. [Crossref] [PubMed]
- Trubey R, Huang C, Lugg-Widger FV, et al. Validity and effectiveness of pediatric early warning systems and track and trigger tools for identifying and reducing clinical deterioration in hospitalised children: a systematic review. BMJ Open 2019;9:e022105. [Crossref] [PubMed]
- Seiger N, Maconochie I, Oostenbrink R, et al. Validity of different pediatric early warning scores in the emergency department. Pediatrics 2013;132:e841-50. [Crossref] [PubMed]
- Fleming S, Thompson M, Stevens R, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 2011;377:1011-8. [Crossref] [PubMed]
- O’Leary F, Hayen A, Lockie F, et al. Defining normal ranges and centiles for heart and respiratory rates in infants and children: a cross-sectional study of patients attending an Australian tertiary hospital pediatric emergency department. Arch Dis Child 2015;100:733-7. [Crossref] [PubMed]
- Bonafide CP, Brady PW, Keren R, et al. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics 2013;131:e1150. [Crossref] [PubMed]
- Advanced Life Support Group. Advanced Pediatric Life Support: The Practical Approach. 4th ed. Wiley Blackwell, 2004.
- American Heart Association. Pediatric Advanced Life Support Provider Manual. American Heart Association, 2006.
- Goel VV, Poole SF, Longhurst CA, et al. Safety analysis of proposed data‐driven physiologic alarm parameters for hospitalized children. J Hosp Med 2016;11:817-23. [Crossref] [PubMed]
- Chong SL, Ong GY, Chin WY, et al. A retrospective review of vital signs and clinical outcomes of febrile infants younger than 3 months old presenting to the emergency department. PLoS One 2018;13:e0190649. [Crossref] [PubMed]
- Eytan D, Goodwin AJ, Greer R, et al. Distributions and behavior of vital signs in critically ill children by admission diagnosis. Pediatr Crit Care Med 2018;19:115-24. [Crossref] [PubMed]
- Task Force of the European Society of Cardiology. the North American Society of Pacing and Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996;93:1043-65. [Crossref] [PubMed]
- Badke CM, Marsillio LE, Weese-Mayer DE, et al. Autonomic Nervous System Dysfunction in Pediatric Sepsis. Front Pediatr 2018;6:280. [Crossref] [PubMed]
- Goldstein B, Fiser DH, Kelly MM, et al. Decomplexification in critical illness and injury: relationship between heart rate variability, severity of illness, and outcome. Crit Care Med 1998;26:352-7. [Crossref] [PubMed]
- Wu L, Jiang Z, Li C, et al. Prediction of heart rate variability on cardiac sudden death in heart failure patients: a systematic review. Int J Cardiol 2014;174:857-60. [Crossref] [PubMed]
- Fauchier L, Babuty D, Cosnay P, et al. Prognostic value of heart rate variability for sudden death and major arrhythmic events in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 1999;33:1203-7. [Crossref] [PubMed]
- Mäkikallio TH, Huikuri HV, Mäkikallio A, et al. Prediction of sudden cardiac death by fractal analysis of heart rate variability in elderly subjects. J Am Coll Cardiol 2001;37:1395-402. [Crossref] [PubMed]
- Kleiger RE, Miller JP, Bigger JT Jr, et al. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987;59:256-62. [Crossref] [PubMed]
- Tapanainen JM, Thomsen PE, Køber L, et al. Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. Am J Cardiol 2002;90:347-52. [Crossref] [PubMed]
- Dekker JM, Crow RS, Folsom AR, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Circulation 2000;102:1239-44. [Crossref] [PubMed]
- Kubota Y, Chen LY, Whitsel EA, et al. Heart rate variability and lifetime risk of cardiovascular disease: the Atherosclerosis Risk in Communities Study. Ann Epidemiol 2017;27:619-25.e2. [Crossref] [PubMed]
- Heldeweg ML, Liu N, Koh ZX, et al. A novel cardiovascular risk stratification model incorporating ECG and heart rate variability for patients presenting to the emergency department with chest pain. Crit Care 2016;20:179. [Crossref] [PubMed]
- Sakamoto JT, Liu N, Koh ZX, et al. Integrating heart rate variability, vital signs, electrocardiogram, and troponin to triage chest pain patients in the ED. Am J Emerg Med 2018;36:185-92. [Crossref] [PubMed]
- Fujimoto Y, Fukuki M, Hoshio A, et al. Decreased heart rate variability in patients with diabetes mellitus and ischemic heart disease. Jpn Circ J 1996;60:925-32. [Crossref] [PubMed]
- Schönauer M, Thomas A, Morbach S, et al. Cardiac autonomic diabetic neuropathy. Diab Vasc Dis Res 2008;5:336-44. [Crossref] [PubMed]
- Pagani M, Malfatto G, Pierini S, et al. Spectral analysis of heart rate variability in the assessment of autonomic diabetic neuropathy. J Auton Nerv Syst 1988;23:143-53. [Crossref] [PubMed]
- Anderson TA. Heart rate variability: implications for perioperative anesthesia care. Curr Opin Anaesthesiol 2017;30:691-7. [Crossref] [PubMed]
- Samsudin MI, Liu N, Prabhakar SM, et al. A novel heart rate variability based risk prediction model for septic patients presenting to the emergency department. Medicine (Baltimore) 2018;97:e10866. [Crossref] [PubMed]
- Chiew CJ, Liu N, Tagami T, et al. Heart rate variability based machine learning models for risk prediction of suspected sepsis patients in the emergency department. Medicine (Baltimore) 2019;98:e14197. [Crossref] [PubMed]
- Chen WL, Kuo CD. Characteristics of heart rate variability can predict impending septic shock in emergency department patients with sepsis. Acad Emerg Med 2007;14:392-7. [Crossref] [PubMed]
- Bonjorno Junior JC, Caruso FR, Mendes RG, et al. Noninvasive measurements of hemodynamic, autonomic and endothelial function as predictors of mortality in sepsis: A prospective cohort study. PLoS One 2019;14:e0213239. [Crossref] [PubMed]
- Barnaby D, Ferrick K, Kaplan DT, et al. Heart rate variability in emergency department patients with sepsis. Acad Emerg Med 2002;9:661-70. [Crossref] [PubMed]
- Barnaby DP, Fernando SM, Ferrick KJ, et al. Use of the low-frequency/high-frequency ratio of heart rate variability to predict short-term deterioration in emergency department patients with sepsis. Emerg Med J 2018;35:96-102. [Crossref] [PubMed]
- Pong JZ, Fook-Chong S, Koh ZX, et al. Combining Heart Rate Variability with Disease Severity Score Variables for Mortality Risk Stratification in Septic Patients Presenting at the Emergency Department. Int J Environ Res Public Health 2019;16:1725. [Crossref] [PubMed]
- Roussel B, Behar J, Oster J. A Recurrent Neural Network for the Prediction of Vital Sign Evolution and Sepsis in ICU. In2019 Computing in Cardiology (CinC) 2019 IEEE.
- Kamaleswaran R, Akbilgic O, Hallman MA, et al. Applying artificial intelligence to identify physiomarkers predicting severe sepsis in the PICU. Pediatr Crit Care Med 2018;19:e495-503. [Crossref] [PubMed]
- van Wyk F, Khojandi A, Kamaleswaran R, et al. How much data should we collect? A case study in sepsis detection using deep learning. In 2017 IEEE Healthcare Innovations and Point of Care Technologies (HI-POCT) 2017.
- van Wyk F, Khojandi A, Mohammed A, et al. A minimal set of physiomarkers in continuous high frequency data streams predict adult sepsis onset earlier. Int J Med Inform 2019;122:55-62. [Crossref] [PubMed]
- Shashikumar SP, Stanley MD, Sadiq I, et al. Early sepsis detection in critical care patients using multiscale blood pressure and heart rate dynamics. J Electrocardiol 2017;50:739-43. [Crossref] [PubMed]
- Ruminski CM, Clark MT, Lake DE, et al. Impact of predictive analytics based on continuous cardiorespiratory monitoring in a surgical and trauma intensive care unit. J Clin Monit Comput 2019;33:703-11. [Crossref] [PubMed]
- Arbo JE, Lessing JK, Ford WJ, et al. Heart rate variability measures for prediction of severity of illness and poor outcome in ED patients with sepsis. Am J Emerg Med 2020:S0735-6757(20)30012-7.
- de Castilho FM, Ribeiro AL, Nobre V, et al. Heart rate variability as predictor of mortality in sepsis: A systematic review. PLoS One 2018;13:e0203487. [Crossref] [PubMed]
- Quinten VM, van Meurs M, Renes MH, et al. Protocol of the sepsivit study: a prospective observational study to determine whether continuous heart rate variability measurement during the first 48 hours of hospitalisation provides an early warning for deterioration in patients presenting with infection or sepsis to the emergency department of a Dutch academic teaching hospital. BMJ Open 2017;7:e018259. [Crossref] [PubMed]
- Wynn JL, Wong HR, Shanley TP, et al. Time for a neonatal-specific consensus definition for sepsis. Pediatr Crit Care Med 2014;15:523. [Crossref] [PubMed]
- Wynn JL, Polin RA. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr Res 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Wynn JL, Polin RA. Progress in the management of neonatal sepsis: the importance of a consensus definition. Pediatr Res 2018;83:13-5. [Crossref] [PubMed]
- Joshi R, Kommers D, Oosterwijk L, et al. Predicting Neonatal Sepsis Using Features of Heart Rate Variability, Respiratory Characteristics and ECG-Derived Estimates of Infant Motion. IEEE J Biomed Health Inform 2020;24:681-92. [Crossref] [PubMed]
- Moorman JR, Carlo WA, Kattwinkel J, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J Pediatr 2011;159:900-6.e1. [Crossref] [PubMed]
- Griffin MP, O’Shea TM, Bissonette EA, et al. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr Res 2003;53:920-6. [Crossref] [PubMed]
- Griffin MP, Lake DE, Bissonette EA, et al. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics 2005;116:1070-4. [Crossref] [PubMed]
- Fairchild KD, Schelonka RL, Kaufman DA, et al. Septicemia mortality reduction in neonates in a heart rate characteristics monitoring trial. Pediatr Res 2013;74:570-5. [Crossref] [PubMed]
- Bohanon FJ, Mrazek AA, Shabana MT, et al. Heart rate variability analysis is more sensitive at identifying neonatal sepsis than conventional vital signs. Am J Surg 2015;210:661-7. [Crossref] [PubMed]
- Nguyen N, Vandenbroucke L, Hernandez A, et al. Early‐onset neonatal sepsis is associated with a high heart rate during automatically selected stationary periods. Acta Paediatr 2017;106:749-54. [Crossref] [PubMed]
- Ellenby MS, McNames J, Lai S, et al. Uncoupling and recoupling of autonomic regulation of the heart beat in pediatric septic shock. Shock 2001;16:274-7. [Crossref] [PubMed]
- Amiri P, Derakhshan A, Gharib B, et al. Identifying Optimal Features from Heart Rate Variability for Early Detection of Sepsis in Pediatric Intensive Care. Conf Proc IEEE Eng Med Biol Soc 2019;2019:1425-8.
- Eytan D, Jegatheeswaran A, Mazwi ML, et al. Temporal variability in the sampling of vital sign data limits the accuracy of patient state estimation. Pediatr Crit Care Med 2019;20:e333-41. [Crossref] [PubMed]
- Coggins SA, Weitkamp JH, Grunwald L, et al. Heart rate characteristic index monitoring for bloodstream infection in an NICU: a 3-year experience. Arch Dis Child Fetal Neonatal Ed 2016;101:F329-32. [Crossref] [PubMed]
- Sameshima H, Ikenoue T, Ikeda T, et al. Association of nonreassuring fetal heart rate patterns and subsequent cerebral palsy in pregnancies with intrauterine bacterial infection. Am J Perinatol 2005;22:181-7. [Crossref] [PubMed]