
Diabetes induces hepatocyte pyroptosis by promoting oxidative stress-mediated NLRP3 inflammasome activation during liver ischaemia and reperfusion injury
Introduction
Hepatic ischaemia and reperfusion (IR) injury has been classified into cold and warm ischaemia. The occurrence of cold ischaemia is associated with the preservation and storage of organs prior to transplantation (1,2). Warm ischaemia is associated with shock, trauma, transplantation, and robotic liver surgery, during which a temporary interruption of the blood supply may occur. The mechanism of warm hepatic IR injury is complex. Some studies suggest that tissue inflammatory immune response and oxidative stress play key roles in the pathogenesis of warm liver IR injury (3,4).
Diabetes is a universal disease, and almost a quarter of patients undergoing liver transplantation have pre-existing diabetes mellitus (5). Pre-transplant diabetes can predict poor prognosis after transplant (6). Higher risk of rejection has been found in diabetic patients post liver transplant (7). Diabetes is also a major risk factor associated with the heart (8), brain (9), kidney (10), and liver ischemic injury (11,12). The inflammatory response caused by immune activation plays a major role in transplant rejection. The most significant sign of diabetes is hyperglycaemia and it has been proved to cause chronic inflammation (13). Markers of diabetes, such as hyperlipidaemia and hyperglycaemia, have been shown to promote the phenotype of inflammatory macrophages (14). In addition, under inflammatory conditions, hyperglycaemic mice have exhibited a growing number of macrophage in the liver, kidney, intestine, and peritoneal cavity (15). Studies have indicated that hepatic IR injury increases NOD-like receptor family pyrin domain-containing 3 protein (NLRP3) inflammasome activation (16,17). Our previous research proved that hyperglycaemia promotes the activation of NLRP3 inflammasome in liver-resident macrophage and then aggravates acute liver injury. Furthermore, the excessive reactive oxygen species (ROS) and following disorder of redox balance also play an important role in liver ischemic injury. Various liver cells can produce ROS post IR-stress (16). Whether diabetes increases NLRP3 inflammasome activation during warm hepatic IR injury and the effects of ROS on murine hepatic IR injury remain unknown.
Herein, we found that diabetes induces hepatocyte pyroptosis by promoting oxidative stress-mediated NLRP3 inflammasome activation during liver IR injury. The ROS antagonist NAC could mitigate hepatic IR injury by suppressing NLRP3 inflammasome activation. Strategies targeting ROS and NLRP3 inflammasome activation would be beneficial for preventing liver IR injury in diabetic patients. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1839).
Methods
Animals
Male C57BL/KsJ-leprdb/leprdb (db/db) and C57BL/6 control mice (eight-week-old) were provided by the Model Animal Research Center of Nanjing University. The Institutional Animal Care and Use Committee of Nanjing Medical University approved this animal experiment protocol (protocol number NMU08-092).
Mouse liver partial warm IR model
Mice were randomly separated into two groups: sham group and IR group (n=6 mice/group). Inhaled isoflurane (1.5%) was used to anesthetize mice. A midline laparotomy was performed on the mice. Seventy percent of the hepatic portal blood supply was blocked for 90 min by an atraumatic clip. The clip was then removed to allow hepatic reperfusion. The same procedure was performed in sham controls but without the step of vascular occlusion. Serum and liver tissues were collected for further analysis (18). CY09 (2.5 mg/kg), N-Acetyl-L-cysteine (NAC, 150 mg/kg, MedChemExpress, Monmouth Junction, MN, USA), or the vehicle control, was intraperitoneally administered 1 h prior to ischaemia.
Serum biochemical measurements
Serum alanine aminotransaminase (sALT) and aspartate aminotransaminase (sAST) were detected using an AU5400 automated chemical analyser (Olympus, Tokyo, Japan).
Histopathology
Liver sections (4 µm) were stained with haematoxylin and eosin (H&E), oil red o, and Masson. The severity of liver injury was scored according to Suzuki’s criteria as described previously (19).
Transferase dUTP nick end labelling (TUNEL) staining
A fluorescence detection kit (Roche, Basel, Switzerland) were used for terminal deoxynucleotidyl TUNEL staining according to the manufacturer’s protocols.
Measurement of measurement of malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD) and ROS levels
MDA, GSH, SOD and ROS were conducted by using commercial kits. Liver tissues were washed with PBS, homogenized in lysis buffer and sonicated. After being sonicated, the lysed tissue was centrifuged (10,000 ×g, 10 min) to remove debris and retain the supernatant. A microplate reader was used to measure the levels of MDA, GSH, SOD and ROS in the supernatant. In addition, MDA, GSH, SOD and ROS levels were normalized according to the protein concentration. Two-step collagenase perfusion was used to obtain mouse primary hepatocytes (20).With reference to the specification, the DHR123 fluorescent probe was used to detect the content of ROS-producing cells by flow cytometry.
Western blot analysis
The protein (20 µg per sample) extracted from liver tissues. Next liver proteins were electrophoresed. Primary antibodies directed against NLRP3, pro-caspasae-1, caspase-3, cleaved caspase-1/3/7/9, p-p65, p-IκBα and GAPDH (Cell Signaling Technology, San Diego, CA, USA) were used.
Quantitative RT-PCR analysis (qRT-PCR)
Total RNA extract from liver tissue. RNA from each sample was reversetranscribed into first-strand cDNA by using a Prime Script RT reagent Kit (Takara Bio). Quantitative PCR was performed using the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) (21). Each experiment was repeated independently three times. Target gene expression was analyzed by the ratio to the hypoxanthine phosphoribosyl transferase (HPRT).
ELISA
Interleukin-1β (IL-1β), TNF-α and IL-6 levels in sera were measured using an ELISA kit (eBiosciences, San Diego, CA, USA) according to the manufacturer’s protocols.
Statistical analysis
Data are expressed as the mean ± SEM. Two-group comparisons were performed using a t-test. And for multiple group comparisons, ANOVA with Bonferroni corrections using STAT software, version 11.0 was used. P values <0.05 were considered statistically significant.
Results
Diabetes exacerbates liver IR injury
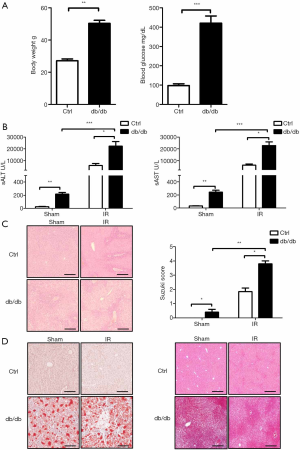
Db/db mice at 8 weeks of age displayed obesity and had an increased body weight compared with C57BL/6 mice (50.3±1.9 vs. 27.1±1.1 g). Blood glucose was higher in db/db than C57BL/6 mice (420±37 vs. 98±9 mg/dL) (Figure 1A). Firstly we assessed whether liver IR injury is exacerbated in db/db mice. A 90-min liver ischaemia or sham procedure was performed on db/db and C57BL/6 (control) mice, and then reperfused for 6 h. We compared liver injury among these groups. Certainly, the db/db mouse group showed meaningfully higher sALT and sAST levels (Figure 1B) and less-well preserved liver architecture with higher Suzuki scores than the sham mouse group (Figure 1C) than the control group. Through oil red o and Masson staining, the fat accumulation and fibrosis levels in liver tissues of db/db mice were significantly higher than those of the control group, but IR injury did not affect this difference (Figure 1D). These results demonstrate that diabetes exacerbates liver IR injury.
Diabetes exacerbates liver IR damage by activating NLRP3 inflammasome and hepatocyte pyroptosis
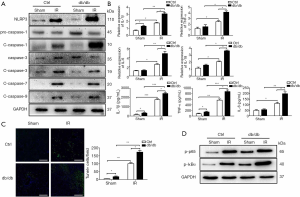
To assess the effect of diabetes associated with liver IR injury, we compared activation of NLRP3 inflammasome in different groups. Interestingly, protein levels of NLRP3 and cleaved caspase-1 were fairly increased in liver tissue isolated from db/db mice after 6 h of IR injury (Figure 2A). At the same time, the activation of caspase-3/7/9 was also detected and found to increase after IR, but no significantly differences were observed between db/db and control mice. The livers of db/db mice also had increased IL-1β, TNF-α, IL-6 and IL-18 gene induction and TUNEL-positive hepatocytes (Figure 2B,C). In addition we analyzed the activation of NF-κB by western blot and found that increased NF-κB activation in db/db diabetic mice (Figure 2D). These results suggest that there is an increase in apoptosis in both db/db and control mice, but NF-κB-mediated activation of NLRP3 and cell pyrolysis play a more important role. Thus, diabetes induces activation of NLRP3 inflammasome and hepatocyte pyroptosis in liver IR injury.
Diabetes induces hepatocyte pyroptosis by promoting NLRP3 inflammasome activation during liver IR injury
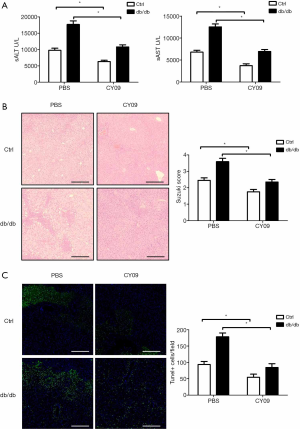
CY09 was used to obstruct NLRP3 inflammasome activation in order to research the function of enhanced activating NLRP3 inflammasome in diabetes during liver IR injury. Compared to the control treatment, NLRP3 inhibition prevented liver IR injury in db/db mice, as illustrated by decreased sALT and sAST levels (Figure 3A) and reduced liver structural damage (Figure 3B). CY09 pretreatment also reduced the quantity of TUNEL-positive hepatocytes (Figure 3C). These results suggest that diabetes induces pyroptosis in hepatocytes by promoting NLRP3 inflammasome activation during liver IR injury.
Oxidative stress induces hepatocyte pyrolysis by promoting NLRP3 inflammasome activation during diabetic mouse liver IR injury
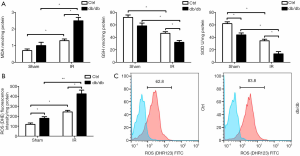
Considering oxidative stress is the essential mechanism of liver IR injury, we studied the function of oxidative stress during liver IR injury in db/db mouse. Liver IR injury induced significant increases in the MDA level and reduced the GSH content and SOD activity in db/db mouse livers compared to the control mouse livers (Figure 4A). Then, we further detected ROS levels in liver tissue. Through DHE fluorescence and DHR123 fluorescence probe, the ROS level and the proportion of ROS-producing cells in the liver tissue of db/db mice were significantly increased (Figure 4B,C). Liver IR injury significantly elevated the ROS content in the db/db mouse groups.
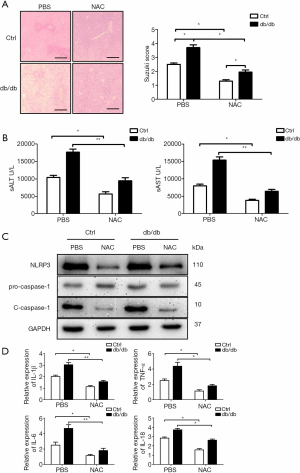
To explore the functional role of ROS in liver IR injury in diabetic mice, NAC was utilized to protect against oxidative stress. NAC pretreatment significantly alleviated liver injury in db/db mice, as indicated by the reduced liver architecture damage and Suzuki scores (Figure 5A) and decreased sALT and sAST levels (Figure 5B). Moreover, activation of the NLRP3 inflammasome was decreased in liver tissue of db/db mice after liver IR injury due to NAC pretreatment, as shown by the decreased protein expression levels of NLRP3 and cleaved caspase-1 (Figure 5C), and gene expression levels of IL-1β, TNF-α, IL-6 and IL-18 (Figure 5D). These results demonstrate that diabetes induces pyroptosis in hepatocytes by promoting oxidative stress-mediated NLRP3 inflammasome activation during liver IR injury.
Discussion
Diabetes mellitus is a significant public health problem that threatens people around the world (22,23). Diabetes is a chronic condition that causes various complications, including atherosclerosis (24), diabetic nephropathy (25), diabetic retinopathy (26), and neural damage (22). Some studies have suggested that diabetes aggravates myocardial IR injury (27,28). Other studies have demonstrated that diabetes enhances renal IR injury (29-31). Our study addressed the questions of whether and how diabetes affects warm hepatic IR injury.
Two different stages of tissue injury have been defined in liver IR injury. Oxygen and nutrition depletion caused by ischemia result in direct injury of hepatocytes, which triggers activation of various immune cells and subsequent inflammatory tissue injury. In previous studies, we have reported strategies to protect against liver IR injury by targeting both the parenchymal liver cells (32) and proinflammatory immune activation (19,33-35).
ROS play a critical role in maintaining normal cellular physiological functions, including cellular development, growth, and differentiation. ROS are maintained in cells at baseline levels that maintain cell proliferation and metabolism. Meanwhile ROS also play the key role to regulate many significant regulatory and metabolic pathways as signal transduction molecules in cells (36). Essential roles of oxidative stress in the occurrence of diabetes complications have been reported in recent studies (37,38). Studies have shown that hyperglycaemia triggers ROS formation in macrophages (22,39,40). Under diabetic conditions, the endothelium secretes monocyte chemoattractant protein-1 (MCP-1) and attracts monocytes (39). Migrating monocytes differentiate into macrophages and produce high levels of ROS, exacerbating inflammation and tissue damage (39). Other studies have also shown that macrophage mitochondrial dysfunction and abnormal activation of cytoplasmic NADPH oxidase (NOX) can increase ROS production under hyperglycemic conditions (41,42). Contrary to the regulation of ROS in the antimicrobial response, the metabolic ROS production in diabetics is more unstable and maladjusted (43). Studies have indicated that hepatic IR injury increases ROS production (16,44). Our study focused on the role of ROS in murine hepatic IR injury accompanied by diabetes.
Various pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) released from injured cells could activate NLRP3 and promote caspase-1 and IL-1β activation (16,45). Studies have shown that hepatic IR injury activates the NLRP3 inflammasome (16,17,46). Additionally, one study demonstrated that the production of intracellular ROS induces NLRP3 translocation to the cytoplasm from the nucleus in LPS-treated neonatal rat cardiomyocytes. NLRP3 cytoplasmic translocation is prevented by elimination of ROS (47). In a previous study, we demonstrated that hyperglycemia aggravated acute liver injury by promoting NLRP3 inflammasome activation in liver-resident macrophages (48). NLRP3 inflammasome inhibition protected the liver from liver damage, inflammation and steatosis of experimental steatohepatitis with diabetes (49). Although increasing evidence indicates that NLRP3 inflammasome activation plays an important role in liver IR injury in the setting of hyperglycemia/diabetes, the precise effects of NLRP3 regulation by hyperglycemia/diabetes on hepatocellular pyroptosis remains largely unclear.
According to the above studies, we investigated how diabetes aggravates hepatic IR injury. We first performed liver IR experiments. The results showed that diabetes exacerbated hepatic IR injury. It has been reported that the perioperative hyperglycemia/diabetes resulted in a poor organ function and increased the rate of liver graft rejection in patients post liver transplantation, which could be improved by intensive insulin treatment (50-52). However, we did not analyze the impact of hyperglycemia/diabetes on liver IR injury in humans. The results also suggested that NLRP3 inflammasome activation and hepatocyte pyroptosis were increased in diabetic livers post IR. The NLRP3 inflammasome antagonist CY09 alleviated hepatic injury. Further study demonstrated a significant increase in ROS expression in livers from db/db mice at 6 h after reperfusion. The ROS antagonist NAC suppressed the activation of NLRP3 inflammasome and hepatocyte pyroptosis and attenuated hepatic IR injury. These results show that diabetes aggravates hepatic IR injury by enhancing ROS expression and increasing NLRP3 inflammasome activation.
In conclusion, our results demonstrate that diabetes induces hepatocyte pyroptosis by promoting oxidative stress-mediated NLRP3 inflammasome activation during liver IR injury, providing a promising strategy for attenuating liver injury in patients with diabetes undergoing hepatic trauma, resection and transplantation.
Acknowledgments
Funding: This study was supported by grants from the National Natural Science Foundation of China (81971495, 81600450, 81901628), CAMS Innovation Fund for Medical Sciences (No. 2019-I2M-5-035), the National Science Foundation of Jiangsu Province (BK20191490) and the Foundation of Jiangsu Collaborative Innovation Center of Biomedical Functional Materials.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1839
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1839
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-1839
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1839). LL serves as an unpaid Associate Editor-in-Chief of Annals of Translational Medicine from Jun 2019 to May 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animals received humane care, and all animal procedures met the relevant legal and ethical requirements according to a protocol (number NMU08-092) approved by the Institutional Animal Care and Use Committee of Nanjing Medical University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li J, Li RJ, Lv GY, et al. The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci 2015;19:2036-47. [PubMed]
- von Heesen M, Muller S, Keppler U, et al. Preconditioning by cilostazol protects against cold hepatic ischemia-reperfusion injury. Ann Transplant 2015;20:160-8. [Crossref] [PubMed]
- Rao J, Yue S, Fu Y, et al. ATF6 mediates a pro-inflammatory synergy between ER stress and TLR activation in the pathogenesis of liver ischemia-reperfusion injury. Am J Transplant 2014;14:1552-61. [Crossref] [PubMed]
- Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant 2011;11:1563-9. [Crossref] [PubMed]
- Kim WR, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual Data Report: liver. Am J Transplant 2014;14 Suppl 1:69-96. [Crossref] [PubMed]
- Li P, Fan H, He Q. Pretransplant diabetes mellitus predicts worse outcomes of liver transplantation: evidence from meta-analysis. J Endocrinol Invest 2018;41:211-21. [Crossref] [PubMed]
- Ramos-Prol A, Hervas-Marin D, Garcia-Castell A, et al. Outcomes in patients with diabetes 10 years after liver transplantation. J Diabetes 2017;9:1033-9. [Crossref] [PubMed]
- Zhao D, Yang J, Yang L. Insights for Oxidative Stress and mTOR Signaling in Myocardial Ischemia/Reperfusion Injury under Diabetes. Oxid Med Cell Longev 2017;2017:6437467.
- Shukla V, Shakya AK, Perez-Pinzon MA, et al. Cerebral ischemic damage in diabetes: an inflammatory perspective. J Neuroinflammation 2017;14:21. [Crossref] [PubMed]
- Shen X, Hu B, Xu G, et al. Activation of Nrf2/HO-1 Pathway by Glycogen Synthase Kinase-3beta Inhibition Attenuates Renal Ischemia/Reperfusion Injury in Diabetic Rats. Kidney Blood Press Res 2017;42:369-78. [Crossref] [PubMed]
- Yue S, Zhou HM, Zhu JJ, et al. Hyperglycemia and liver ischemia reperfusion injury: a role for the advanced glycation endproduct and its receptor pathway. Am J Transplant 2015;15:2877-87. [Crossref] [PubMed]
- Zhang Y, Yuan D, Yao W, et al. Hyperglycemia Aggravates Hepatic Ischemia Reperfusion Injury by Inducing Chronic Oxidative Stress and Inflammation. Oxid Med Cell Longev 2016;2016:3919627.
- Chang SC, Yang WV. Hyperglycemia, tumorigenesis, and chronic inflammation. Crit Rev Oncol Hematol 2016;108:146-53. [Crossref] [PubMed]
- Ahmed M, de Winther MPJ, Van den Bossche J. Epigenetic mechanisms of macrophage activation in type 2 diabetes. Immunobiology 2017;222:937-43. [Crossref] [PubMed]
- Niu S, Bian Z, Tremblay A, et al. Broad Infiltration of Macrophages Leads to a Proinflammatory State in Streptozotocin-Induced Hyperglycemic Mice. J Immunol 2016;197:3293-301. [Crossref] [PubMed]
- Yue S, Zhu J, Zhang M, et al. The myeloid heat shock transcription factor 1/beta-catenin axis regulates NLR family, pyrin domain-containing 3 inflammasome activation in mouse liver ischemia/reperfusion injury. Hepatology 2016;64:1683-98. [Crossref] [PubMed]
- Li C, Jin Y, Wei S, et al. Hippo Signaling Controls NLR Family Pyrin Domain Containing 3 Activation and Governs Immunoregulation of Mesenchymal Stem Cells in Mouse Liver Injury. Hepatology 2019;70:1714-31. [Crossref] [PubMed]
- Zhou H, Wang H, Ni M, et al. Glycogen synthase kinase 3beta promotes liver innate immune activation by restraining AMP-activated protein kinase activation. J Hepatol 2018;69:99-109. [Crossref] [PubMed]
- Yue S, Zhou H, Wang X, et al. Prolonged Ischemia Triggers Necrotic Depletion of Tissue-Resident Macrophages To Facilitate Inflammatory Immune Activation in Liver Ischemia Reperfusion Injury. J Immunol 2017;198:3588-95. [Crossref] [PubMed]
- Mudra DR, Parkinson A. Preparation of hepatocytes. Curr Protoc Toxicol 2001;Chapter 14:Unit 14.2.
- Wei S, Zhou H, Wang Q, et al. RIP3 deficiency alleviates liver fibrosis by inhibiting ROCK1-TLR4-NF-kappaB pathway in macrophages. FASEB J 2019;33:11180-93. [Crossref] [PubMed]
- Rendra E, Riabov V, Mossel DM, et al. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019;224:242-53. [Crossref] [PubMed]
- Kim CH. Microbiota or short-chain fatty acids: which regulates diabetes? Cell Mol Immunol 2018;15:88-91. [Crossref] [PubMed]
- Gray SP, Di Marco E, Okabe J, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013;127:1888-902. [Crossref] [PubMed]
- Bichet DG, Lussier Y. Mice deficient for ERAD machinery component Sel1L develop central diabetes insipidus. J Clin Invest 2017;127:3591-3. [Crossref] [PubMed]
- Retnakaran R, Shah BR. Role of Type 2 Diabetes in Determining Retinal, Renal, and Cardiovascular Outcomes in Women With Previous Gestational Diabetes Mellitus. Diabetes Care 2017;40:101-8. [Crossref] [PubMed]
- Baumgardt SL, Paterson M, Leucker TM, et al. Chronic Co-Administration of Sepiapterin and L-Citrulline Ameliorates Diabetic Cardiomyopathy and Myocardial Ischemia/Reperfusion Injury in Obese Type 2 Diabetic Mice. Circ Heart Fail 2016;9:e002424. [Crossref] [PubMed]
- Ge ZD, Li Y, Qiao S, et al. Failure of Isoflurane Cardiac Preconditioning in Obese Type 2 Diabetic Mice Involves Aberrant Regulation of MicroRNA-21, Endothelial Nitric-oxide Synthase, and Mitochondrial Complex I. Anesthesiology 2018;128:117-29. [Crossref] [PubMed]
- Muroya Y, He X, Fan L, et al. Enhanced renal ischemia-reperfusion injury in aging and diabetes. Am J Physiol Renal Physiol 2018;315:F1843-54. [Crossref] [PubMed]
- Yang YY, Gong DJ, Zhang JJ, et al. Diabetes aggravates renal ischemia-reperfusion injury by repressing mitochondrial function and PINK1/Parkin-mediated mitophagy. Am J Physiol Renal Physiol 2019;317:F852-64. [Crossref] [PubMed]
- Muratsubaki S, Kuno A, Tanno M, et al. Suppressed autophagic response underlies augmentation of renal ischemia/reperfusion injury by type 2 diabetes. Sci Rep 2017;7:5311. [Crossref] [PubMed]
- Rao Z, Pan X, Zhang H, et al. Isoflurane Preconditioning Alleviated Murine Liver Ischemia and Reperfusion Injury by Restoring AMPK/mTOR-Mediated Autophagy. Anesth Analg 2017;125:1355-63. [Crossref] [PubMed]
- Rao Z, Sun J, Pan X, et al. Hyperglycemia Aggravates Hepatic Ischemia and Reperfusion Injury by Inhibiting Liver-Resident Macrophage M2 Polarization via C/EBP Homologous Protein-Mediated Endoplasmic Reticulum Stress. Front Immunol 2017;8:1299. [Crossref] [PubMed]
- Lu L, Yue S, Jiang L, et al. Myeloid Notch1 deficiency activates the RhoA/ROCK pathway and aggravates hepatocellular damage in mouse ischemic livers. Hepatology 2018;67:1041-55. [Crossref] [PubMed]
- Yang H, Zhou H, Zhuang L, et al. Plasma membrane-bound G protein-coupled bile acid receptor attenuates liver ischemia/reperfusion injury via the inhibition of toll-like receptor 4 signaling in mice. Liver Transpl 2017;23:63-74. [Crossref] [PubMed]
- Zandalinas SI, Mittler R. ROS-induced ROS release in plant and animal cells. Free Radic Biol Med 2018;122:21-7. [Crossref] [PubMed]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058-70. [Crossref] [PubMed]
- Rajesh M, Mukhopadhyay P, Batkai S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol 2010;56:2115-25. [Crossref] [PubMed]
- Tesch GH. Role of macrophages in complications of type 2 diabetes. Clinical and experimental pharmacology & physiology 2007;34:1016-9. [Crossref] [PubMed]
- Wang Q, Wei S, Zhou H, et al. Hyperglycemia exacerbates acetaminophen-induced acute liver injury by promoting liver-resident macrophage proinflammatory response via AMPK/PI3K/AKT-mediated oxidative stress. Cell Death Discov 2019;5:119. [Crossref] [PubMed]
- Widlansky ME, Wang J, Shenouda SM, et al. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl Res 2010;156:15-25. [Crossref] [PubMed]
- Huang X, Sun M, Li D, et al. Augmented NADPH oxidase activity and p22phox expression in monocytes underlie oxidative stress of patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2011;91:371-80. [Crossref] [PubMed]
- Kumar P, Swain MM, Pal A. Hyperglycemia-induced inflammation caused down-regulation of 8-oxoG-DNA glycosylase levels in murine macrophages is mediated by oxidative-nitrosative stress-dependent pathways. Int J Biochem Cell Biol 2016;73:82-98. [Crossref] [PubMed]
- Nakamura K, Kageyama S, Kaldas FM, et al. Hepatic CEACAM1 expression indicates donor liver quality and prevents early transplantation injury. J Clin Invest 2020. [Crossref] [PubMed]
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol 2009;27:229-65. [Crossref] [PubMed]
- Zhu H, Cao X. NLR members in inflammation-associated carcinogenesis. Cell Mol Immunol 2017;14:403-5. [Crossref] [PubMed]
- Li N, Zhou H, Wu H, et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol 2019;24:101215. [Crossref] [PubMed]
- Wang Q, Wei S, Zhou S, et al. Hyperglycemia aggravates acute liver injury by promoting liver-resident macrophage NLRP3 inflammasome activation via the inhibition of AMPK/mTOR-mediated autophagy induction. Immunol Cell Biol 2020;98:54-66. [Crossref] [PubMed]
- Leng W, Wu M, Pan H, et al. The SGLT2 inhibitor dapagliflozin attenuates the activity of ROS-NLRP3 inflammasome axis in steatohepatitis with diabetes mellitus. Ann Transl Med 2019;7:429. [Crossref] [PubMed]
- Ramos-Prol A, Hervas-Marin D, Rodriguez-Medina B, et al. Intensified blood glucose treatment in diabetic patients undergoing a liver transplant: impact on graft evolution at 3 months and at 5 years. J Endocrinol Invest 2018;41:821-9. [Crossref] [PubMed]
- Zant R, Melter M, Beck D, et al. Glucose Metabolism and Associated Outcome After Pediatric Liver Transplantation. Transplant Proc 2016;48:2709-13. [Crossref] [PubMed]
- Kang R, Han S, Lee KW, et al. Portland Intensive Insulin Therapy During Living Donor Liver Transplantation: Association with Postreperfusion Hyperglycemia and Clinical Outcomes. Sci Rep 2018;8:16306. [Crossref] [PubMed]


