
Rectal cancer patients with downstaging after neoadjuvant chemoradiotherapy and radical resection do not benefit from adjuvant chemotherapy
Introduction
For patients with locally advanced rectal cancer (LARC), total mesorectal excision (TME) and neoadjuvant chemoradiotherapy (NCRT) have been shown to significantly decrease the local recurrence rate and improve the overall survival (OS) rate (1). However, approximately 25–30% of patients still develop distant metastasis postoperatively (2,3). Adjuvant chemotherapy prevents and clears circulating tumor cells and micro-metastases, thereby reducing the risk of developing distant metastases. Current guidelines state that all patients with locally advanced rectal cancer who receive NCRT and radical resection should undergo adjuvant chemotherapy (4,5). However, recent research has supplied little evidence to suggest that adjuvant chemotherapy is beneficial for rectal cancer patients treated with NCRT and radical resection (6-9), especially for those who have already responded well to treatment (10,11). Currently, adjuvant chemotherapy is recommended for patients after NCRT and radical resection based on pretreatment clinical staging; however, pretreatment clinical staging can be inaccurate, and postoperative TNM staging is easier. Some researchers have suggested that adjuvant chemotherapy should be used selectively, based on the final pathological stage. According to the literature, pathological stage has a better predictive value than clinical stage or tumor regression classification in tumor prognosis (12-14). Patients with ypT0-2N0 rectal cancer are a subgroup that responds well to NCRT and have favorable oncological prognosis, with a 5-year disease-free survival (DFS) rate reaching 83–95% (12-14). Nevertheless, studies have shown that not all ypT0-2N0 rectal cancer patients benefit from adjuvant chemotherapy after NCRT and surgery, and controversy still surrounds the use of adjuvant chemotherapy for these patients (15-18), the prognostic factors of whom are rarely reported.
This study aimed to evaluate the effect of adjuvant chemotherapy on the oncological prognosis and prognostic factors of ypT0-2N0 rectal cancer patients after NCRT and radical resection.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1278).
Methods
Patients and evaluation before the treatment
This retrospective study was conducted at the Department of Colorectal Surgery, Changhai Hospital, Shanghai, China. The study was performed in accordance with the ethical standards of our institutional research committee, and the principles of the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from each individual participant included in the study.
The data of resectable locally advanced rectal cancer patients who received NCRT in the Department of Colorectal Surgery in Changhai Hospital between January, 2010 and June, 2018 were retrospectively analyzed. The inclusion criteria were: (I) low or middle rectal carcinoma (a distance of <10 cm between the inferior tumor edge and the anal verge); (II) pretreatment clinical stage was II/III; (III) no obvious distant metastasis; (IV) postoperative pathological results showed R0 resection; (V) pathological diagnosis of ypT0-2N0 after NCRT and radical resection; and (VI) completed neoadjuvant treatment and adjuvant treatment. The exclusion criteria were: (I) other malignant tumors present (except for locally advanced rectal cancers); (II) a history of malignant tumor or relapse; (III) managed by a watch-and-wait strategy after NCRT; or (IV) pathological results showed tumor deposits.
All of the patients underwent colonoscopy and pathological consultation before treatment to confirm the pathological diagnosis. Before treatment, chest computed tomography, magnetic resonance imaging, or computed tomography with intravenous contrast of the liver and pelvis were also performed for clinical staging. Clinicopathological classification and staging were based on the American Joint Committee on Cancer tumor-node-metastasis (TNM) staging system (19).
Treatment
All of the patients received intensity-modulated radiation therapy with concurrent oral administration of capecitabine: the total dosage was 45–50.4 Gy (1.8–2.0 Gy per time, 25–28 fractions). In the chemotherapy group (the chemo group), 48 patients underwent NCRT alone and 42 patients underwent combined chemotherapy. In the non-chemotherapy group (the non-chemo group), NCRT alone and combined chemotherapy was received by 18 and 13 patients, respectively.
The chemotherapy regimens included: oral capecitabine alone during radiotherapy (n=66) (825 mg/m2 orally, twice a day, 5 days a week for 5 weeks); CapeOx as consolidation chemotherapy (n=48) (oxaliplatin 130 mg/m2, intravenous infusion 2 h, day 1; capecitabine 1,000 mg/m2 orally, twice a day, 1–14 days, repeated every 3 weeks), FOLFOX as consolidation chemotherapy (n=7) (oxaliplatin 85 mg/m2 intravenous infusion for 2 h, day 1, LV 400 mg/m2 intravenous infusion for 2 h, day 1, 5-Fu 400 mg/m2 intravenous infusion, day 1, then 1,200 mg/m2/day × continuous intravenous infusion for 2 days).
All patients underwent radical total mesorectal excision (TME). The adjuvant chemo group comprised 90 patients (74.4%) including: (I) oral capecitabine (n=22); (II) CapeOx (n=59); (III) FOLFOX (n=9). The non-chemo group comprised 31 (25.6%) patients, including 8 patients who received fewer than 3 cycles of chemotherapy due to poor performance status, the other 23 patients in the non-chemo group did not receive adjuvant chemotherapy including 15 patients who had favorable pathology, 5 patients who refused chemotherapy, and 3 patients who experienced postoperative complications.
Follow up
Follow-up data were retrospectively obtained from the medical records. The follow-up ended on July 21, 2019. Each patient was followed-up every three months for the first two years, every six months for the next three years, and once a year thereafter. Digital rectal examination was performed and the levels of carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 were determined at every follow-up visit. Chest computed tomography, magnetic resonance imaging, or computed tomography with intravenous contrast of the liver and pelvis, and full colonoscopy were regularly undertaken. Disease-free survival was defined as the time between the surgery and tumor recurrence or distant metastasis. Overall survival was defined as the time between surgery and death or last follow-up.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics 22.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY, USA). Categorical values were reported as frequency and percentage, and continuous values were reported as the mean ± standard deviation (SD) or median with range, depending on whether the values were normally distributed or not. Categorical variables were statistically analyzed by the chi-square test and continuous variables were compared using the Student’s t-test or the Mann-Whitney test. Survival analysis was performed using the Kaplan-Meier curve method, and differences in survival between the groups were compared with the log-rank test. Multivariate analysis, using a Cox proportional hazard model, was performed to identify independent predictors of overall survival (OS) and disease-free survival (DFS). P<0.05 (two sided) was considered statistically significant.
Results
This study included 121 patients, of whom 88 (72.7%) were male and 33 (27.3%) were female. The patients had an average age of 57.0±10.8 (range, 25–81) years old. There were 14, 67, and 40 cases of cT2, cT3, cT4 patients, respectively; 66 cases underwent NCRT, and 55 cases underwent consolidation chemotherapy after NCRT with CapeOx or FOLFOX. The median interval between the end of radiotherapy and surgery was 8.9 weeks (range: 2.7–16 weeks). All of the patients received R0 resection with negative distal and circumferential margins. The results of postoperative pathology showed there were 47, 8, and 66 patients with ypT0, ypT1, and ypT2, respectively. There were 90 (74.4%) and 31 (25.6%) patients in the chemo group and non-chemo group, respectively. The age of patients in the chemo group was significantly lower than that in the non-chemo group (55.6±10.6 vs. 61.2±10.4 years, P=0.012). The incidence of anastomotic leakage in the non-chemo group was significantly higher than that in the chemo group (19.4% vs. 6.7%, P=0.042) (Table 1).
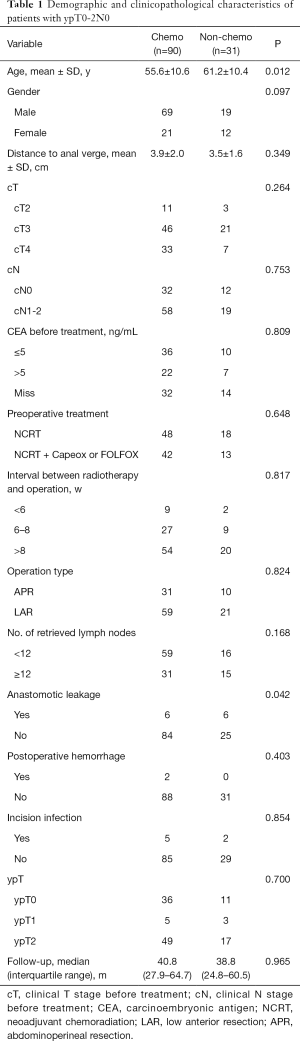
Full table
The median follow-up time for all patients was 40.1 months (IQR, 26.2–63.2). In the chemo and non-chemo groups, the median follow-up time was 40.4 (IQR, 27.9–64.7) and 39.2 (IQR, 24.8–60.5) months, respectively. There was no significant difference between the two groups (P=0.642). During follow-up, 24 patients relapsed, of whom 3 were local recurrences, and 21 were distant metastases. The median relapse time was 37.5 (range, 5.3–113.1) months. There were 19 cases of recurrence in the chemo group, of which 16 cases were distant metastasis, and 3 cases were pelvic recurrence. In the non-chemo group, 4 cases had distant metastasis, and 1 case had concurrent distant metastasis and pelvic recurrence. During follow-up, 12 patients died including 9 in the chemo group and 3 in the non-chemo group.
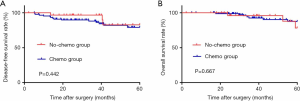
The 5-year DFS and OS rates for all patients were 80.2% and 85.0%, respectively. In the chemo group and non-chemo group, the 5-year DFS and OS rates were 79.1% and 82.9% (P=0.442), and 87.5% and 78.2% (P=0.667), respectively (Figure 1).
Cox univariate analysis revealed that cT, preoperative treatment, and number of retrieved lymph nodes were the prognostic factors for DFS (P=0.002, 0.005, and 0.007, respectively). Meanwhile, cT and ypT were the prognostic factors for OS (P=0.014 and 0.046). Cox multivariate analysis showed that cT4 (HR =4.227, 95% CI: 1.128–15.838, P=0.02) is an independent risk factor for OS, as well as an independent risk factor for DFS (HR =4.878, 95% CI: 1.752–13.578, P=0.002). Preoperative consolidation chemotherapy with CapeOx or FOLFOX (HR =0.212, 95% CI: 0.058–0.776, P=0.019) after NCRT significantly improved DFS (Tables 2-4).
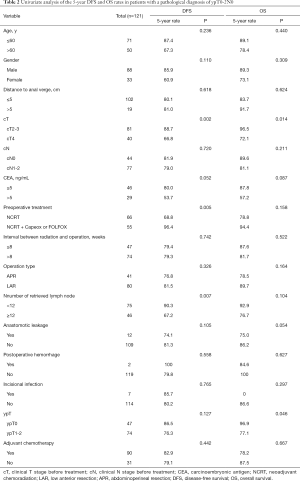
Full table

Full table
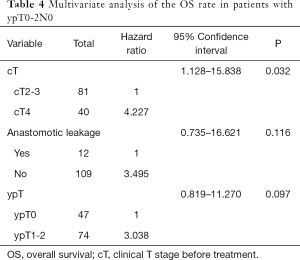
Full table
Discussion
This study showed that rectal cancer patients who underwent NCRT and radical resection with postoperative pathological diagnosis of ypT0-2N0 did not benefit significantly from adjuvant chemotherapy.
For locally advanced rectal cancer, the standard treatment is NCRT and surgery, followed by adjuvant chemotherapy. The theoretical basis for postoperative chemotherapy for rectal cancer stems from patients with colon cancer benefiting from postoperative chemotherapy (20-23). However, the efficacy of adjuvant chemotherapy for treating rectal cancer is not clear (24). Previous studies that have shown adjuvant chemotherapy to benefit patients with rectal cancer have involved patients with rectal cancer who did not receive NCRT before radical resection (25-27). However, studies involving patients with rectal cancer who received NCRT and radical resection have failed to prove that these patients can benefit from adjuvant chemotherapy (6-9).
The prognosis of patients with tumor regression and T or N downstaging after NCRT for rectal cancer has been shown to improve significantly. Rödel et al. analyzed 385 patients with rectal cancer who underwent NCRT before surgery in the CAO/ARO/AIO-94 study and found that the 5-year disease-free survival rates of TRG4, TRG2 + 3, and TRG0 + 1 patients according to postoperative pathology were 86%, 75%, and 63%, respectively (P=0.006) (12). Multivariate analysis revealed postoperative ypT to be an important independent prognostic factor for disease-free survival (12). Rectal cancer patients with ypT1-2N0 after NCRT and surgery have a better prognosis than patients with ypT3-4N0 (14). Postoperative pathological T and N staging is significantly better for predicting the prognosis than clinical stage before NCRT (12-14). This study showed that ypT0-2N0 rectal cancer patients had favorable prognosis after NCRT and surgery; however, 11 patients (12.4%) still had distant metastases during the follow-up period, including 4 patients whose pathological results showed pathological complete response (pCR). In Cox multivariate analysis, cT instead of ypT was found to be an independent prognostic factor for DFS and OS. This is probably because we only recruited ypT0-2N0 patients with good response to neoadjuvant chemoradiotherapy. Consistent with our results, Shahab et al. found that cT is an independent prognostic factor of OS for patients who show good response to preoperative chemoradiotherapy and surgery (28). Our further analysis showed that cN is not an independent risk factor for these patients, which can possibly be attributed to the accuracy of lymph node metastasis assessment being lower than that of cT staging with MRI (29,30). Shahab et al., also did not find cN to be an independent risk factor of OS in the patients with good response to preoperative radiotherapy (28).
In Zhao et al.’s comparison of prognoses between patients with ypT0-2 rectal cancer, there was no significant difference in OS rate or recurrence-free survival (RFS) rate in patients with or without adjuvant chemotherapy (15). This suggests that the ypT0-2 patients in that study did not benefit from postoperative adjuvant chemotherapy (15). Lee et al. also reported that adjuvant chemotherapy failed to improve the local recurrence, DFS, and OS rates in patients with ypT0-2N0, but that adjuvant chemotherapy could significantly decrease the local recurrence rate in patients with ypT3-4N0 (16). A multicenter retrospective study of 1,016 patients with ypT0-2 rectal cancer after NCRT and surgical resection showed that adjuvant chemotherapy failed to significantly improve the 5-year local recurrence and distant metastasis rates (17). These findings are consistent with our results that rectal cancer patients with ypT0-2N0 who underwent NCRT and radical resection did not benefit significantly from postoperative adjuvant chemotherapy. Despite the 5-year OS rate of the chemo group being 9%, which was higher than that of the non-chemo group, there was no significant difference (P>0.05).
Few previous studies have found that consolidation chemotherapy can improve the DFS rate, although most of them have shown that consolidation chemotherapy can increase the pathological complete response (pCR) rate (31,32). However, finding out if consolidation chemotherapy can improve DFS was not the direct focus of these studies. Our study shows preoperative consolidation chemotherapy with CapeOx or FOLFOX after NCRT can significantly improve DFS.
There are several limitations in the current study. Firstly, this is a single-center, retrospective study with a small number of patients. Besides, there were fewer patients in the non-chemo group than in the chemo group. Secondly, some baseline characteristics, including age and anastomotic leakage, were different in the non-chemo and chemo groups. Thus, multivariate analysis was utilized to avoid the possible bias. Thirdly, the data of the level of differentiation in the tumors and serum CEA of patients before NCRT were incomplete. As ypT0-2N0 patients represent a large proportion of patients with rectal cancer after neoadjuvant chemoradiotherapy, randomized clinical trials should be performed in the future.
Conclusions
Rectal cancer patients with a pathological diagnosis of ypT0-2N0 after NCRT and radical resection did not benefit significantly from postoperative adjuvant chemotherapy. cT4 is a high risk factor for patients with ypT0-2N0 rectal cancer. Preoperative chemotherapy with CapeOx or FOLFOX can significantly improve the DFS rate for patients.
Acknowledgments
The authors thank research assistant Yan Cai at the Department of Colorectal Surgery of Changhai Hospital for helpful data support.
Funding: This study was supported in part by grants from the Application Research of Precision Medicine Translation (No. 2017jz06) and National Natural Science Foundation of China (No. 81572358).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1278
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1278
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-1278
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1278). WZ serves as an unpaid Section Editor of Annals of Translational Medicine from Oct 2019 to Sep 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval from the Ethics Committee of Changhai Hospital was not required, because of the study’s retrospective case-control nature. The study was conducted in accordance with the Declaration of Helsinki (as is revised in 2013) and approved by the ethical standards of our institutional research committee. Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fleming FJ, Pahlman L, Monson JRT. Neoadjuvant therapy in rectal cancer. Dis Colon Rectum 2011;54:901-12. [Crossref] [PubMed]
- Van Gijn W, Marijnen CAM, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575-82. [Crossref] [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [Crossref] [PubMed]
- National Comprehensive Cancer Network Guidelines, Rectal Cancer, Version 1.2019. Available online: (Last accessed 06/11/2019).https://www.nccn.org
- Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv22-iv40. [Crossref]
- Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol 2014;15:184-90. [Crossref] [PubMed]
- Breugom AJ, Van Gijn W, Muller EW, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: A Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol 2015;26:696-701. [Crossref] [PubMed]
- Glynne-Jones R, Counsell N, Quirke P, et al. Chronicle: Results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol 2014;25:1356-62. [Crossref] [PubMed]
- Sainato A, Cernusco LNV, Valentini V, et al. No benefit of adjuvant Fluorouracil Leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): Long term results of a randomized trial (I-CNR-RT). Radiother Oncol 2014;113:223-9. [Crossref] [PubMed]
- Geva R, Itzkovich E, Shamai S, et al. Is there a role for adjuvant chemotherapy in pathological complete response rectal cancer tumors following neoadjuvant chemoradiotherapy? J Cancer Res Clin Oncol. 2014;140:1489-94. [Crossref] [PubMed]
- Hu X, Li YQ, Ma XJ, et al. Adjuvant chemotherapy for rectal cancer with complete pathological response (pCR) may not be necessary: A pooled analysis of 5491 patients. Cancer Cell Int 2019;19:127. [Crossref] [PubMed]
- Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005;23:8688-96. [Crossref] [PubMed]
- Valentini V, Coco C, Picciocchi A, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys 2002;53:664-74. [Crossref] [PubMed]
- Quah HM, Chou JF, Gonen M, et al. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer 2008;113:57-64. [Crossref] [PubMed]
- Lu Z, Cheng P, Zhang MG, et al. Is adjuvant chemotherapy necessary for patients with ypT0–2N0 rectal cancer treated with neoadjuvant chemoradiotherapy and curative surgery? Gastroenterol Rep (Oxf) 2018;6:277-83. [Crossref] [PubMed]
- Lee KH, Kim JC, Kim JY, et al. Oncologic results and prognostic predictors of patients with locally advanced rectal cancer showing ypN0 after radical surgery following neoadjuvant chemoradiotherapy. Int J Colorectal Dis 2015;30:1041-50. [Crossref] [PubMed]
- Park IJ, Kim DY, Kim HC, et al. Role of adjuvant chemotherapy in ypT0-2N0 patients treated with preoperative chemoradiation therapy and radical resection for rectal cancer. Int J Radiat Oncol Biol Phys 2015;92:540-7. [Crossref] [PubMed]
- Lichthardt S, Zenorini L, Wagner J, et al. Impact of adjuvant chemotherapy after neoadjuvant radio- or radiochemotherapy for patients with locally advanced rectal cancer. J Cancer Res Clin Oncol 2017;143:2363-73. [Crossref] [PubMed]
- Edge SB, Compton CC. The american joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. [Crossref] [PubMed]
- Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352-8. [Crossref] [PubMed]
- O’Connell MJ, Mailliard JA, Kahn MJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 1997;15:246-50. [Crossref] [PubMed]
- Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696-704. [Crossref] [PubMed]
- Bujko K, Glynne-Jones R, Bujko M. Does adjuvant fluoropyrimidine-based chemotherapy provide a benefit for patients with resected rectal cancer who have already received neoadjuvant radiochemotherapy? A systematic review of randomised trials. Ann Oncol 2010;21:1743-50. [Crossref] [PubMed]
- Akasu T, Moriya Y, Ohashi Y, et al. Adjuvant chemotherapy with uracil-tegafur for pathological stage III rectal cancer after mesorectal excision with selective lateral pelvic lymphadenectomy: A multicenter randomized controlled trial. Jpn J Clin Oncol 2006;36:237-44. [Crossref] [PubMed]
- QUASAR Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020-9. [Crossref] [PubMed]
- Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: Results from NSABP protocol r-01. J Natl Cancer Inst 1988;80:21-9. [Crossref] [PubMed]
- Shahab D, Gabriel E, Attwood K, et al. Adjuvant Chemotherapy Is Associated With Improved Overall Survival in Locally Advanced Rectal Cancer After Achievement of a Pathologic Complete Response to Chemoradiation. Clin Colorectal Cancer 2017;16:300-7. [Crossref] [PubMed]
- Ogawa S. Selection of Lymph Node–Positive Cases Based on Perirectal and Lateral Pelvic Lymph Nodes Using Magnetic Resonance Imaging: Study of the Japanese Society for Cancer of the Colon and Rectum. Ann Surg Oncol 2016;23:1187-94. [Crossref] [PubMed]
- Torkzad MR, Påhlman L, Glimelius B. Magnetic resonance imaging (MRI) in rectal cancer: a comprehensive review. Insights Imaging 2010;1:245-67. [Crossref] [PubMed]
- Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: A multicentre, phase 2 trial. Lancet Oncol 2015;16:957-66. [Crossref] [PubMed]
- Fokas E, Allgäuer M, Polat B, et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J Clin Oncol 2019;37:3212-22. [Crossref] [PubMed]


