Luminal thrombosis in middle cerebral artery occlusions: a high-resolution MRI study
Introduction
In recent years, high-resolution magnetic resonance imaging (HR-MRI) has been developed as a novel tool for the investigation of extracranial and intracranial atherosclerosis (1,2). Using this technique, the vessel wall boundary, lumen, and plaque components can be identified in vivo (2,3). Regarding the occluded vessels, HR-MRI is helpful for identifying luminal thrombosis which shows high signals on non-contrast T1 weighted-images (HST1) in extracranial vessels (4-6). In the studies of acute coronary syndrome, intracoronary thrombus diagnosed by HR-MRI was perfectly consistent with the findings of X-ray coronary angiography and optical coherence tomography (4,5). Until now, however, in vivo observations on intracranial artery occlusions have been lacking. In this study, we sought to investigate the feasibility of in vivo identification of luminal thrombosis in middle cerebral artery (MCA) occlusions on HR-MRI.
Methods
Patients
We retrospectively reviewed our institutional HR-MRI database (from January 2007 to August 2012). MCA atherosclerotic occlusion was defined as a complete signal loss of MCA trunk on magnetic resonance angiography (MRA) in the absence of an embolic cardiac or proximal large arterial source. Patients with unilateral symptomatic MCA occlusion were recruited if there was an ischemic stroke in the distribution of the occluded MCA within 1 month of stroke onset. All patients received thorough evaluations, including diffusion-weighted imaging, carotid duplex, transcranial Doppler, electrocardiogram and echocardiogram. Patients were excluded if they had any of the following characteristics: (I) coexistent >50% ipsilateral internal carotid artery stenosis; (II) evidence of cardioembolism, including atrial fibrillation or recent myocardial infarction within 1 month; (III) age <40 years; (IV) non-atherosclerotic vasculopathy such as vasculitis and arterial dissection, diagnosed by comprehensive laboratory work (such as erythrocyte sedimentation rate or C-reactive protein elevations, antinuclear antibody, or antiphospholipid antibody positivity), vascular imaging (MRA, computerized tomographic angiography, and digital subtraction angiography), and clinical evaluation; or (V) poor image quality due to motion artifact. The ethics committee of the Peking Union Medical College Hospital approved this study, with all patients signing a written consent to participate.
HR-MR imaging
All patients were imaged using a 3T magnetic resonance scanner (Signa VH/i, GE Medical Systems, Milwaukee, WI, USA) and a standard 8-channel head coil. Conventional 3-dimensional time-of-flight MRA was obtained. 3-dimensional MRA localizer (choosing maximum intensity projection images and source images of 3-dimensional time of-flight MRA as topogram) was used to ensure the cross-sectional HR-MRI images were perpendicular to the M1 segment of MCA. When the signals of the occluded MCA were completely absent, the contralateral MCA was considered as a symmetric counterpart and used as the reference. The detailed imaging parameters of MRA, T1-weighted fat-suppressed and T2-weighted sequence of HR-MRI were described previously (3,7).
Image analysis
Image analysis was performed using a standard workstation (ADW4.2, GE Medical Systems, USA). All the cross-sectional image slices of bilateral MCA on HR-MRI were analyzed for the arterial wall abnormalities. HST1 were defined as an area of high signal within the cross-section of occluded MCA, the intensity of which was >150% of the signal of adjacent muscles (Figures 1,2) (7,8).
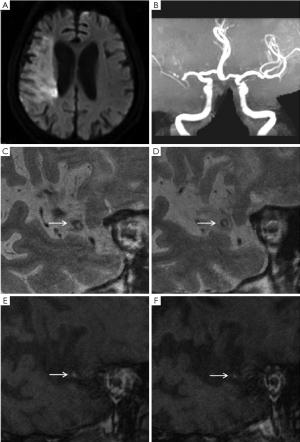
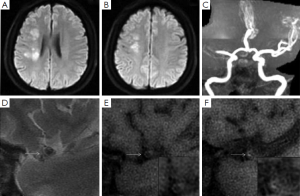
Ischemic lesions were classified as a large territorial infarct (≥1/3 ipsilateral MCA distribution on T2-weighted images), or not. In patients with non-large territory infarcts, the distribution of infarcts on diffusion-weighted images was categorized as cortical infarct, border zone infarct, or perforating artery infarct on the basis of the location of the infarct (9). Border zone infarcts include anterior border zone infarct when the infarct occurred between the anterior cerebral artery and MCA territories, posterior border zone infarct when the infarct occurred between the MCA and posterior cerebral artery territories, and internal border zone infarct between the deep and superficial perforators of the MCA (9). The non-large territory infarcts were also classified as single or multiple on the basis of the number of infarcts. Multiple infarcts were defined as more than one lesion that was topographically distinct (separated in space or discrete) on contiguous slices of diffusion-weighted images (9).
All images were reviewed by two experienced readers (W-H Xu and M-L Li) who were blinded to the clinical details. Differences between the two observers were solved by consensus.
Statistical analysis
Quantitative data were described as mean ± standard deviation or percentage. Comparisons of data were conducted using student’s t-test. The Chi-square test was used to compare several rates or proportions. SPSS 12.0 software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
A total of 34 stroke patients (mean age 55±11 years old) were considered for recruitment. Two patients with poor image quality due to motion artifact and seven patients with unavailable T1-weighted imaging were excluded. The final analyses included 25 patients. Demographic data of the patients are described in Table S1.
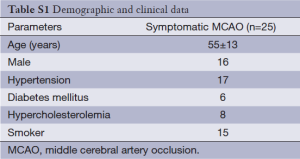
Full table
There were seven patients with a large territory infarction and with mean National Institutes of Health Stroke Scale (NIHSS) 14±5. The remaining 18 patients had mean NIHSS 8±5. The average time from stroke onset to HR-MRI examination was 9±6 days. In 13 (52%) patients, other intracranial artery stenosis in addition to the occluded MCA was observed on MRA. In the 18 patients with a non-large territory infarct, multiple infarcts were seen in 16 patients, while a single perforating artery infarct was observed in 2 patients.
Table 1 summarized the infarct patterns in relation to the presence of HST1. There were 18 (72%) patients with HST1 on HR-MRI. HST1 was observed 5/7 patients with a large territory infarct and 13/18 patients without (P=0.37). In patients with non-large territory infarcts, the presence of HST1 was similar in those with and without border zone infarcts (9/13 vs. 4/5, P=0.42).
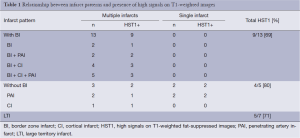
Full table
Discussion
HST1 represent methemoglobin production in the early stages of thrombus formation, which can persist for several weeks to 6 months (10). In carotid occlusive diseases, both intraplaque hemorrhage and luminal thrombosis show HST1 on HR-MRI, which can be reliably differentiated (11,12). In advanced MCA atherosclerosis, the prevalence of HST1 within plaques is low (7). In a recent study, a total of 109 severe stenotic MCAs (>70%) on 981 image slices of HR-MRI were analyzed (7). HST1 were revealed in only 19.6% of symptomatic vessels (7). With similar imaging time from stroke onset, HST1 were observed in as high as 72% of symptomatic MCA occlusions in current study. We hypothesize a higher prevalence of luminal thrombosis contribute the most to the difference. Supporting evidence from traditional vascular imaging studies suggested spontaneous recanalization of acute MCA occlusions occurs in up to 52.7% by 1 week after stroke (13). It’s reasonable that remnant luminal thrombosis is common within the occluded vessels in the early period of stroke.
For the strokes due to intracranial occlusive diseases, hypoperfusion has been suspected as a cause. Misery brain perfusion is a predictor of subsequent stroke and prone to involve border zone area (14). On the other hand, Caplan et al. hypothesized that embolism are the more common explanations for cerebral infarcts than hypoperfusion alone (15). In this study, perfusion imaging and collaterals evaluation were not performed which prevent further analysis. However, it was observed HST1 were very common in patients with symptomatic MCA occlusions, irrespective of infarct sizes and patterns. The results appeared supportive to the predominance of thrombosis/emboli mechanisms, although the HST1 alone couldn’t account for the heterogeneity of infarct sizes and patterns.
Our study with retrospective design has limitations. First, since 9 out of 34 stroke patients were excluded from analysis, the prevalence of HST1 reported in our study should be treated with caution. However, even if HST1 were absent in all those nine patients, the occurrence of HST1 would be 53%, remaining much higher than that of symptomatic MCA stenosis. Second, there are no studies that provide pathological verification of HR-MRI defined luminal thrombosis in the intracranial arteries to date. The HST1 definition used for luminal thrombosis by our group is extrapolated from carotid pathology/HR-MRI studies, but has not yet been proven in intracranial arteries.
In conclusions, our study suggests it’s feasible to identify luminal thrombosis in occluded MCA. HR-MRI is potentially powerful tool for investigating the mechanisms of stroke due to MCA occlusions.
Acknowledgements
Funding: Our study is supported by Peking Union Medical College (PUMC) Youth Fund and the Fundamental Research Funds for the Central Universities of China (NCET-12-0069).
Disclosure: The authors declare no conflict of interest.
References
- Klein IF, Lavallée PC, Schouman-Claeys E, et al. High-resolution MRI identifies basilar artery plaques in paramedian pontine infarct. Neurology 2005;64:551-2. [PubMed]
- Moody AR, Murphy RE, Morgan PS, et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation 2003;107:3047-52. [PubMed]
- Xu WH, Li ML, Gao S, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis 2010;212:507-11. [PubMed]
- Jansen CH, Perera D, Makowski MR, et al. Detection of intracoronary thrombus by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 2011;124:416-24. [PubMed]
- Ehara S, Hasegawa T, Nakata S, et al. Hyperintense plaque identified by magnetic resonance imaging relates to intracoronary thrombus as detected by optical coherence tomography in patients with angina pectoris. Eur Heart J Cardiovasc Imaging 2012;13:394-9. [PubMed]
- Corti R, Osende JI, Fayad ZA, et al. In vivo noninvasive detection and age definition of arterial thrombus by MRI. J Am Coll Cardiol 2002;39:1366-73. [PubMed]
- Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol 2012;71:195-8. [PubMed]
- Turan TN, Bonilha L, Morgan PS, et al. Intraplaque hemorrhage in symptomatic intracranial atherosclerotic disease. J Neuroimaging 2011;21:e159-61. [PubMed]
- Wong KS, Gao S, Chan YL, et al. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol 2002;52:74-81. [PubMed]
- Kelly J, Hunt BJ, Moody A. Magnetic resonance direct thrombus imaging: a novel technique for imaging venous thromboemboli. Thromb Haemost 2003;89:773-82. [PubMed]
- Altaf N, MacSweeney ST, Gladman J, et al. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke 2007;38:1633-5. [PubMed]
- Kampschulte A, Ferguson MS, Kerwin WS, et al. Differentiation of intraplaque versus juxtaluminal hemorrhage/thrombus in advanced human carotid atherosclerotic lesions by in vivo magnetic resonance imaging. Circulation 2004;110:3239-44. [PubMed]
- Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967-73. [PubMed]
- Yamauchi H, Higashi T, Kagawa S, et al. Is misery perfusion still a predictor of stroke in symptomatic major cerebral artery disease? Brain 2012;135:2515-26. [PubMed]
- Caplan LR, Wong KS, Gao S, et al. Is hypoperfusion an important cause of strokes? If so, how? Cerebrovasc Dis 2006;21:145-53. [PubMed]


