Second primary malignancies among cancer patients
Introduction
Cancer has become the leading cause of death. In 2016, the death toll from cancer accounted for 21.8% (1). Fortunately, with the improvement of the comprehensive level of diagnosis and treatment, cancer-related mortality has declined by about 1.5% annually in both men and women, 2006–2015 (2), besides, 5-year relative survival for both sex about 67.1%, 2009–2015 (3). Cancer survivors have also increased dramatically as cancer survival rates have increased. It is reported that an estimated 13.7 million Americans with a history of cancer were alive on January 1, 2012 (4,5), However, by 2017 this figure has risen to 15.5 and that number is expected to increase to 20 million by the year 2024 (6).
Second primary malignancy (SPM) is not a phenomenon of cancer recurrence or metastasis, but it is suffering from another cancer [different from first primary malignancy (FPM)] (7,8). The prolongation of the survival time of patients and the increase in the number of survivors have led to a significant increase in the chances of SPM in this group (9), which also provides an opportunity for us to study SPM. Yang et al. reported that SPMs are common in cancer patients with an overall cumulative incidence of 14% at 25 years of follow-up (10). Different FPMs have different characteristics and the probability of SPM reported varies from 5.5% to 16% (9,11,12).
Most of the previous studies have observed the risk of SPM in specific cancers and each study has its own focus and there were no uniform evaluation criteria. Nicholas selected SPM cases of 10 common cancers between 1992 to 2008 and found that 1 in 12 patients were prone to SPM (13). But after ten years, the medical level and social environment have undergone tremendous changes. Updated cognition, advancing assessment, effective measures are needed. Therefore, we selected 16 common cancer cases from 2000 to 2016 from the SEER database to analyze the spectrum of SPM prevalence and studied the impact of common prognostic factors on survival time.
Methods
Study population selection
Data was acquired using the SEER database, which comprises 18 cancer registries and covers approximately 30% of U.S population. The SEER database was accessed via SEER*Stat software (Version 8.3.5; National Cancer Institute Cancer Statistics Branch).
We identified all patients diagnosed with FPM among the 16 cancer sites with the Site Recode B ICD-O-3/WHO 2008 Definition [including prostate, female breast, lung and bronchus, brain and cranial nervous system (brain, CNS), thyroid, esophagus, stomach, liver, pancreas, colorectal, bladder, kidney, corpus uteri, cervix uteri, ovary and non-Hodgkin lymphoma (NHL)]. SPM also uses Site Recode B ICD-O-3/WHO 2008 Definition, a total of 16 sites of tumors are included in the events, including prostate, female breast, lung and bronchus, brain and cranial nervous system (brain, CNS), thyroid, esophagus, stomach, liver, pancreas, colorectal, bladder, kidney, female genital system, melanoma of skin, leukemia and non-Hodgkin lymphoma (NHL). Among them, colorectal includes ascending colon, descending colon, transverse colon, sigmoid colon, hepatic flexure, splenic flexure, rectosigmoid junction and rectum. Female genital system refers to corpus uteri, cervix uteri, ovary, uteri, not of special (NOS), vagina and vulva. Acute/chronic myeloid leukemia, monocytic leukemia and lymphocytic leukemia are outlined as leukemia. NHL includes nodal and extra-nodal NHL.
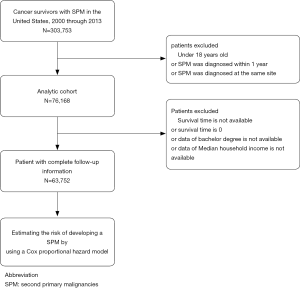
Although data were available through 2016, we limited the current study cohort to patients diagnosed between 2000 and 2013 to ensure 3 years of follow-up after a cancer diagnosis. Similar to previously published studies (13), we excluded patients with SPM diagnosed within one year or at the same site to avoid misidentifying metastatic malignancies as SPM. Cases younger than 18 years old are also excluded.
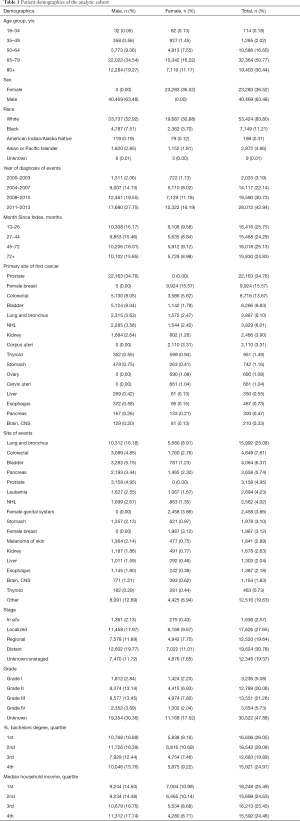
Patient demographics included age in years (18–34, 35–49, 50–64, 65–79, and 80+), race (white, black, American Indian, Alaska Native, Asian/Pacific Islander and unknown), sex (male or female) and year of diagnosis (2000–2003, 2004–2007, 2008–2010 and 2011–2013). The time interval between the time of diagnosis of SPM and FPM is defined as Month Since Index, and it was divided into 13–26, 27–44, 45–71, 72+ months by using the X-tile program (Yale University, New Haven, Connecticut, USA; Figure S1). Tumor characteristics included grade (Grade I, well differentiated; Grade II, moderately differentiated; Grade III, poorly differentiated; Grade IV, undifferentiated and unknown) and stage (in situ, localized, regional, distant and unstaged/unknown). SEER merged ZIP code-level data for educational level and annual household income from the 2008 US Census data. Individual-level data were imputed from the percentage of patients holding a Bachelor’s degree and the median annual household income in each patient’s ZIP code, which was then stratified into quartiles (14).

Statistical analysis
We provided an overview of the spectral distribution of FPM and SPM and showed a more specific and detailed information about the site of the SPM distribution. Data on primary site of FPM, primary site of SPM, age groups, gender, race, year of diagnosis of SPM, grade, stage, Month Since Index, bachelor’s degree, median household income were incorporated in the multivariate Cox proportional hazards model for overall survival. Hazard ratios (HRs) of each variable with corresponding 95% confidence intervals (CI) were calculated stratified by sex. We also calculated the distribution of causes of death (COD) in the analysis cohort and compared the proportion of COD in primary or secondary malignancies. Median survival time was estimated using Kaplan-Meier (K-M) method and the differences between the survival curves were compared using the log-rank test.
Standard incidence rate of multiple primary (MP-SIR), which is also known as the relative risk, is a relative measure of the strength of association between two cancers. It is calculated by dividing the observed number of SPM cases by the expected number [observed/expected (O/E) ratio] based on general population rates. Risks were considered significant when corresponding 95% confidence interval did not include the null value (15,16).
All statistical analyses were finished in the R software (version 3.6.0; http://www.r-project.org/). In all statistical analyses, a P value of <0.05 was considered significant.
Results
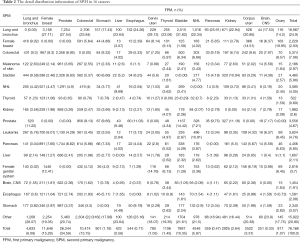
We identified 303,753 patients with SPM diagnosed between 2000 and 2013, among whom 76,168 patients (25.08%) meet our inclusion criteria, after excluding case of developing SPM within a year or at the same site as FPM or patients under the age of 18 (Figure 1). In the analytic cohort, the majority of patients (81.21%) were aged >65 years. And of those patients with SPM, 83.80% were white; 63.48% were male; and 74.67% were diagnosis after 2008. 41.32% of patients were diagnosis as Grade II, moderately differentiated or Grade III, poorly differentiated. Total of 30.22% patients are at stage of in situ/localized and 30.78% were distant metastasis. The specific demographic data of patients, the characteristics of tumors and other related information were showed in Table 1.
Full table
The composition of the analytic cohort
The specific value of each FPM in this study and the cohort is visually shown in Figure 2A. Obviously, the number of patients with prostate cancer, female breast cancer, or colorectal cancer respectively ranks in the top three. Similarly, the Sector Graph in Figure 2B shows the proportion of FPM in the analytic cohort, and prostate cancer survivors have the largest number (34.59%) of cases of SPM. Classified according to where the events occur, Figure 2C shows the common location of 16 SPMs, with lung and bronchus accounting for the largest proportion (24.90%). Figure 2D reveal the ranking which is based on the proportion of each site of the FPM or SPM groups. The ranking shows the different performance of the same location in FPM and SPM. For example, prostate cancer ranks No.1 in FPM, but it ranks No.6 in SPM, besides, lung and bronchus are ranked No.5 in FPM, but it is the most prone to SPM (it ranks No. 1). It is worth mentioning that although there are few cases of pancreas cancer (ranked No. 15), it is the fourth site that is prone to SPM.
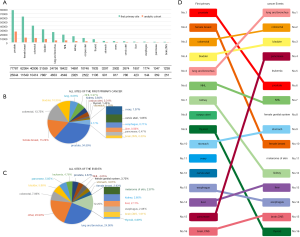
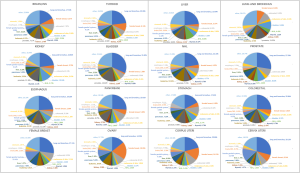
Table 2 shows the detail distribution information of SPM in 16 cancers. Figure S2 shows the results in the form of Sector Graph to make the results more intuitive. As the picture shows, nearly one-fourth of the SPM of each tumor is lung and bronchus, and different tumors have different distribution preferences. Among bladder cancer survivors, lung and bronchus disease was particularly common, representing 31.82% of all SPM in this group. Breast cancer was especially common in ovary cancer, corpus uteri cancer and cervix uteri cancer survivors at 19.95%, 14.51% and 8.14%, respectively.
Full table
Follow-up characteristics of the analytic cohort
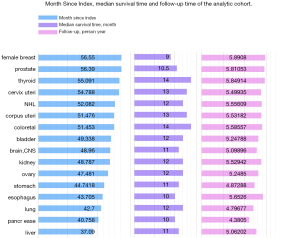
Figure 3 shows the Month Since Index (which is also recognized as month at events), the median survival time and the time of follow-up. Among them, liver cancer has the shortest time interval for developing SPM, which is usually 37.09 months. The median survival time is usually about 1 year. The prognosis of female breast cancer survivors is the worst with the median survival time is only 9 months. The mean follow-up of the entire cohort was 5.35 years (standard deviation, 0.42 years). The detailed magnitude with standard deviation value were displayed in Table S1.

Full table
SPM risk assessment
Multivariate Cox proportional hazards model for overall survival was performed to estimate the impact of various factors on survival. HRs of each variable with corresponding 95% CI were displayed in the Table 3. The diagnosis of Liver Neoplasms is an obvious risk factor for prognosis (HR =1.64, 95% CI, 1.54–1.75, P<0.001; patients with bladder neoplasms were recognized as reference), the results of both men and women also confirm this. Interestingly, with the progress of the times, the prognosis of patients with secondary cancer is improving year by year (2011–2013: HR =0.20, 95% CI, 0.15–0.25, P<0.001; patients diagnosed at 2000–2003 were recognized as reference).
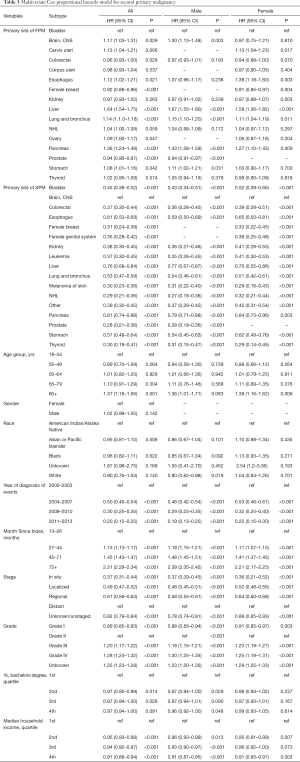
Full table
The heat map can simply aggregate a large amount of data, and use a progressive color band to visually show the density or frequency of spatial data. The final result is generally better than the direct display of discrete points. In our heat map, where the data is large, the area is red and the small amount data is blue. Figure 4A shows the raw data of the SPM case count. The MP-SIR data obtained from the SEER database is shown in Figure 4B. We found that the risk of being infected with gastric cancer after esophageal cancer is highest (SIR =5.08). The normalized SIR data is shown in Figure 4C, which can more specifically demonstrate the risk of SPM in different parts of the same cancer, as well as highlighting the potential link between the diseases and primary site.

Survival analysis of the analysis cohort
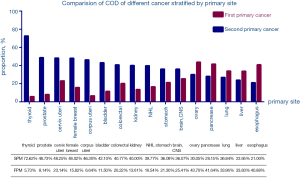
In addition, we calculated the probability that the COD of 16 cancers is FPM or SPM (Figure 5), the data shows that the probability of esophageal cancer dying from FPM is 5.73%, but the probability of dying from SPM is as high as 72.62%. K-M method was used to find the difference between different 16 FPMs and 16 SPMs and K-M survival curves were made separately (Figure S3), but due to the excessive amount of data, curves overlapped and intersected and no obvious conclusions can be drawn.

Discussion
The era of big data means that the formulation of clinical decisions and the implementation of health care policies require evidence-based medicine support, especially in the field of cancer research. We used the SPM data of the SEER database to study the following three aspects: (I) compare rate and type of SPM in 16 common FPMs. (II) Investigate the time of occurrence and median survival time of SPM. (III) Analysis of the risk of SPM in 16 common cancers and made a preliminary comparison.
Nicholas pointed out that the most common SPM is lung cancer (13), which is consistent with our findings, indicating that after ten years, lung cancer remains the difficult problem in the SPM research field. Nicholas also said that the second primary cancer caused at least 50% of all of our malignant tumor survivors (13). This conclusion is still true today. The probability of thyroid cancer dying from SPM has risen from 63% five years ago to 72.62%, and lung cancer has dropped from 36% to 26.84%. This may be related to the increased sensitivity to radiation of cells in younger patients and the longer life-span in which an SPM may be diagnosed (7,8,12).
Lifestyle, environment, host factors and interactions and other influences are recognized as multiple primary cancers etiologic factors (9). In particular, radiation therapy is thought to play an important role in the pathogenesis of SPM and the risk of radiation-related malignancies has been investigated (17-19). Among the survivors of several primary malignancies, the most obvious are Hodgkin’s lymphoma, as well as testes, breast, cervical and prostate cancers. Of course, some scholars have proposed different opinions that even if radiotherapy increases the radiation dose of adjacent organs, the second cancer risks from radiotherapy in adulthood are relatively small, especially when compared with the treatment benefits (20). Kier et al. draws similar conclusions in a retrospective study of 5,190 patients with germ cell cancer (21).
We found that there is a systematic connection between the recurrence position and the primary position. For example, patients with primary gynecologic tumors, SPM also prone to occur in the female genital system or female breast. Another interesting phenomenon is that gastric cancer accounts for 9.19% of patients with SPM in esophageal cancer. Similarly, esophageal cancer accounts for 5.11% of gastric cancer patients with SPM (higher than normal level), which is consistent with the result of SIR (SIR =5.08) and COX analysis (HR =1.12, 95% CI, 1.02–1.21, P=0.021). There may be some association between the two tumors. Excessive drinking and tobacco intaking is considered a synergistic factor affecting upper digestive tract and upper respiratory tract cancer (22,23), which may explain this phenomenon.
In addition, we found that the tumors of the digestive system (except for colorectal tumors) have a lower probability of developing SPM. Possible causes include poor prognosis of digestive tract tumors and short survival time. Another possible reason is that most of these tumors are based on surgery and the intervention of radiotherapy and chemotherapy is less than other tumors (24-26).
The advantage of our research is that we have included a large number of samples between 2000 and 2013, depicting the location of SPM and updating and enriching the relevant data. At the same time, we also conducted COX analysis on related factors and identified some risk factors. However, due to the limited permission of the SEER database and the inherent flaws in retrospective research, our data must have some limitations, although we try our best to avoid potential bias. First, we excluded the same site and cases of SPM diagnosed within one year to avoid misclassification of metastasis and SPM, but this also directly led to the gap in the field of SPM in the primary site. To this end, we have retained the calculation of the SIR in the primary part of the SPM. The heat map can also show that this risk does exist and cannot be ignored (Figure S4). Second, because of the limited access to information, we have no access to patient-specific treatment information and lifestyle habits, such as tobacco and alcohol intake, radiation doses in radiation regimens, specific chemotherapy regimens and genetic mutations. These factors are considered to be closely related to the development of SPM. Third, when we chose primary site of FPM, we did not include some non-solid tumors, including Hodgkin's lymphoma, leukemia. When we chose SPM, we did not include some important parts, including the head and neck. And these are considered to be the location of SPM, and some are often fatal (27-29).

The occurrence of SPM may be the result of a combination of factors, so it is necessary to find a study of the individual factors affecting its occurrence, but more important is the grasp of the overall situation of patients and comprehensive analysis of multiple factors. It is foreseeable that with the increase in the number of cancer survivors and the longevity of cancer survivors, SPM will occur more frequently and become a medical problem and a social burden. Awareness, evaluation, counseling, and amelioration strategies are recommended (30,31). Recommendations for a research agenda, study methods, and infrastructure required to advance our understanding of SPMs and to establish the basis for evidence-based approaches to patient management and intervention strategies were derived from an NCI-sponsored workshop that included clinicians, researchers, and patient advocates (32). Therefore, more specific and long-term follow-up research is needed, and more rational clinical decisions guidelines and medical policies need to be developed and implemented.
Conclusions
The improvement of medical level has allowed the life of cancer patients to continue. At the same time, cancer survivors are also at risk of developing secondary primary malignancies. Prostate cancer patients have the highest probability of SPM and lung and bronchus are the most prone site to develop SPM. Once the SPM is diagnosed, the median survival time is usually around one year. Therefore, it is particularly important to rationally adjust the treatment plan, better define high-risk groups and strengthen targeted interventions and clinical interventions. Our research on the population distribution of SPM and its impact on survival can provide a reference for prevention, screening, treatment and survival recommendations for specific ages.
Acknowledgments
We thank the contribution of the SEER database and the 18 registries supplying cancer research information.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2059). XL serves as an unpaid section editor of Annals of Translational Medicine from Jan 2020 to Dec 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- US Mortality Files, National Center for Health Statistics, Centers for Disease Control and Prevention 2016.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. editors. SEER Cancer Statistics Review, 1975-2016, National Cancer Institute. Bethesda, MD, , based on November 2018 SEER data submission, posted to the SEER web site, April 2019.https://seer.cancer.gov/csr/1975_2016/
- de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev 2013;22:561-70. [Crossref] [PubMed]
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures: 2014–2015. Atlanta, GA: American Cancer Society; 2014.
- American Cancer Society. Cancer Facts & Figures 2017. In: Atlanta: American Cancer Society. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html
- Silva-Vieira M, Carrilho Vaz S, Esteves S, et al. Second Primary Cancer in Patients with Differentiated Thyroid Cancer: Does Radioiodine Play a Role?. Thyroid 2017;27:1068-76. [Crossref] [PubMed]
- Izkhakov E, Barchana M, Liphshitz I, et al. Trends of Second Primary Malignancy in Patients with Thyroid Cancer: A Population-Based Cohort Study in Israel. Thyroid 2017;27:793-801. [Crossref] [PubMed]
- Wood ME, Vogel V, Ng A, et al. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol 2012;30:3734-45. [Crossref] [PubMed]
- Yang J, Terebelo HR, Zonder JA. Secondary primary malignancies in multiple myeloma: an old NEMESIS revisited. Adv Hematol 2012;2012:801495. [Crossref] [PubMed]
- Li Z, Wang K, Shi Y, et al. Incidence of second primary malignancy after breast cancer and related risk factors-Is breast-conserving surgery safe? A nested case-control study. Int J Cancer 2020;146:352-62. [Crossref] [PubMed]
- Marti JL, Jain KS, Morris LG. Increased risk of second primary malignancy in pediatric and young adult patients treated with radioactive iodine for differentiated thyroid cancer. Thyroid 2015;25:681-7. [Crossref] [PubMed]
- Donin NM, Kwan L, Lenis AT, et al. Second primary lung cancer in United States Cancer Survivors, 1992-2008. Cancer Causes Control 2019;30:465-75. [Crossref] [PubMed]
- Wang W. Increased incidence of second primary malignancy in patients with malignant astrocytoma: a population-based study. Biosci Rep 2019;39:BSR20181968. [Crossref] [PubMed]
- Breslow NE, Day NE. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. IARC Sci Publ 1987.1-406. [PubMed]
- Rothman KJ, Boice Jr JD. Epidemiologic analysis with a programmable calculator. Bethesda: MD. NIH Publication; 1979.
- Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst 2005;97:1354-65. [Crossref] [PubMed]
- Wallis CJ, Mahar AL, Choo R, et al. Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis. BMJ 2016;352:i851. [Crossref] [PubMed]
- Jin T, Song T, Deng S, Wang K. Radiation-induced secondary malignancy in prostate cancer: a systematic review and meta-analysis. Urol Int 2014;93:279-88. [Crossref] [PubMed]
- Berrington de Gonzalez A, Curtis RE, Kry SF, et al. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol 2011;12:353-60. [Crossref] [PubMed]
- Kier MG, Hansen MK, Lauritsen J, et al. Second Malignant Neoplasms and Cause of Death in Patients With Germ Cell Cancer: A Danish Nationwide Cohort Study. JAMA Oncol 2016;2:1624-7. [Crossref] [PubMed]
- Schottenfeld D. Alcohol as a co-factor in the etiology of cancer. Cancer 1979;43:1962-6. [Crossref] [PubMed]
- Adjei Boakye E, Buchanan P, Hinyard L, et al. Trends in the risk and burden of second primary malignancy among survivors of smoking-related cancers in the United States. Int J Cancer 2019;145:143-53. [Crossref] [PubMed]
- Chen SC, Liu CJ, Hu YW, et al. Second primary malignancy risk among patients with gastric cancer: a nationwide population-based study in Taiwan. Gastric Cancer 2016;19:490-7. [Crossref] [PubMed]
- Kim JW, Jang JY, Chang YW, et al. Clinical features of second primary cancers arising in early gastric cancer patients after endoscopic resection. World J Gastroenterol 2015;21:8358-65. [Crossref] [PubMed]
- Chen QW, Li HJ, Chen YN, et al. Hepatic Lesions Detected after Mastectomy, in Breast Cancer Patients with Hepatitis Background May Need to Undergo Liver Biopsy to Rule Out Second Primary Hepatocellular Carcinoma. PLoS One 2016;11:e0139782. [Crossref] [PubMed]
- Baxi SS, Pinheiro LC, Patil SM, et al. Causes of death in long-term survivors of head and neck cancer. Cancer 2014;120:1507-13. [Crossref] [PubMed]
- Tadmor T, Liphshitz I, Silverman B, et al. Incidence and epidemiology of non-Hodgkin lymphoma and risk of second malignancy among 22 466 survivors in Israel with 30 years of follow-up. Hematol Oncol 2017;35:599-607. [Crossref] [PubMed]
- Chien SH, Liu CJ, Hong YC, et al. Development of second primary malignancy in patients with non-Hodgkin lymphoma: a nationwide population-based study. J Cancer Res Clin Oncol 2015;141:1995-2004. [Crossref] [PubMed]
- Travis LB, Ng AK, Allan JM, et al. Second malignant neoplasms and cardiovascular disease following radiotherapy. J Natl Cancer Inst 2012;104:357-70. [Crossref] [PubMed]
- Nathan PC, Ness KK, Mahoney MC, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Ann Intern Med 2010;153:442-51. [Crossref] [PubMed]
- Travis LB, Rabkin CS, Brown LM, et al. Cancer survivorship--genetic susceptibility and second primary cancers: research strategies and recommendations. J Natl Cancer Inst 2006;98:15-25. [Crossref] [PubMed]