
Folate receptor-positive circulating tumor cells as a predictive biomarker for the efficacy of first-line pemetrexed-based chemotherapy in patients with non-squamous non-small cell lung cancer
Introduction
Platinum-based doublet chemotherapy remains the standard first-line treatment for advanced non-small cell lung cancer (NSCLC) patients, especially for those who are ineligible for targeted therapy (1). Pemetrexed, an anti-folate agent, is one of the most commonly used drugs for treating patients with non-squamous NSCLC (nsNSCLC) (2). Pemetrexed primarily targets thymidylate synthase (TS) and also inhibits folate-dependent enzymes such as dihydrofolate reductase (DHFR) and glycinamide ribonucleotide formyltransferase (GARFT) (3). Through inhibiting those enzymes, pemetrexed interferes with the biosynthesis of thymidine and purine nucleosides, and thus blocks tumor growth (3). Nowadays, many clinical guidelines, including NCCN, ESMO, and ASCO (2,4,5), have recommended pemetrexed in combination with platinum as a first-line treatment option for patients with advanced nsNSCLC. Despite the clinical benefits of pemetrexed, it lacks an effective biomarker currently. TS and folate receptor alpha (FRA) have been found to be correlated to the efficacy of pemetrexed (6-10). Recently, Tie et al. found that FRβ was overexpression in tumor-associated macrophages and was associated with poor prognosis in lung cancer (11). However, the clinical value of these biomarkers remains controversial (12,13). More importantly, these biomarkers require tumor tissue for analysis while the majority of advanced lung cancer patients may not have sufficient tumor tissue for the analysis.
Recently, liquid biopsy has become a research hotspot in oncology. Many studies have investigated the clinical significance of circulating tumor cells (CTCs) in cancer management (14). CTCs are tumor cells that shed from the primary or metastatic lesions and enter the blood circulation. Comparing with conventional tumor tissue, CTCs can be easily obtained in a non-invasive approach. Hence, it can be collected repeatedly for molecular test and dynamic monitoring. “CytoploRare detection kit” is a diagnostic kit developed by Genosaber Biotech Co. Ltd. (Shanghai, China). The kit utilizes negative enrichment and ligand-targeted PCR (LT-PCR) method to enumerate FR-positive CTCs. Our previous study has proven the diagnostic efficiency of FR-positive CTCs in the diagnosis of lung cancer (15) and in 2016, China Food and Drug Administration has approved the clinical application of the kit in lung cancer diagnosis. In the present study, we prospectively investigated the predictive capability of FR-positive CTCs for the efficacy of pemetrexed-based chemotherapy.
Methods
Study design
This was a prospective, single-institution, phase II clinical trial approved by the Institutional Review Board of the Shanghai Pulmonary Hospital (No. K13-112). Inclusion criteria included: (I) pathologically or cytologically confirmed advanced nsNSCLC based on the International Association for the Study of Lung Cancer 8th TNM Staging System; (II) received pemetrexed-based chemotherapy for at least one cycle; and (III) had sufficient blood samples prior to initial treatment. Eligible patients were treated with pemetrexed (500 mg/m2, d1) with cisplatin (75 mg/m2, d1) or carboplatin [area under the curve (AUC) =5, d1] or oxaliplatin (100 mg/m2, d1), or pemetrexed alone (500 mg/m2, d1) every 3 weeks for at least one cycle.
This trial was registered on the Chinese Clinical Trial Registry Web site (ChiCTR-ONC-13003475).
FR-positive CTC analysis
Three milliliters of peripheral blood were collected from each patient before the initiation of treatment for FR-positive CTC analysis. FR-positive CTC analysis was performed using “CytoploRare detection kit” (GenoSaber Biotech Co., Ltd., Shanghai, China) as previously described (15). Whole blood samples from eligible patients were collected in 3 mL ethylene diamine tetraacetic acid (EDTA) anticoagulant tubes and stored at 4 °C. FR-positive CTC analysis was performed within 24 hours of collection, according to the manufacturer’s instructions. In brief, CTCs were enriched from 3 mL of whole blood by immunomagnetic depletion of leukocytes and then labeled with conjugates of a tumor-specific ligand folic acid and a synthesized oligonucleotide. After washing off free conjugates, the stripped bound conjugates were analyzed by quantitative PCR. In this study, the quantity of FR-positive CTC was expressed as an arbitrarily defined “FR Unit” (FU), which was defined as the number of FR-positive CTC detected in 3 mL of blood. A serial of standards containing oligonucleotides (10−14 to 10−19 M, corresponding to 2 to 2×105 FU/3 mL) was used for FR-positive CTC quantification.
Statistical analysis
The categorical variables were compared using chi-square tests, or Fisher’s exact tests when needed. Progression-free survival (PFS) was defined as the time from the date of first-line treatment to the date of systemic progression or death. Overall survival (OS) was defined as the time from the date of first-line treatment to the date of death and was censored at the date of the last follow-up if the patient was alive. Tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (16). Kaplan-Meier curve and two-sided log-rank test were used for survival analyses. Multivariate Cox proportional hazards regression analyses were used to determine the independently predictive and prognostic value of FR-positive CTC. P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using SPSS 20.0 software (SPSS Inc., IL, USA) and Prism 7.0 (GraphPad Software Inc., CA, USA).
Results
Study population
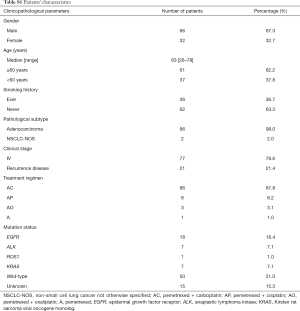
A total of 98 patients with nsNSCLC were enrolled in this study (the patients’ baseline clinicopathological characteristics were presented in Table S1). The majority of the enrolled patients had stage IV (77/98, 78.6%) adenocarcinoma (96/98, 98.0%). Most of the patients received first-line (84/98, 85.7%) pemetrexed/carboplatin (86/98, 87.8%) treatment. All of the 98 patients commenced pemetrexed treatment between May 2014 and May 2018. The median follow-up duration was 150 days (ranging from 2 to 1,204 days). At the date of analysis, 47 patients had disease progression. The median PFS and OS were 229 and 1,122 days, respectively.
Full table
Association of FR-positive CTC level with PFS and OS
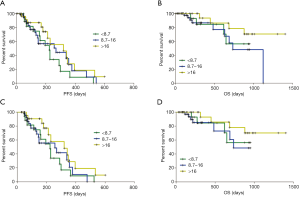
The median CTC level of the enrolled patients was 11.9 FU/3 mL (0.4–35.5 FU/3 mL). According to the manufacturer’s instruction, the optimal cutoff threshold of FR-positive CTC count in lung cancer diagnosis was 8.7 FU/3 mL. In this study, the positive rate was 80.6% (79/98). According to our previous report (17), another cutoff threshold (16 FU/3 mL) was chosen to classify high and low FR-positive CTC levels.
The median PFS of the “<8.7 group”, “8.7–16 group”, and “>16 group” were 224, 269, and 320 days, respectively (Figure 1A). The median OS of the “8.7–16 group” was 735 days, while that of the “<8.7 group” and “>16 group” had not yet reached at the date of analysis (Figure 1B). The PFS and OS showed no significant differences among the three subgroups. When patients who received only one cycle of treatment or received pemetrexed-based chemotherapy as second-line treatment were excluded, the PFS and OS among the three CTC subgroups remained statistically insignificant (Figure 1C,D).
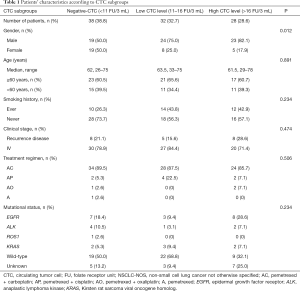
Since the cutoff threshold of 8.7 FU/3 mL was determined to differentiate lung cancer from benign lung disease, a new cutoff threshold was chosen. Our previous has demonstrated that the median level of CTC in lung cancer patients was 11.64 FU/3 mL (15). We further divided patients into three groups, “‘negative-CTC group” (<11 FU/3 mL), “the low CTC level group” (11–16 FU/3 mL), and “the high CTC level group” (>16 FU/3 mL). The baseline clinicopathological features of patients among the three CTC subgroups were well balanced, except for gender, as shown in Table 1.
Full table
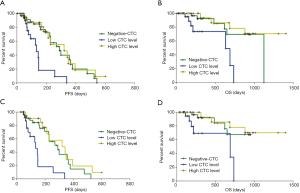
Interestingly, the median PFS of the three CTC subgroups were 290, 133, and 320 days, respectively (Figure 2A). And the median OS were 1,122, 632 days and not reached, respectively (Figure 2B). Further univariate and multivariate Cox proportional hazards regression analyses (Table 2) demonstrated that “high CTC level” was an independent factor that was significantly associated with better PFS [hazard ratio (HR) =0.26, 95% confidence interval (CI), 0.12–0.58, P=0.001] and OS (HR =0.23, 95% CI, 0.06–0.92, P=0.037). Furthermore, EGFR/ALK/ROS1 molecular alterations was identified as an independent factor that was significantly associated with better PFS (HR =0.27, 95% CI, 0.10–0.71, P=0.008).
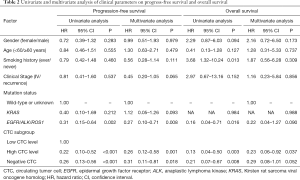
Full table
When patients who received only 1 cycle of treatment (n=10) were excluded, the median PFS of the three CTC subgroups were 290, 133, and 320 days, respectively (Figure 2C). Patients with low CTC level had a significantly shorter PFS than those with high CTC level (Log-rank P=0.0002). The median OS of the “low CTC level group” was 688 days, while that of the “negative-CTC group” and the “high CTC level group” had not yet reached at the date of analysis (Figure 2D).
Association of FR-positive CTC level with objective response rate (ORR) and disease control rate (DCR)
The ORR of the three CTC subgroups were 21.2%, 9.5% and 40.9%, respectively (Table 3, Figure 3A). The ORR of patients with high CTC level was significantly higher than those with low CTC level (P=0.0339), whereas the ORR between the “low CTC level group” and the “negative-CTC group” (P=0.4559), and that between the “high CTC level group” and the “negative-CTC group” (P=0.1390) were not significantly different.

Full table
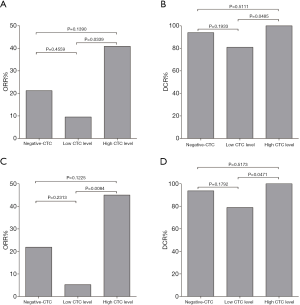
The DCR of the three CTC subgroups were 93.9%, 81.0% and 100.0%, respectively (Table 3, Figure 3B). The DCR of patients with high CTC level was significantly higher than those with low CTC level (P=0.0485), whereas the DCR between the “low CTC level group” and the “negative-CTC group” (P=0.1933), and that between the “high CTC level group” and the “negative-CTC group” (P=0.5111) were not significantly different.
When patients who received only 1 cycle of treatment (n=10) were excluded, the differences were still significant in terms of ORR and DCR. The ORR of the three CTC subgroups were 21.9%, 5.3% and 45.0%, respectively (P=0.0137, Figure 3C). The DCR of the three CTC subgroups were 93.8%, 79.0% and 100%, respectively (P=0.0511, Figure 3D).
Discussion
A wide variety of biomarkers have been investigated to predict the efficacy of chemotherapy during the past decade, unfortunately, none of these biomarkers has been prospectively and appropriately validated in clinical trials (18). CTCs as a non-invasive source that reflects the molecular underpinnings of the originating tumors represent a valuable resource for predicting treatment efficacy (19-22), including in advanced NSCLC patients treated with targeted therapy, chemotherapy or immunotherapy. However, no studies dedicatedly focus on the predictive value of CTC for the efficacy of pemetrexed-based chemotherapy.
In the present study, we focused on the association of FR-positive CTCs with the efficacy of pemetrexed-based chemotherapy. Our results demonstrated that, using 11 and 16 FU/3 mL as the cutoff threshold, patients with high CTC level had better clinical outcomes over those with low CTC level, in terms of ORR, DCR, PFS, and OS. As previous studies have proven that, 8.5–8.94 FU/3 mL was the established optimal cutoff threshold chosen for differentiating lung cancer from lung benign disease (15,23-25). When using the cutoff threshold of 8.7 FU/3 mL, although a trend towards better OS was observed in patients with high CTC level over those in the “8.7–16 group” (HR =2.69, P=0.0812), it lacks of statistical significance. For predicting the efficacy of pemetrexed, the cutoff threshold may be different. Therefore, a different cutoff threshold is required. In the present study, using 11 FU/3 mL as the cutoff threshold can better discriminate the clinical outcomes, in term of ORR, DCR, PFS and OS, therefore, we chose 11 FU/3 mL as the cutoff threshold for defining FR-positive or negative CTCs.
The ORR and median PFS of patients with low CTC level in our study was 9.5% and 4.4 months, which was significantly inferior to the results from PARAMOUNT study (26,27). With use of this non-invasive and convenient method, we could potentially identify approximately 30% patients with nsNSCLC may not derive the expected benefits from pemetrexed-based chemotherapy. If only 9.5% with low CTC level responded to pemetrexed-based chemotherapy, other chemotherapeutic regimens should be assessed in this subtype populations.
To explain the better clinical outcome of pemetrexed in patients with high CTC level, we proposed the following hypotheses: (I) since FRA is responsible for the transport of pemetrexed into cells, tumor cells with a high FRA expression will retain a higher concentration of pemetrexed within the cells (28), which leads to the superior efficacy. (II) FRA also transports folic acid into cells. Folic acid is a key substrate for TS to synthesize deoxythymidine monophosphate, hence regulating DNA biosynthesis (29). When tumor cells have high expression level of FRA, it is more likely that these cells rely on this pathway to proliferate. Thus, pemetrexed which inhibits the TS activity would be more effective in killing these tumor cells.
For patients with negative-CTC (<11 FU/3 mL), the clinical outcome of pemetrexed fell between the “high CTC level group” and the “low CTC level group” in this study. Theoretically, low CTC level may indicate a favorable prognosis and a less aggressive disease. Therefore, the better prognosis of patients in negative-CTC group when treated with pemetrexed-based chemotherapy may be attributed to a favorable prognosis and a less aggressive nature of this subgroup of patients themselves, rather than correlating with the sensitivity to pemetrexed.
In conclusion, FR-positive CTC is an attractive biomarker to predict the clinical outcomes of advanced nsNSCLC patients when receiving pemetrexed-based chemotherapy. Enumeration of FR-positive CTC could help select suitable patients for pemetrexed treatment and possibly improve patients’ prognosis by avoiding ineffective treatment. Further investigation of this biomarker in well-designed prospective studies may propel the advance of precision therapy in chemotherapy.
Acknowledgments
Presented in part at the European Society for Medical Oncology Annual Meeting in Barcelona, Spain, on 29th September-1st October, 2019.
Funding: This study was partially supported by Shanghai Municipal Science and Technology Commission Medical Guidance Project (No. 17411969200). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-19-4680
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-19-4680). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This was a prospective, single-institution, phase II clinical trial approved by the Institutional Review Board of the Shanghai Pulmonary Hospital (No. K13-112). The written informed consent was obtained from each participant to use the clinical data for research before any medical interventions.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Su C, Zhou F, Shen J, et al. Treatment of elderly patients or patients who are performance status 2 (PS2) with advanced Non-Small Cell Lung Cancer without epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) translocations - Still a daily challenge. Eur J Cancer 2017;83:266-78. [Crossref] [PubMed]
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw 2018;16:807-21. [Crossref] [PubMed]
- Curtin NJ, Hughes AN. Pemetrexed disodium, a novel antifolate with multiple targets. Lancet Oncol 2001;2:298-306. [Crossref] [PubMed]
- Besse B, Adjei A, Baas P, et al. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol 2014;25:1475-84. [Crossref] [PubMed]
- Hanna N, Johnson D, Temin S, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484-515. [Crossref] [PubMed]
- Gronberg BH, Lund-Iversen M, Strom EH, et al. Associations between TS, TTF-1, FR-alpha, FPGS, and overall survival in patients with advanced non-small-cell lung cancer receiving pemetrexed plus carboplatin or gemcitabine plus carboplatin as first-line chemotherapy. J Thorac Oncol 2013;8:1255-64. [Crossref] [PubMed]
- Lee SH, Noh KB, Lee JS, et al. Thymidylate synthase and ERCC1 as predictive markers in patients with pulmonary adenocarcinoma treated with pemetrexed and cisplatin. Lung Cancer 2013;81:102-8. [Crossref] [PubMed]
- Nicolson MC, Fennell DA, Ferry D, et al. Thymidylate synthase expression and outcome of patients receiving pemetrexed for advanced nonsquamous non-small-cell lung cancer in a prospective blinded assessment phase II clinical trial. J Thorac Oncol 2013;8:930-9. [Crossref] [PubMed]
- Christoph DC, Reyna-Asuncion B, Hassan B, et al. Assessment of folate receptor-alpha and epidermal growth factor receptor expression in pemetrexed-treated non-small-cell lung cancer patients. Clin Lung Cancer 2014;15:320-30.e1-3.
- Sun JM, Ahn JS, Jung SH, et al. Pemetrexed Plus Cisplatin Versus Gemcitabine Plus Cisplatin According to Thymidylate Synthase Expression in Nonsquamous Non-Small-Cell Lung Cancer: A Biomarker-Stratified Randomized Phase II Trial. J Clin Oncol 2015;33:2450-6. [Crossref] [PubMed]
- Tie Y, Zheng H, He Z, et al. Targeting folate receptor beta positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct Target Ther 2020;5:6. [Crossref] [PubMed]
- Shimizu T, Nakanishi Y, Nakagawa Y, et al. Association between expression of thymidylate synthase, dihydrofolate reductase, and glycinamide ribonucleotide formyltransferase and efficacy of pemetrexed in advanced non-small cell lung cancer. Anticancer Res 2012;32:4589-96. [PubMed]
- Shimizu T, Nakagawa Y, Takahashi N, et al. Thymidylate synthase gene amplification predicts pemetrexed resistance in patients with advanced non-small cell lung cancer. Clin Transl Oncol 2016;18:107-12. [Crossref] [PubMed]
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017;14:531-48. [Crossref] [PubMed]
- Chen X, Zhou F, Li X, et al. Folate Receptor-Positive Circulating Tumor Cell Detected by LT-PCR-Based Method as a Diagnostic Biomarker for Non-Small-Cell Lung Cancer. J Thorac Oncol 2015;10:1163-71. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Chen X, Zhou C, Xuefei. Correlation of baseline value of folate receptor-positive circulating tumor cells and efficacy of pemetrexed and dynamic monitoring study in non-small cell lung cancer patients receiving first-line platinum-based chemotherapy. Cancer Res 2016;76:abstr nr 2252.
- Olaussen KA, Postel-Vinay S. Predictors of chemotherapy efficacy in non-small-cell lung cancer: a challenging landscape. Ann Oncol 2016;27:2004-16. [Crossref] [PubMed]
- Jiang T, Zhao J, Zhao C, et al. Dynamic Monitoring and Predictive Value of Circulating Tumor Cells in EGFR-Mutated Advanced Non-Small-Cell Lung Cancer Patients Treated With First-Line EGFR Tyrosine Kinase Inhibitors. Clin Lung Cancer 2019;20:124-133.e2. [Crossref] [PubMed]
- Tong B, Xu Y, Zhao J, et al. Prognostic role of circulating tumor cells in patients with EGFR-mutated or ALK-rearranged non-small cell lung cancer. Thorac Cancer 2018;9:640-5. [Crossref] [PubMed]
- Alama A, Coco S, Genova C, et al. Prognostic Relevance of Circulating Tumor Cells and Circulating Cell-Free DNA Association in Metastatic Non-Small Cell Lung Cancer Treated with Nivolumab. J Clin Med 2019;8:1011. [Crossref] [PubMed]
- Shishido SN, Carlsson A, Nieva J, et al. Circulating tumor cells as a response monitor in stage IV non-small cell lung cancer. J Transl Med 2019;17:294. [Crossref] [PubMed]
- Wang L, Wu C, Qiao L, et al. Clinical Significance of Folate Receptor-positive Circulating Tumor Cells Detected by Ligand-targeted Polymerase Chain Reaction in Lung Cancer. J Cancer 2017;8:104-10. [Crossref] [PubMed]
- Yu Y, Chen Z, Dong J, et al. Folate receptor-positive circulating tumor cells as a novel diagnostic biomarker in non-small cell lung cancer. Transl Oncol 2013;6:697-702. [Crossref] [PubMed]
- Zito Marino F, Ronchi A, Accardo M, et al. Detection of folate receptor-positive circulating tumor cells by ligand-targeted polymerase chain reaction in non-small cell lung cancer patients. J Thorac Dis 2016;8:1437-9. [Crossref] [PubMed]
- Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247-55. [Crossref] [PubMed]
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:2895-902. [Crossref] [PubMed]
- Theti DS, Jackman AL. The role of alpha-folate receptor-mediated transport in the antitumor activity of antifolate drugs. Clin Cancer Res 2004;10:1080-9. [Crossref] [PubMed]
- Stanger O. Physiology of folic acid in health and disease. Curr Drug Metab 2002;3:211-23. [Crossref] [PubMed]


