
Translating KEYNOTE-048 into practice recommendations for head and neck cancer
In 2016, immune checkpoint inhibitors entered the therapeutic portfolio of squamous cell carcinoma of the head and neck (SCCHN) in the second-line recurrent and/or metastatic setting, and it took another three years that the long-awaited results of the first-line KEYNOTE-048 trial demonstrated superiority relative to the standard cytotoxic doublet with cetuximab (1-3). As for a brief historical review, the combination of a platinum derivate, preferably cisplatin, and 5-fluorouracil emerged as a reference regimen for recurrent and/or metastatic SCCHN already in 1980s, although its impact on overall survival has rather been assumed on a basis of extrapolations than proved in large randomized trials (4). The major turning point came when the results of the EXTREME trial (Erbitux in first-line treatment of recurrent or metastatic head and neck cancer) were published back in 2008. Adding cetuximab, a monoclonal antibody against the epidermal growth factor receptor (EGFR), to a platinum/5-fluorouracil combination improved overall survival by almost 3 months at acceptable toxicity rates and no cetuximab-related deaths (3). Since then, the 10-month median survival mark achieved by EXTREME has repeatedly been confirmed in several large randomized trials using this regimen in the comparator arm (2,5,6). However, this progress related only to the first-line setting in platinum-sensitive patients. At that time, the outcomes of second-line treatment remained bleak with an expected median survival of 3 to 6 months (7).
This changed in 2016. The immune checkpoint inhibitor nivolumab, an immune-modulating monoclonal antibody against programmed cell death protein-1 (PD-1), became the first drug ever to significantly prolong median overall survival to about 8 months in platinum-resistant patients enrolled in the CheckMate-141 trial (1). Using pembrolizumab, another anti-PD-1 agent, this benefit was reproduced in the succeeding KEYNOTE-040 trial. Besides that, the latter trial introduced a long-awaited predictive biomarker, albeit originating from an exploratory analysis. With a P value for interaction of 0.015, the tumour proportion score (TPS), corresponding with the percentage of tumour cells with membranous staining of the ligand for PD-1 (PD-L1), of 50% or more was associated with improved benefit in comparison to a lower expression, particularly with respect to survival and tumour response (8). Subsequently, high hopes were put into the first-line administration of immune checkpoint inhibitors. The KEYNOTE-048 trial randomly assigned platinum-sensitive patients to receive either the standard of care, i.e., the EXTREME regimen, or a single-agent pembrolizumab, or a combination of the two, i.e., a platinum/5-fluorouracil doublet with pembrolizumab (2).
While the primary hypothesis of the two former immunotherapy studies focused solely on the superiority of the respective immune checkpoint inhibitors for overall survival, there were in total 14 primary hypotheses tested in KEYNOTE-048 both for progression-free and overall survivals, which emerged after four changes in the original protocol of the latter study. Using superiority or non-inferiority design according to selected hypothesis, the applied statistical analyses compared the standard-of-care arm with either pembrolizumab alone or pembrolizumab associated with chemotherapy, but not the two immunotherapy arms between each other. In 2016, more than one year after the KEYNOTE-048 start date, the fifth out of ten protocol amendments updated the pre-specified biomarker from TPS to the combined positive score (CPS). This modification was supported by preliminary results from two previous phase I-II studies, KEYNOTE-012 and KEYNOTE-055, which showed that inclusion of inflammatory cells, next to neoplastic cells, into PD-L1 scoring improves its predictive capacity for response, particularly when related to CPS cutpoints 1 and 20. Subsequently, the analyses were run separately in CPS ≥20, CPS ≥1, and total populations. The final efficacy and toxicity outcomes are summarized in Table 1 (1,2,8,9). Based on overall survival results, the authors concluded that pembrolizumab with chemotherapy should be considered the new standard in the first-line palliative setting, whereas pembrolizumab alone can be recommended for PD-L1 positive tumours (2).

Full table
However, the seemingly decreasing efficacy in analyses of populations with more patients having lower CPS, which becomes apparent when moving from CPS ≥20 to CPS ≥1 and then to total populations, incited questions about the benefit of immunotherapy in tumours with lower PD-L1 expression. Such subgroup analyses were not included in the peer-review publication but are available in an assessment report of the European Medicines Agency dating from October 17, 2019 (10). Here, the following information can be retrieved. In the CPS <1 subgroup, overall survival advantage was found neither in the comparison between pembrolizumab alone and EXTREME [7.9 vs. 11.3 months, respectively, hazard ratio (HR), 1.51; 95% confidence interval (CI), 0.96–2.37] nor between pembrolizumab/chemotherapy and EXTREME (11.3 vs. 10.7 months, respectively, HR, 1.21; 95% CI, 0.76–1.94). In the CPS ≥1 to CPS <20 population, the benefit was confined only to the pembrolizumab/chemotherapy combination (12.7 vs. 9.9 months, respectively, HR, 0.71; 95% CI, 0.54–0.94) but not pembrolizumab monotherapy (10.8 vs. 10.1 months, respectively, HR, 0.86; 95% CI, 0.66–1.12). Owing to insufficient power, the results of such post-hoc analyses are likely to be biased and should be thus regarded with caution. Nonetheless, they can still be considered hypothesis generating and provide in fact a plausible explanation for the observed correlation between PD-L1 expression and treatment effect.
Pros and cons of immunotherapy
Beyond any doubt, immune checkpoint inhibitors represent the therapeutic highlight of the past decade. They demonstrated clinical activity in heavily pre-treated patients after several previous chemotherapy lines, including platinum-resistant cases (1,8,11). Their generally mild toxicity profile exempt from most of the typical chemotherapy-related adverse events allow a safe and effective administration in elderly and probably also frail patients as shown in non-small-cell lung and urothelial cancers, respectively (12-15). Furthermore, nivolumab delayed time to quality of life deterioration in CheckMate-141 (1). However, they won recognition primarily for the encouraging long-term outcomes and complete responses, particularly in malignant melanoma. In CheckMate-141, less than 10% of patient population were expected to be alive after 3 years from treatment initiation (9). In KEYNOTE-048, longer follow-up is still needed, yet the plateau-phase of the presented survival curves strongly suggests the existence of a larger proportion of patients who may still be alive even at 5 years (2).
Although the rationale behind immunotherapy, that is to strengthen the immune system in the defence against cancer, seems to be a reasonable approach in almost every individual, the current armamentarium leaves many questions open. One of the greatest fears when treating patients with symptomatic unresectable tumour is the risk of progression. Local and/or regional recurrences of head and neck cancer often fulfil these criteria due to their growth in a relatively small, aesthetically exposed area near vital structures. The KEYNOTE-048 trial showed that the rate of progressive disease in both immunotherapy arms was numerically higher than in the EXTREME arm irrespective of CPS. Noteworthy, this rate remains relatively constant at about 40% when anti-PD-1 agents are used alone in both the first- and second-line settings (Table 1). We may speculate about the relationship between such progression and the lack of survival benefit brought by immunotherapy in patients presenting with local and/or regional recurrence only as seen in all KEYNOTE-048 subgroups (2). The perils of progression are further amplified by the risk of an accelerated tumour growth, also known as hyper progression, which may occur in as much as 25–29% of cases (16,17).
Despite the overall reassuring toxicity profile of immune checkpoint inhibitors, the adverse events might be more frequent than first thought and the reported treatment-related severe acute toxicity of 12% to 16% in the single-agent use thus underestimated (Table 1). This assumption is based on administrative claims data, on health-related quality of life scores, and more recently on the rate of all-cause grade 3–4 toxicity in KEYNOTE-048 reaching up to 46% in the pembrolizumab alone arm (2,4). Although data on all-cause toxicity are missing in CheckMate-141 and KEYNOTE-040, the discrepancy between the treatment-related and all-cause side effects in KEYNOTE-048 may reflect the downside of subjective toxicity evaluation by investigators but also the difficulty of recognizing the so called immune-related adverse events. Treatment-related mortality remains very low in the experimental arms but is still numerically higher than in the chemotherapy arms when looking at the two second-line trials (2 vs. 1 and 4 vs. 2 deaths, respectively) and at the pembrolizumab/chemotherapy arm against EXTREME in KEYNOTE-048 (11 vs. 8, respectively). Whether this subtle but noticeable increment in the risk of death has any meaning is really speculative, yet a consistent finding (1,2,8).
Moreover, mounting evidence, albeit retrospective or arising from indirect prospective comparisons, suggests non-inferiority of simultaneous versus sequential administration of chemotherapy and immunotherapy. This is in line with recent pharmacological findings suggesting that at a mechanistical level, clinical efficacy of many combination regimens is conferred by independent drug action without interaction (i.e., without additivity or synergy) (18). Indeed, the efficacy seems to be preserved or even improved with sequential versus simultaneous treatment and the risks of premature progression or immune-related side-effects reduced (4,19). In this respect, the choice of first-line cytotoxics, such as taxanes, may play an important role (20). It’s also worth mentioning that in KEYNOTE-048, patients allocated to EXTREME received more frequently second-line therapy (in 53%) than it was the case in the other two arms (49% after pembrolizumab monotherapy and 41% after pembrolizumab/chemotherapy regimen) (2).
However, there are some more unanswered questions following the KEYNOTE-048 report. First, a direct comparison between the two immunotherapy arms would help us better define the role of the individual therapeutic approaches. While in the total population and the CPS ≥1 group, overall survival was the highest in the pembrolizumab/chemotherapy arm, intermediate in the pembrolizumab arm, and the lowest in the EXTREME arm; in the CPS ≥20 group, pembrolizumab monotherapy yielded the longest survival, even better than pembrolizumab/chemotherapy. Understanding the shorter-than-expected survival in the latter arm could be crucial for treatment sequencing. In addition, the authors stated that there were more long-term survivors than was the proportion of long-term responders. Again, more detailed information would be welcomed, including disease characteristics and subsequent treatments in the respective subgroups. This holds also for the used platinum derivates because we learned from the EXTREME trial that cisplatin generates more responses than carboplatin and the overall survival benefit of adding cetuximab to platinum/5-fluorouracil doublet was limited to those who received cisplatin (3,7). Given that carboplatin was chosen for the majority of chemotherapy-receiving patients in KEYNOTE-048, it is of major interest to know the differences in responses and survival between these two drugs. In particular, it might have important implications if cisplatin was able to overcome at least some of the (hyper)progressions on immunotherapy, especially in local and/or regional recurrences.
New treatment algorithm for 2020
Due to the distinct pharmacodynamic features of immune checkpoint inhibitors, there are several factors impacting on the decision-making process in the recurrent and/or metastatic setting as depicted in Figure 1. Platinum compounds represent the chemotherapeutic backbone in palliative but also curative protocols and a distinction between platinum-sensitive and platinum-refractory disease has to be therefore made in the first place. Then we implemented four continuous functions which help steer our therapeutic preference to either chemotherapy or immunotherapy or in the context of first-line platinum-sensitive disease also to a combination of these two. These include pathologically determined CPS (from high to low) and clinically assessed biological age (from fitness to frailty), disease burden (from high to low), and pace of the disease (from fast to slow). The clinical utility of CPS has already been described above. We would like to stress that the given cut-off values are not to be regarded as a dogma. The results can be biased by tumour heterogeneity, inter-observer variability, and other possible methodological issues (21,22). This comes forward when dealing with borderline values around the predefined cut-off levels. At the same time, we should not forget that high CPS does not equate with tumour response, albeit we lack detailed analyses stratified according to CPS above the cutpoint 20.
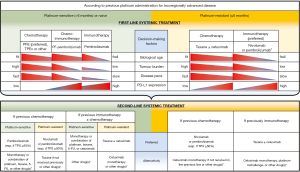
Biological age has already been mentioned in connection with arguments in favour of immunotherapy in frail persons. Although typically associated with advancing age (≥70 years) and involuntary weight loss (≥5% in one year), frailty can even exist in younger people, especially cancer patients. Appropriate indication of screening tests and geriatric assessment tools that encompass a thorough interdisciplinary appraisal contributes to personalisation of cancer care (23). The last two variables, burden and pace of the disease, have been introduced to address the low response rate and high risk of progression observed in patients treated with immunotherapy alone in comparison with cytotoxic agents. Typically relying on individual clinical judgement and imaging modalities, no standardized methods or scales have been validated for their measurement. Therefore, patients with a bulky, widespread, or rapidly progressive disease might rather be considered for a regimen incorporating chemotherapy. In this respect, the presence or absence of symptoms, organ dysfunctions, or their imminent threat can serve as a good guiding clue. Notably, the median time to response was comparable between immunotherapy and chemotherapy in KEYNOTE-048 and CheckMate-141 but was longer in the pembrolizumab arm in KEYNOTE-040 (median: 4.5 vs. 2.2 months) (1,2,8). A different concept applies to an indolent or oligoprogressive disease. Here, early systemic treatment initiation should be weighed against the use of local ablation strategies (4). Some investigators even question that in all circumstances early intervention is always better than delayed initiation of treatment (24).
Opting for a drug class or a combination thereof is followed by selecting a treatment regimen or enrolling the patient in a clinical trial. Immune checkpoint inhibitors validated for SCCHN have been limited to two anti-PD-1 agents so far. In principle, nivolumab and pembrolizumab are interchangeable, and the inconsistency in biomarker recommendations (CPS, TPS, or none in CheckMate-141) is primarily driven by the respective trial designs (25). On the other hand, the choice of chemotherapeutics can be more challenging. According to local regulations and policies, the standard first-line regimen for platinum-sensitive patients, EXTREME, has often been modified or replaced by taxane-containing protocols. Prioritizing cisplatin before carboplatin can improve local control in symptomatic and/or bulky disease. In the second line, no randomized trials demonstrated a superiority of one chemotherapy over another. With this limitation in mind, taxanes alone or in combination, for example, with cetuximab seem to provide a clinically relevant benefit (4). In BERIL-1, a randomized phase II trial, paclitaxel plus placebo achieved a disease control rate of 70% with only one quarter of patients who had progression as their best response (26). A subgroup analysis of both second-line immunotherapy trials, CheckMate-141 and KEYNOTE-040, found no survival difference between the PD-1 inhibitor and single-agent docetaxel (1,8). Last but not the least, based on disease-control rates of about 50% and acceptable toxicity profile, cetuximab monotherapy is reimbursed in some countries in the second line, although its benefit might be limited to human papillomavirus (HPV)-negative disease (27).
Conclusions and future outlooks
The purpose of our treatment algorithm is to show the complexity of therapeutic landscape in recurrent and/or metastatic SCCHN and propose possible options in sequencing of systemic treatment. It is critical to recognize that all of the presented decision-making factors (excluding platinum-sensitivity/resistance) are continuous variables and should all be taken into account conjointly while respecting patient autonomy and local resources. In view of profound inequities in the accessibility of modern therapeutic approaches across the globe, the latter factor cannot be underestimated. However, good availability and affordability of immunotherapy per se do not guarantee an optimal cancer care and may sometimes even be harmful, such as when treatment recommendations are adopted prior to peer-review publications, especially in the context of accumulating negative results from other immunotherapy trials (Active8, CONDOR, EAGLE, and CheckMate-714) (28). Nevertheless, we remain optimistic and look forward to the upcoming reports of KEYNOTE-048 and other exciting news in the field of cancer immunotherapy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.164). PS has had in the last three years consulting/advisory relationships with Merck-Serono, Servier, and BMS and has received honoraria from Merck-Serono. JBV has had in the last three years consulting/advisory relationships with Immunomedics, Innate Pharma, Merck-Serono, Merck Sharp & Dome Corp, PCI Biotech, Synthon Biopharmaceuticals, Debiopharm, Cue Biopharma, and WntResearch and has received honoraria from Merck-Serono, MSD, and BMS.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375:1856-67. [Crossref] [PubMed]
- Burtness B, Harrington KJ, Greil R. at al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915-28. [Crossref] [PubMed]
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116-27. [Crossref] [PubMed]
- Szturz P, Vermorken JB. Management of recurrent and metastatic oral cavity cancer: Raising the bar a step higher. Oral Oncol 2020;101:104492. [Crossref] [PubMed]
- Vermorken JB, Peyrade F, Krauss J, et al. Cisplatin, 5-fluorouracil, and cetuximab (PFE) with or without cilengitide in recurrent/metastatic squamous cell carcinoma of the head and neck: results of the randomized phase I/II ADVANTAGE trial (phase II part). Ann Oncol 2014;25:682-8. [Crossref] [PubMed]
- Ferris RL, Saba NF, Gitlitz BJ, et al. Effect of Adding Motolimod to Standard Combination Chemotherapy and Cetuximab Treatment of Patients With Squamous Cell Carcinoma of the Head and Neck: The Active8 Randomized Clinical Trial. JAMA Oncol 2018;4:1583-8. [Crossref] [PubMed]
- Szturz P, Vermorken JB. Systemic Treatment of Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck. In: Bernier J, editor. Head and Neck Cancer: Multimodality Management (2nd edition). Cham: Springer International Publishing AG 2016:711-29.
- Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156-67. [Crossref] [PubMed]
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45-51. [Crossref] [PubMed]
- European Medicines Agency. Assessment report EMA/CHMP/591139/2019. Available online: https://www.ema.europa.eu/en/documents/variation-report/keytruda-h-c-3820-ii-0065-epar-assessment-report-variation_en.pdf. Accessed on January 19, 2020.
- Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016;17:956-65. [Crossref] [PubMed]
- Grossi F, Crinò L, Logroscino A, et al. Use of nivolumab in elderly patients with advanced squamous non-small-cell lung cancer: results from the Italian cohort of an expanded access programme. Eur J Cancer 2018;100:126-34. [Crossref] [PubMed]
- Nosaki K, Saka H, Hosomi Y, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 2019;135:188-95. [Crossref] [PubMed]
- Balar AV, Castellano D, O'Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483-92. [Crossref] [PubMed]
- Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67-76. [Crossref] [PubMed]
- Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605-11. [Crossref] [PubMed]
- Lo Russo G, Moro M, Sommariva M, et al. Antibody-Fc/FcR Interaction on Macrophages as a Mechanism for Hyperprogressive Disease in Non-small Cell Lung Cancer Subsequent to PD-1/PD-L1 Blockade. Clin Cancer Res 2019;25:989-99. [Crossref] [PubMed]
- Palmer AC, Sorger PK. Combination Cancer Therapy Can Confer Benefit via Patient-to-Patient Variability without Drug Additivity or Synergy. Cell 2017;171:1678-91. [Crossref] [PubMed]
- Even C, Torossian N, Ibrahim T, et al. First-line versus second-line immunotherapy in recurrent/metastatic squamous cell carcinoma of the head and neck. Ann Oncol 2019.30. abstract 1138P.
- Guigay J, Fayette J, Mesia R, et al. TPExtreme randomized trial: TPEx versus Extreme regimen in 1st line recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol 2019;37:abstr 6002.
- Rasmussen JH, Lelkaitis G, Håkansson K, et al. Intratumor heterogeneity of PD-L1 expression in head and neck squamous cell carcinoma. Br J Cancer 2019;120:1003-6. [Crossref] [PubMed]
- Butter R, 't Hart NA, Hooijer GKJ, et al. Multicentre study on the consistency of PD-L1 immunohistochemistry as predictive test for immunotherapy in non-small cell lung cancer. J Clin Pathol 2020;73:423-30. [Crossref] [PubMed]
- Szturz P, Bossi P, Vermorken JB. Systemic treatment in elderly head and neck cancer patients: recommendations for clinical practice. Curr Opin Otolaryngol Head Neck Surg 2019;27:142-50. [Crossref] [PubMed]
- Sacco AG, Cohen EE. Current Treatment Options for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. J Clin Oncol 2015;33:3305-13. [Crossref] [PubMed]
- Fessas P, Lee H, Ikemizu S, et al. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin Oncol 2017;44:136-40. [Crossref] [PubMed]
- Soulières D, Faivre S, Mesía R, et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol 2017;18:323-35. [Crossref] [PubMed]
- Szturz P, Seiwert TY, Vermorken JB. How Standard Is Second-Line Cetuximab in Recurrent or Metastatic Head and Neck Cancer in 2017? J Clin Oncol 2017;35:2229-31. [Crossref] [PubMed]
- Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol 2019;99:104460. [Crossref] [PubMed]


