
The anti-tumor effect of taxifolin on lung cancer via suppressing stemness and epithelial-mesenchymal transition in vitro and oncogenesis in nude mice
Introduction
Lung cancer, of which non-small cell lung cancer (NSCLC) comprises the vast majority (85%) of cases, is the leading global cause of cancer-related death in both men and women (1). Many patients have already developed metastasis by the time they are diagnosed; consequently, the five-year survival rate is less than 20% (2,3). Therefore, for patients with the disease, proper classification-based administration as well as early diagnosis are desperately needed, as is the development of more effective targeted therapies (4).
Cancer stem cells (CSCs) are cancer cells which possess stem cell-like properties. As well as being responsible for tumor growth, CSCs are also considered to be a cause of drug resistance, tumor recurrence, and epithelial-to-mesenchymal (EMT) reprogramming-enabled metastasis (5). For patients with NSCLC, there is currently no effective CSCs targeted therapy. Some signal transduction pathways have been studied as potential therapeutic targets of lung cancer, including the VEGF, p38 MAPK, Wnt, NF-κB, and PI3K signaling pathways and recent immune checkpoints (6,7); however, more therapeutic improvement is needed to improve the efficacy of such therapies. Thus, in-depth study into the effects of targeting CSCs is vital.
Taxifolin (3,5,7,3’,4’-pentahydroxyflavanone or dihydroquercetin), a member of the flavonoid family, has been shown to possess antioxidant, anti-inflammatory, hepatoprotective, anti-Alzheimer’s disease, and anti-angiogenic properties (8). Recently, taxifolin has also attracted attention for its antitumor activity. The antitumor mechanism of taxifolin mainly includes the inhibition of angiogenesis, cytochrome P450 enzymes, P-glycoprotein, reactive oxidative species (ROS), and cell cycle regulators, as well as the induction of apoptosis (8). These multiple effects in a single compound present taxifolin as a possible therapeutic agent or an adjuvant in cancer therapy.
The inhibition of stemness and EMT of taxifolin has been directly or indirectly indicated in several tumors. Taxifolin modifies bioactive biomaterials to promote the differentiation of human umbilical cord-derived mesenchymal stem cells to osteoblasts (9). Osteogenic differentiation, which is enhanced by taxifolin, was shown to be a result of the inhibition of the NF-κB signaling pathway. Other signaling pathways involved in the stemness of tumors include the Janus family tyrosine kinase (JAK)/signal transducer and activator of transcription (STAT), Notch, PI3K/AKT serine/threonine kinase, SHH, and Wnt/β-catenin pathways (5). The involvement of taxifolin in most of these signaling pathways has been demonstrated in recent studies (10-12). Remarkably, Li et al. recently found that taxifolin enhanced MET of highly aggressive breast cancer cells via β-catenin signaling (13), which directly supported the EMT regulating role of taxifolin. In this paper, we investigated the possible effects of taxifolin in two NSCLC cell lines, A549 and H1975, in vitro and in vivo, focusing in particular on its potential regulatory activity in stemness and EMT.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3329).
Methods
Animal experiments
Twelve six-week-old male BALB/c null nude mice were purchased from Guangdong Medical Laboratory Animal Center (Guangdong, China). The mice were acclimated for two weeks before the study; they were housed in climate-controlled quarters with a 12 h/12 h light/dark cycle, with free access to food and water. Approximately 1×106 A549 cells with matrigel (BD Biosciences, San Jose, CA, USA) at 1:1 dilution were injected subcutaneously into the right flank region of 12 of the mice. After 5 days, the mice were randomly divided into two groups and intraperitoneally injected with 1 mg/kg of taxifolin or saline once a day for 25 days, as described in previous experiments (14). After 25 days, the mice were anesthetized and sacrificed, and the tumors were harvested for photographing and subsequent experiments.
The animal experiments in this study were approved by the Ethics Committee of the People’s Hospital of Zhangqiu. Each experiment was performed in strict accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition, published by the National Research Council (US) Committee.
Cell culture and reagents
A549 and H1975 cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were kept in RPMI-1640 medium (HyClone, UT, USA) with 9% fetal bovine serum (Thermo Fisher Scientific, MA, USA) and 1% Penicillin-Streptomycin Solution (Solarbio, Beijing, China). Cell Counting Kit-8 (CCK-8) was purchased from Beijing Solarbio Science & Technology Co., Ltd. B27, epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) were obtained from Invitrogen (CA, USA). Primary antibodies, including anti-Ki67 (ab92742), anti-PCNA (ab92552), anti-CD133 (ab16518), anti-SOX2 (ab97959), anti-OCT4 (ab181557), anti-E-cadherin (ab40772), anti-N-cadherin (ab18203), anti-Vimentin (ab8069), anti-PI3K (ab32089), anti-p-PI3K (ab182651), anti-TCF4 (ab217668), anti-P65 (ab16502), anti-p-P65 (ab86299), anti-STAT3 (ab119352), and anti-p-STAT3 (ab76315) were purchased from Abcam (Cambridge, UK). Anti-Actin (#3700) and all secondary antibodies were bought from PTG Company (Rosemont, IL, USA). Crystal Violet Staining Solution and other reagents were purchased from Beyotime Biotechnology (Shanghai, China).
Cell viability
The viability of A549 and H1975 cells under treatment of taxifolin at different concentrations was tested. A549 and H1975 cells in good condition were digested and seeded into 96-well plates at a density of 1×104 cells/well in 100 µL medium. Different concentrations of taxifolin were obtained by mixing 100 µL culture medium with a 500 mM/L taxifolin stock solution. At 24 h after seeding, the mixtures containing different concentrations of taxifolin were added to the wells for a further 23 h incubation. Next, 20 µL of CCK-8 was added to each well. One hour later, the optical density (OD) was read measured by a microplate reader (Bio-Rad, Hercules, USA) at a wavelength of 450 nm. There were five replicates for each concentration.
Colony formation
Resuspended A549 and H1975 cells were randomly seeded into 6-well plates in 1 mL culture medium at a density of 1×103 cells/well. After the first 6 h 2 mL medium containing 0, 25, 50, or 100 µM/L of taxifolin was refreshed. The culture mediums containing different concentrations of taxifolin were refreshed every three days. The cells were then incubated for 10 days, before staining with 0.1% (W/V) crystal violet.
Sphere formation assay
A549 and H1975 cells were seeded in low adherent 24-well culture plates at a density of 2×103 cells per well, and incubated in 0, 25, 50 or 100 µM/L taxifolin with RPMI 1640 containing 20 µL/mL of B27, 20 ng/mL of EGF, 20 ng/mL of bFGF, and 1% of penicillin-streptomycin, in serum-free conditions. After 10 days of incubation at 37 °C in 5% CO2, pictures were taken under a microscope, and the number of spheres was counted in three fields.
Western blotting
A549 and H1975 cells or tissues were lysed and kept on ice 24 h after 0, 25, 50 or 100 µM/L taxifolin was added or after photographing of the tumors. Bradford assay was then applied to calculate the protein concentration of each sample and balanced with PBS. For each sample, approximately 40 µg in 20 µL of proteins was used and the proteins were separated by SDS-PAGE gel electrophoresis. Then the separated proteins was transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Primary and secondary antibodies were incubated according to the manufacturers' protocols. The proteins of interest were visualized by enhanced chemiluminescence reagents with ChemiDoc XRS (Bio−Rad, Hercules, USA). Blots were analyzed with Image J software (National Institutes of Health, Bethesda, USA). Actin was used as endogenous control.
Flow cytometry
Fluorescence-activated cell-sorting (FACS) assay was performed to test A549 and H1975 cell proliferation under treatment of 0, 25, 50, or 100 µM/L taxifolin for 24 h. For each well, 100 µL of cells at a density of 1×107 were incubated with 10 µL anti-CD133-PE in the dark at 4 °C for 10 min. Then, the cells were washed twice with buffer and suspended in 500 µL of buffer for analysis by flow cytometry.
Transwell assay
A549 and H1975 cell invasion was analyzed by Transwell assay. Briefly, A549 and H1975 cells were seeded in the upper chamber of the Transwell with DMEM supplemented with 0.1% FBS and 0, 25, 50, or 100 µM/L taxifolin was added. The lower chamber was filled with DMEM supplemented with 10% FBS with 0, 25, 50 or 100 µM/L taxifolin. After 24 h of incubation, the A549 and H1975 cells in the bottom chamber were fixed in 95% ethanol, stained with hematoxylin, and the number of invaded A549 and H1975 cells were counted using a DM2500 bright field microscope at 40× fields on 10 random fields of each well.
Immunohistochemical (IHC) analysis
Immunohistochemical (IHC) staining was performed (Dako Envision plus System, Dako, Carpinteria, CA, USA) according to the manufacturer’s instructions. Briefly, the tumors were fixed in 4% paraformaldehyde overnight and dehydrated with a series of ethanol and xylene solutions, then embedded in paraffin wax. Next, 5-µm-thick sections were sliced, the wax was washed out, and the sections were rehydrated with a series of xylene and ethanol solutions. The samples were blocked with 10% goat serum and then incubated with SOX2 and OCT4 primary antibodies overnight. Then, the samples were incubated with secondary antibody for 30 min. Finally, the positively stained cells were evaluated by digital image analysis with Image J software.
Statistical analysis
Data were presented as mean ± standard deviation (SD) and analyzed using analysis of variance (ANOVA) or Student’s t-test with GraphPad Prism 7.0. Statistical significance was represented by P<0.05. All in vitro experiments were independently repeated at least 3 times.
Results
Taxifolin inhibited proliferation of A549 and H1975
The viability of A549 and H1975 cells was tested by cell counting kit-8 assay. Under treatment of taxifolin for 24 h, cell viability was decreased dose dependently in both cell lines (Figure 1A). Treatment with about 200 µM/L of taxifolin started to show significantly lower viability compared with the control, so 0, 25, 50 and 100 µM/L of taxifolin were used in later experiments to show its effects and mechanisms. Colony formation was further applied to validate the inhibitive effect of taxifolin on proliferation. As expected, taxifolin dose dependently inhibited colony formation in A549 and H1975 cells (Figure 1B,C). In both experiments, the A549 cells appeared to be more sensitive to taxifolin compared to the H1975 cells. Then the inhibited proliferation was validated with protein expression of Ki67 and PCNA (Figure 1D). As expected, adding of taxifolin significantly reduced Ki67 and PCNA in both cell lines (Figure 1E,F).
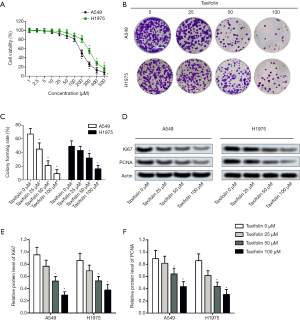
Taxifolin inhibited stemness of A549 and H1975 cells
Colony formation was then tested in A549 and H1975 cells treated with taxifolin. As indicated in Figure 2A, taxifolin dose dependently inhibited sphere formation of both cell lines. The diameter of spheres (Figure 2B) and the number of spheres per 100 cells (Figure 2C) were significantly decreased with 50 µM of taxifolin. Next, markers of stem-like properties including SOX2 and OCT4 were tested by Western blotting (Figure 2D). Treatment with 25 µM of taxifolin was able to markedly reduce the expression of SOX2 and OCT4 in both cell lines (Figure 2E,F). CD133-positive cells among the taxifolin-treated cells were further tested by flow cytometry to validate the inhibited stemness. As expected, taxifolin reduced the number of CD133 positive cells in both S549 and H1975 cells in a dose-dependent manner (Figure 2G,H). All these results reflected the inhibitive effect of taxifolin on the stemness of A549 and H1975 lung cancer cell lines.
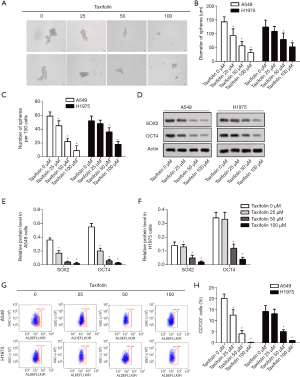
Taxifolin inhibited epithelial-mesenchymal transition of A549 and H1975 cells
The invasive cells were dose-dependently decreased in both the A549 and H1975 cells (Figure 3A,B). The inhibition of epithelial-mesenchymal transition was further validated by Western blotting (Figure 3C). Taxifolin dose-dependently increased E-cadherin while N-cadherin and vimentin were decreased in both A549 (Figure 3D) and H1975 (Figure 3E) cells.
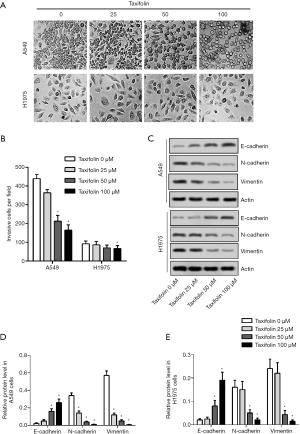
Inactivation of PI3K and TCF4 was involved in the mechanism of action of taxifolin
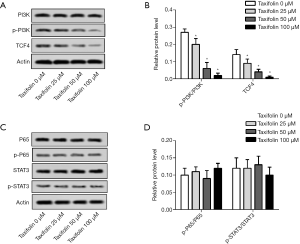
To explore taxifolin’s mechanism of action in the inhibition of A549 and H1975 cells, the activation of PI3K, TCF4, NF-κB P65, and STAT3 was evaluated by Western blotting. P-PI3K/PI3K and TCF4 were decreased in the A549 cells (Figure 4A,B), but no significant change was observed in NF-κB P65 and STAT3 (Figure 4C,D).
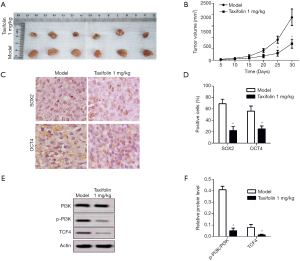
Taxifolin inhibited tumor growth in xenograft mice
Furthermore, the antitumor effects of taxifolin were investigated in A549 Xenograft BALB/c null nude mice. After 25 days of treatment with 1 mk/kg/day of taxifolin, A549 xenografts were significantly inhibited (Figure 5A), and the tumor volumes of the treatment group were reduced compared to the model (Figure 5B). The levels of OCT4 and SOX2 in the tumor tissues were also decreased in the taxifolin treatment group by IHC staining (Figure 5C,D). Finally, the expression levels of PI3K, p-PI3K, and TCF4 in the tumor tissues were tested. The relative expression levels of p-PI3K/PI3K and TCF4 were significantly inhibited with 1mk/kg/day of taxifolin (Figure 5E,F). Altogether, these results indicated that taxifolin suppresses tumor growth, by decreasing epithelial-mesenchymal transition and inhibiting PI3K and TCF4 signaling.
Discussion
Lung cancer affects more people than any other cancer, which means the discovery of effective therapeutics is crucial (15). The heterogeneity of small cell lung CSCs makes it difficult for a single compound to achieve an inhibitive effect (4). Consequently, multi-targeted therapeutics have long been an important aspect of new drug development. In the present study, we showed that taxifolin dose-dependently suppressed viability, stem-like properties, and EMT in A549 and H1975 cells. The suppressive effect of taxifolin in lung cancer cell lines involves PI3K and TCF4 inhibition, but not the inhibition of NF-κB P65 or STAT3. The administration of taxifolin also suppressed tumor growth in A549 Xenograft BALB/c null nude mice, which was accompanied with decreased expression of SOX2, OCT2, p-PI3K/PI3K, and TCF4.
The tumor suppressive role of taxifolin has been characterized across several cell lines and in vivo studies. The mechanism of action of taxifolin mainly includes the suppression of cytochrome P450, CDKs, the generation of ROS, angiogenesis, as well as the inhibition of autophagy and induction of apoptosis (8). Taxifolin usually targets several molecules to inhibit tumors. For example, taxifolin suppressed UVB-induced phosphorylation of endothelial growth factor receptor (EGFR) and Akt, subsequently suppressing their signaling pathways; and activates the Nrf2 anti-oxidative stress pathway to inhibit EMT in skin cancer cells (14,16). Taxifolin uncompetitively inhibits P-Glycoprotein to resensitize human multidrug resistant cell lines (17). In breast cancer, taxifolin has been shown to inhibit Wnt signaling to subsequently inhibit EMT and metastasis (13), and downregulate Aryl hydrocarbon receptor (AhR)/CYP1A1 to inhibit tumor growth (18). To some extent, the complex effects of taxifolin were expected, as its structure is similar in part to the inhibitors of a number of receptors, and such small molecules often have many receptors. In this study, we investigated the possible role of taxifolin in lung cancer from the perspective of stemness and EMT regulation, and found taxifolin to be effective in these fields.
Stemness is mostly regulated via the Wnt, Notch, SHH, PI3K/AKT, JAK/STAT and/or NF-κB P65 pathways. Stemness in NSCLC was thought to promote growth, metastasis, and therapy resistance, all of which are the main obstacles for current therapies (5). Taxifolin was reported to be involved in the direct or indirect regulation of stemness via multiple pathways. Taxifolin arrested cell cycle at the G2 phase by regulating the Wnt/beta-catenin and AKT signaling pathways in human colorectal cancer cell lines HCT116 and HT29 (12). Taxifolin could attenuate murine psoriasis by regulating the Notch1 and JAK2/STAT3 signal pathways and Th cell differentiation, decreasing the ratio of proinflammatory Th1 and Th17 cells in both skin lesions and skin draining lymph nodes (10). Other stemness regulating effects of taxifolin include the suppression of skin carcinogenesis via EGFR and PI3K targeting (14), and enhancing osteogenic differentiation via the NF-kappaB pathway (19). All of these previous findings strongly support the possibility that taxifolin could be a regulator of stemness. In this research, we showed that taxifolin did reduce stemness in lung cancer cell lines A549 and H1975, as well as in A549 xenograft mice, as indicated by inhibited sphere formation, decreased expression of SOX2 and OCT4, and reduced CD133-positive cells. Key regulators in the PI3K, Wnt, NF-κB, and JAK/STAT pathways were investigated, and PI3K and Wnt signaling was found to possibly be responsible for the reduced stemness in taxifolin-treated cells.
EMT was widely accepted as an early event in metastasis (20). Generally metastatic tumor cells are considered stem cells, and almost all stemness regulating pathways including Wnt, Notch, SHH, PI3K/AKT, JAK/STAT, and NF-κB P65 are involved in EMT regulation (5). Therefore, taxifolin-regulated stemness could possibly affect EMT in both A549 and H1975 cells. Taxifolin is also involved in EMT regulation in breast cancer cells through the Wnt signaling pathway and in skin cancer via the regulation of Nrf2 signaling (13,16). Other regulators of EMT mainly include some growth factors and MAPK signaling. Taxifolin is a potent angiogenesis inhibitor owing to its inhibitory effect on the specific autophosphorylation sites of vascular endothelial growth factor receptor (VEGFR)-2 (21), and the targeting of EGFR by taxifolin suppressed UV-induced mouse skin cancer (14). In the present study, EMT was verified with Transwell, and reduced expression levels of N-cadherin and vimentin and an increased level of E-cadherin were observed. The reduced EMT seen with taxifolin could be a result of inhibited stemness or a direct consequence of its effect on EMT-regulating signaling pathways, such as EGFR. As this paper is focused purely on the stemness-related effects of taxifolin, these other mechanisms need to be further investigated.
Flavonoids are one of the most abundant naturally occurring products. Flavonoids derived structures are seen in numerous bioactive chemicals (22). Fruits and vegetables containing flavonoids have been reported as having a cancer chemopreventive effect. The mechanism of action of flavonoids mainly includes the regulation of Ras and P53 protein (23), cell cycle arrest (24), tyrosine kinase inhibition (25), the inhibition of heat shock proteins (26), and Estrogen receptor binding capacity (27). Taxifolin has been used in health products, and different formulations of taxifolin have been developed. Taxifolin has even been proved to be effective in suppressing amyloid-beta production and beneficially modulating proinflammatory microglial phenotypes (28), to block PD-1/PD-L1 CTLA-4/CD80 immune checkpoint (29) and to inhibit the activity of carbohydrate-hydrolyzing enzymes, reducing dietary carbohydrate absorption (30). Many structures that are similar to taxifolin have been reported to harbor hepatoprotective and anti-tumor effects. For example silybin, which can be synthesized from coupling of taxifolin and coniferyl alcohol, has been used in a clinical setting for hepatoprotective purposes (31). As most clinical therapeutic agents are harmful to the liver, the co-administration of taxifolin or its analogs/derivatives could potentially be beneficial. However, there are possibilities these previous reports contain bias, and whether or not flavonoids including taxifolin could compete receptors with targeted therapies requires further investigation.
There are some limitations to this study. As the mechanisms of action of almost all flavonoids, including taxifolin, are complicated (22), we only tested its effects on lung cancer cells in relation to the regulation of stemness. Of course, the mechanism of action for taxifolin in inhibiting lung cancer encompasses much more than stemness regulation. Furthermore, the outcome of taxifolin therapy can be affected by liver toxicity, oxidative stress, immune checkpoints, and even the absorption of nutrients. Therefore, the specific health benefits of taxifolin, especially for cancer patients require much more investigation.
In conclusion, taxifolin inhibits the viability, stemness, and EMT of lung cancer in vitro and in vivo. The inhibition of viability and EMT in both A549 and H1975 cells could be a result of stemness suppression. These results highlight the possible beneficial effects of taxifolin as a lung cancer treatment, either alone or as an adjuvant, and support further anticancer drug development based on taxifolin and other flavonoids.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3329
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-3329
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3329). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The animal experiments in this study were approved by the Ethics Committee of People’s Hospital of Zhangqiu.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Duruisseaux M, Esteller M. Lung cancer epigenetics: From knowledge to applications. Semin Cancer Biol 2018;51:116-28. [Crossref] [PubMed]
- Shankar A, Dubey A, Saini D, et al. Environmental and occupational determinants of lung cancer. Transl Lung Cancer Res 2019;8:S31-S49. [Crossref] [PubMed]
- Osmani L, Askin F, Gabrielson E, et al. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol 2018;52:103-9. [Crossref] [PubMed]
- Lin CC. Challenges of the phase I drug development in non-small cell lung cancer. Chin Clin Oncol 2019;8:25. [Crossref] [PubMed]
- Masciale V, Grisendi G, Banchelli F, et al. Correlating tumor-infiltrating lymphocytes and lung cancer stem cells: a cross-sectional study. Ann Transl Med 2019;7:619. [Crossref] [PubMed]
- Shroff GS, de Groot PM, Papadimitrakopoulou VA, et al. Targeted Therapy and Immunotherapy in the Treatment of Non-Small Cell Lung Cancer. Radiol Clin North Am 2018;56:485-95. [Crossref] [PubMed]
- Ai X, Guo X, Wang J, et al. Targeted therapies for advanced non-small cell lung cancer. Oncotarget 2018;9:37589-607. [Crossref] [PubMed]
- Sunil C, Xu B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019;166:112066. [Crossref] [PubMed]
- Córdoba A, Satue M, Gomez-Florit M, et al. Flavonoid-modified surfaces: multifunctional bioactive biomaterials with osteopromotive, anti-inflammatory, and anti-fibrotic potential. Adv Healthc Mater 2015;4:540-9. [Crossref] [PubMed]
- Yuan X, Li N, Zhang M, et al. Taxifolin attenuates IMQ-induced murine psoriasis-like dermatitis by regulating T helper cell responses via Notch1 and JAK2/STAT3 signal pathways. Biomed Pharmacother 2020;123:109747. [Crossref] [PubMed]
- Zhou W, Guo Z. Taxifolin inhibits the scar cell carcinoma growth by inducing apoptosis, cell cycle arrest and suppression of PI3K/AKT/mTOR pathway. J BUON 2019;24:853-8. [PubMed]
- Razak S, Afsar T, Ullah A, et al. Taxifolin, a natural flavonoid interacts with cell cycle regulators causes cell cycle arrest and causes tumor regression by activating Wnt/beta-catenin signaling pathway. BMC Cancer 2018;18:1043. [Crossref] [PubMed]
- Li J, Hu L, Zhou T, et al. Taxifolin inhibits breast cancer cells proliferation, migration and invasion by promoting mesenchymal to epithelial transition via beta-catenin signaling. Life Sci 2019;232:116617. [Crossref] [PubMed]
- Oi N, Chen H, Ok Kim M, et al. Taxifolin suppresses UV-induced skin carcinogenesis by targeting EGFR and PI3K. Cancer Prev Res (Phila) 2012;5:1103-14. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Kuang H, Tang Z, Zhang C, et al. Taxifolin Activates the Nrf2 Anti-Oxidative Stress Pathway in Mouse Skin Epidermal JB6 P+ Cells through Epigenetic Modifications. Int J Mol Sci 2017. [Crossref] [PubMed]
- Chen HJ, Chung YL, Li CY, et al. Taxifolin Resensitizes Multidrug Resistance Cancer Cells via Uncompetitive Inhibition of P-Glycoprotein Function. Molecules 2018. [Crossref] [PubMed]
- Haque MW, Pattanayak SP. Taxifolin Inhibits 7,12-Dimethylbenz(a)anthracene-induced Breast Carcinogenesis by Regulating AhR/CYP1A1 Signaling Pathway. Pharmacogn Mag 2018;13:S749-S755. [PubMed]
- Wang YJ, Zhang HQ, Han HL, et al. Taxifolin enhances osteogenic differentiation of human bone marrow mesenchymal stem cells partially via NF-kappaB pathway. Biochem Biophys Res Commun 2017;490:36-43. [Crossref] [PubMed]
- Yeung KT, Yang J. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol 2017;11:28-39. [Crossref] [PubMed]
- Lamy S, Ouanouki A, Beliveau R, et al. Olive oil compounds inhibit vascular endothelial growth factor receptor-2 phosphorylation. Exp Cell Res 2014;322:89-98. [Crossref] [PubMed]
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal 2013;2013:162750. [Crossref] [PubMed]
- Moon SH, Lee CM, Nam MJ. Cytoprotective effects of taxifolin against cadmium-induced apoptosis in human keratinocytes. Hum Exp Toxicol 2019;38:992-1003. [Crossref] [PubMed]
- Chen X, Gu N, Xue C, et al. Plant flavonoid taxifolin inhibits the growth, migration and invasion of human osteosarcoma cells. Mol Med Rep 2018;17:3239-45. [PubMed]
- Lee WJ, Wu LF, Chen WK, et al. Inhibitory effect of luteolin on hepatocyte growth factor/scatter factor-induced HepG2 cell invasion involving both MAPK/ERKs and PI3K-Akt pathways. Chem Biol Interact 2006;160:123-33. [Crossref] [PubMed]
- Verma S, Singh A, Mishra A. Dual inhibition of chaperoning process by taxifolin: molecular dynamics simulation study. J Mol Graph Model 2012;37:27-38. [Crossref] [PubMed]
- An HJ, Lee Y, Liu L, et al. Physical and Chemical Stability of Formulations Loaded with Taxifolin Tetra-octanoate. Chem Pharm Bull (Tokyo) 2019;67:985-91. [Crossref] [PubMed]
- Inoue T, Saito S, Tanaka M. Pleiotropic neuroprotective effects of taxifolin in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A 2019;116:10031-8. [Crossref] [PubMed]
- Li W, Kim TI. Immune Checkpoint PD-1/PD-L1 CTLA-4/CD80 are Blocked by Rhus verniciflua Stokes and its Active Compounds. Molecules 2019. [Crossref] [PubMed]
- Yoon KD, Lee JY, Kim TY, et al. In Vitro and in Vivo Anti-Hyperglycemic Activities of Taxifolin and Its Derivatives Isolated from Pigmented Rice (Oryzae sativa L. cv. Superhongmi). J Agric Food Chem 2020;68:742-50. [Crossref] [PubMed]
- Yang J, Liang J, Shao L, et al. Green production of silybin and isosilybin by merging metabolic engineering approaches and enzymatic catalysis. Metab Eng 2020;59:44-52. [Crossref] [PubMed]


