
The clinical application of mesenchymal stem cells in liver disease: the current situation and potential future
Introduction
Liver disease is a major health problem and caused by various etiologies around the world. Acute liver failure (ALF), liver cirrhosis and liver cancer are the main common liver diseases. ALF is a fatal clinical syndrome characterized by extensive hepatocyte necrosis and inflammatory infiltration caused by hepatotoxic drugs, immune-mediated attacks or viral infections (1,2). In the clinical, ALF progressed rapidly with a poor presentation to medical treatment (3). Cirrhosis is the end stage of liver fibrosis and prone to various complications including infection, hemorrhage, hepatic encephalopathy (HE) and spontaneous peritonitis, etc. According to the latest global report, liver cancer ranked sixth for cancer incidence and fourth for cancer deaths in 2015 (4). Hepatocellular carcinoma (HCC) accounts for 85–90% of primary liver cancer (5). At present, liver transplantation is recognized as the most effective treatment for advanced liver diseases. However, there is a prevailing contradiction between urgent clinical need and the shortage of donor livers. Therefore, new effective methods for treating liver diseases is urgently needed.
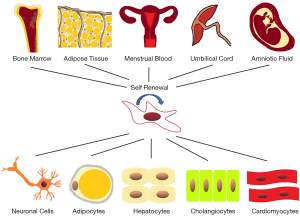
In recent years, mesenchymal stem cells (MSCs) have been proposed as an alternative approach to treat liver diseases. MSCs can be defined as pluripotent cells with the capacity of self-renewal, which can give rise to many unique, differentiated mesenchymal cell types (6). At present, the MSCs applied in clinical therapy and basic experimental research are mainly derived from bone marrow, umbilical cord, adipose tissue, amniotic fluid, menstrual blood, etc. (7-9). As shown in Figure 1, MSCs have the potential to differentiate into chondrocytes, osteocytes, and adipocytes, which show significant effect in regenerative medicine (10). Furthermore, MSCs have low inherent immunogenicity and can modulate immune responses by interacting with various immune cells (11). The homing capacity is the key to the effective application of MSC in clinical treatment, which was defined as blocking MSCs in the tissue vasculature and then migrating across the endothelium (12). The present study demonstrated that stem cell therapy was a therapeutic strategy in liver disease.
In this review, we aim to discuss available evidence and highlight some unsolved questions of stem cells for treating liver disease. This work will focus on the clinical application of MSCs in liver disease.
The mechanism of MSC in tissue repair and regeneration medicine
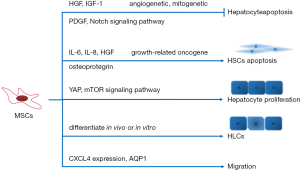
In previous study, stem cell therapy for liver disease has been proved to be effective in both basic and clinical research. MSCs were mostly applied in liver cirrhosis and shown a better therapeutic effect in compensatory period of liver disease. According to present studies, stem cell therapy shown significant improvement in liver function through anti-apoptosis and immune regulation. MSC can differentiate into hepatocytes in vivo and play a key therapeutic role in the treatment of liver fibrosis by secreting various immunomodulatory factors. After MSCs therapy, antiapoptotic factors including hepatocyte growth factor (HGF) and insulin-like growth factor (IGF-1) were elevated with the same promotion in angiogenetic and mito-genetic factors (13).
In vitro experiments, MSCs can promote apoptosis of hepatic stellate cells and inhibit collagen synthesis. Direct coculture of endothelial progenitor cells (EPCs) and MSCs in vitro enhanced cell proliferation and angiogenic capacity, PDGF and Notch signaling pathways were involved in this effect (14). In indirect coculture experiment, MSCs inhibited LX2 (hepatic stellate cell line) proliferation through secretion of inflammatory factors [interleukin-6 (IL-6), IL-8, HGF, growth-related oncogene and osteoprotegerin] (15). MSCs participated in the cell communication directly or indirectly through paracrine function.
Hepatic progenitor cells (HPCs), also named hepatic stem cell, possess an extremely low percentage in adult liver. When liver injured, HPCs can differentiate into both hepatocytes and cholangiocytes.
The application of MSCs in ALF
In the clinical, ALF progressed rapidly with a poor presentation to medical treatment (3). In recent years, the incidence of liver failure caused by herbal medicine has increased significantly.
It has been demonstrated that excessive inflammatory response plays a key role in pathogenesis and prognosis of ALF (16). During the progress of ALF, the domestic Kupffer cells (KCs), dendritic cells (DCs), and natural killer (NK) cells are highly activated, simultaneously the monocytes/macrophagocytes and the neutrophils are recruited in liver tissue (17). Another report demonstrated that activated KCs released high levels of TNF-α, IL-1, and IL-6 in LPS induced ALF models (18). The systemic inflammation could stimulate hepatocytes necrosis and apoptosis.
Many studies have confirmed the role of MSCs in the treatment of ALF animal models. MSCs can reduce the mortality, improve liver functions, inhibit hepatocytes apoptosis and promote proliferation (19,20). Previous studies demonstrated that MSCs showed therapeutic effect in ALF by immunoregulation. Firstly, MSCs can inhibit inflammation and alleviate liver injury by regulating inflammatory cytokine levels. Chen et al. indicated that MenSC could down-regulate the expression of TNF-α, IL-6, and IL-1β in mice models (21). Zhu et al. showed that BM-MSCs reduced the levels of TNF-α, IFN-γ and IL-4 (22). Secondly, MSCs inhibit the levels of inflammatory cytokine released by T cells, B cells, DCs and NKs cells (23-25). On the other hand, MSCs can enhance hepatocyte proliferation in liver failure models. Liu et al. showed that PGE2 secreted by MSCs enhanced hepatocyte proliferation by YAP and mTOR signaling (19). Shi et al. demonstrated that the DLL-4 secreted by Human BM-MSC promoted the proliferation of biliary epithelium cells in ALF pigs and rats (26). Although multiple MSCs can differentiate into hepatocyte-like cells in vitro (27-29), only few part (<4.5%) of transplanted MSCs differentiated into hepatocyte-like cells in ALF pigs (26).
Currently, the application of MSCs in ALF is limited to basic research. But it is obvious that MSCs have the capacity of promoting liver regeneration and suppressing inflammation. Comparing to control group, BM-MSCs effectively reduced the levels of ALT and ALB after 1 week of treatment (30). All these studies indicated the broad prospects of MSCs application in the clinical treatment of ALF.
The application of MSCs in cirrhosis
Liver fibrosis is a chronic disease caused by various etiologies, including viral infection, drug damage, alcohol abuse and immunological diseases. Chronic liver damage leads to excessive extracellular matrix (ECM) deposition through cycles of hepatocytes apoptosis, inflammation and repetitive damage repair. Cirrhosis is the end stage of progressive fibrosis that lack of effective comprehensive medical treatment. MSCs have the capacity of differentiating into hepatocyte-like cells and secreting factors to regulate immune. Thus, MSCs participate in tissue repair through direct and indirect approach.
It has been reported that MSCs alleviate the processes of epithelial-mesenchymal transition (EMT) and contribute to liver regeneration through differentiation, immune regulation and secretion (31). At present, BM-MSCs are the most widely used in clinical application.
A few clinical trials have been conducted to evaluate the curative effect of MSCs treating liver diseases (Table 1). Autologous BM-MSCs transplantation was effective in improving liver function and Child-Pugh scores in patients with liver cirrhosis (32). Autologous BM-MSCs were investigated to improve histologic fibrosis and liver function in patients with alcoholic cirrhosis (34,35). Comparing to one-time transplantation, there was no improved results in fibrosis quantification in two-time BM-MSC transplantation (34). After autologous BM-MSCs therapy, serum albumin levels and total protein were elevated. In SHUJI TERAI’s study, α-Fetoprotein (AFP) and proliferating cell nuclear antigen (PCNA) expression was significantly elevated in liver biopsy tissue after autologous BM-MSCs therapy (32). It was reported that AFP and PCNA participate in the process of hepatocyte proliferation (42). MSCs can differentiate into hepatocytes, effectively promoting liver regeneration. In another clinical trial, regulation of Treg/Th17 cell balance was investigated in treating liver cirrhosis (36). Present evidences demonstrated that autologous BM-MSCs can activate T cell receptors and rebuild immunological tolerance (43). Paracrine function of MSCs regulates immune response and promotes liver regeneration. In Mehdi Mohamad Nejad’s research (37), autologous BM-MSC transplantation probably has no beneficial effect in decompensated cirrhotic patients. Meaningfully, repeated autologous BM-MSCs therapy improved liver function of patients with decompensated liver cirrhosis after splenectomy (44). In HCV-related end-stage liver disease, MSCs therapy also shown a satisfactory tolerability and beneficial effects on liver synthetic functions and hepatic fibrosis (38). The efficacy of autologous BM-MSCs was connected to age and physical condition which make a close relation to differentiative capacity (39,45). Insufficient enrolled patients may be one of the limitations in the study. Future randomized controlled trials need to expand number of enrolled patients to confirm the efficacy of MSCs therapy in liver disease. To be concerned, autologous BM-MSC therapy couldn’t achieve acceptable long-term effects on prognosis (33). The reasons for the poor long-term efficacy may be as follows: the number of autologous BM-MSCs were limited; the homing ability is poor that MSCs could not reach the effective amount in liver (46).
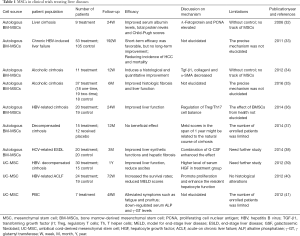
Full table
UC-MSC can be obtained free and amplify to a large number. It has been reported that UC-MSC transplantation alleviate symptoms of various autoimmune diseases (47,48). In a 1-year follow-up clinical research, UC-MSC transplantation has been proved to be safe and could improve liver function and reduce ascites in patients with decompensated liver cirrhosis (39). Even in ACLF patients, Shi et al. (40) have shown that UC-MSC transfusions significantly increased the survival rates. UC-MSCs show a more beneficial immunogenic profile and stronger overall immunosuppressive potential than BM-MSCs (49). After UC-MSC therapy, liver function was improved, that serum albumin levels increased and bilirubin levels decreased. UC-MSC treatment also improved liver function and patient’s quality of life in primary biliary cirrhosis (PBC) (41). UC-MSC therapy lowered serum alkaline phosphatase and γ-glutamyltransferase levels and alleviate fatigue and pruritus symptom in PBC patients. Comparing to BM-MSC, adipose-derived stromal cells (ADSCs) can be obtained from healthy donors freely and cultured to expand sufficient numbers. During cell amplification, ADSCs could maintain the characteristics of stem cells without losing their differentiation capacity (50). During the past few years, ADSCs have been used as a therapeutic strategy for tissue repair and immune modulation (51). ADSCs shown anti-inflammatory effect and secrete various factors to promote regeneration. ADSCs have been displayed as a feasible therapeutic strategy to alleviate liver damage (11,52). But there aren’t many clinical trials using ADSCs to treat liver disease.
Human menstrual blood-derived stem cells (MenSCs) are isolated from menstrual fluids with the advantage of simple operation, easy obtainment, safe and painless. MenSCs have been used to treat several diseases such as stroke, type 1 diabetes, premature ovarian failure and myocardial infarction (53-56). Previous study described that MenSCs can be differentiated into functional hepatocyte-like cells (57). In mice experiment, MenSCs shown an antifibrotic effect in liver fibrosis (9). At present, there are few clinical applications of MenSCs in liver disease. More clinical trials should be conducted in the future study.
The application of MSCs in liver cancer
It has been proved that MSCs have the ability of migrating and integrate into the tumor tissue (58). However, the application of multi-origin MSCs in liver cancer is controversial. Different sources of MSCs play diverse roles in liver cancer, which limits the application of MSCs in clinical treatment. Gardin et al. proved that AD-MSCs inhibited HepG2 and PLC-PRF-5 proliferation while promoted apoptosis in vitro by up-regulating P53 and RB and down-regulating c-Myc and hTER (59). Another report showed that conditioned medium (CMs) delivered from AD-MSCs inhibited hepatoma carcinoma cell proliferation and promoted death in vitro by down-regulating Akt signaling (60).
Yet BM-MSCs showed the opposite effect in HCC. A recent work demonstrated that BM-MSCs stimulated migration and invasion of HCC cells which could be hampered by AQP1 inhibitor (61). BM-MSCs-mediated upregulation of CXCL4 also plays critical role in promoting HCC cell migration and invasion in vitro (62). Interestingly, genetically modified BM-MSCs, which expressed high levels of stTRAIL, could migrate to heat-shocked HCC cells and induce apoptosis in nude mice (63).
Despite the original effect of MSCs on tumors, MSCs are potential tools for transporting drugs or anti-tumor virus due to their ability of tumor chemotaxis, immunosuppressive properties and low immunogenicity. It was reported that oncolytic measles virus infected BM-MSCs could homing to HCC tumors and transfer the virus to HCC via heterofusion to significantly inhibit tumor growth (58). What’s more, MenSCs infected with oncolytic adenovirus also showed their ability to migrate to variety kinds of tumors and suppress tumor growth in vivo. These studies show that using MSCs as gene vehicle could be a novel strategy for tumor-targeted clinical application in the future.
The transduction of secretory substance released by MSCs
It has been reported CM from MSCs show similar protective effects in tissue damage. CM contained paracrine soluble factors and extracellular vesicles (EVs) promoting tissue repair and regeneration (64). EVs can be released by MSCs and transport lipids, proteins, DNA, miRNA, and non-coding RNA. EVs can be classified into three subtypes including exosomes, microvesicles and apoptotic bodies. We partly summarized the cytokines secreted by MSCs and the signal transduction involved in the therapy pathway (Figure 2). In mice experiment, MenSC derived exosomes alleviated fulminant hepatic failure induced by D-GalN/LPS (21). MenSC derived exosomes provided an anti-apoptotic capacity with higher levels of cytokines including ICAM-1, angiopoietin-2, Axl, angiogenin, IGFBP-6, osteoprotegerin, IL-6 and IL-8 (21). MSCs exosomes were proposed as an ideal ‘cell-free’ therapeutic alternative to replace stem cell therapy. Combined with the homing capacity of stem cells, exosomes can be used as vectors for targeted therapy. However, exosome is currently limited to be widely used by the complicated extraction. And it is of great significance to clarify the components of exosomes for further research.
The potential application of MSCs in bioartificial liver (BAL) system
BAL support system incorporating cell source in bioreactor has been proposed as an effective means to treat end-stage liver diseases. With the application of BAL, it suggested to improve the survival time of fulminant hepatic failure pigs (65). Cell source used in BAL should possess the capacity of extensive amplification and maintenance of cellular characteristics. Cell lines derived from hepatomas and genetically engineered hepatocyte-like cells hepatoid cells have been widely used for BAL. Lv et al. study shown that the efficacy of BAL can be enhanced by co-culturing of liver cells with MSCs (65). It suggested that MSCs can modulate cell properties to achieve a better curative effect in BAL application. Adding secretory substance released by MSCs to BAL may enhance the capacity to improve the therapeutic potential.
Safety and efficacy of MSCs treatment
Safety, especially potential of tumorigenesis is the most concerned issue in MSCs clinical applications. However, after analyzing previous studies about chromosomal aberrations of MSCs, Sensebe et al. argued that genomic stability of cultured adult stem cells, especially MSCs is robust, which indicated that MSCs transplantation was less likely to cause tumor. During a follow-up of 11.5 years after BM-MSCs transplantation, neither tumor nor infections were observed among the 41 patients (66). Chen et al. indicated that MSC infusion could significantly reduce the mortality rate of ACLF patients without increasing severe complications (67). According to another meta-analysis, BM-MSCs and UC-MSCs infusion could improve liver function in cirrhosis patients. At the end of the first year, there were no serious side effects or complications.
Fever (37–38 °C) and rash are the most common adverse events reported but recover without additional treatment in clinical trials. Considering that MSCs has the character of low inherent immunogenicity, it may be related to the immune regulation function of MSCs.
Although large amount of studies has demonstrated the therapeutic effect of different MSCs on various liver diseases both in animal models and patients, there is currently no uniform clinical treatment guideline for efficient therapy strategy.
Determination of cells dose and timing is urgently needed for clinical applications (68). In most clinical indications, the dose of human MSCs is generally 1–2 million cells/kg, and never exceeds 12 million cells/kg (69). However, so far, there are no clinical trials reporting treatment effects comparison in different cell doses. On the other hand, there are very few related studies, especially clinical trials about timing of MSCs transplantation in liver diseases. After comparing effect of MSCs infusion in different time points in hepatic schistosomiasis models, El-Shennawy et al. suggested that the earlier injection of MSCs, the better treatment effect (70). These studies suggested necessities for in-depth research of MSCs administration timing in patients.
Optimization and standardization of isolation, culture, expansion and delivering are also key factors to steer MSCs treatment efficacy and safety, which need undergo extensive investigations before applicated in the clinical field.
Conclusions
In present studies, stem cells applied in clinical trials varies from different disease. Autologous BM-MSCs is the most widely used in liver diseases. To some extent, autologous MSCs extraction from patients is still a damaging process. The efficacy of autologous BM-MSCs is limited by physician condition including aging differentiation and deficiency in vitality (45). Comparing to BM-MSCs, UC-MSC are free from these limitations (49). ADSCs clinical application is limited by isolation technique. If ADSCs can be isolated more easily, they can be used in greater number than BMSCs.
In conclusion, MSCs therapy couldn’t achieve beneficial effect in decompensated cirrhosis and the long-term efficacy is poor. By far, liver transplantation is the most effective and the ultimate treatment for end-stage liver disease. Combined with stem cells in the treatment of liver disease, stem cell therapy can improve liver function in the short-term. Stem cell therapy can prolong the waiting time for liver transplantation in patients with end-stage liver disease and become a bridge between end-stage liver disease and liver transplantation. Repeated stem cell therapy may be an effective approach to achieve long-term therapeutic effects. Further clinical trials are needed to clarify the mechanism of stem cell in liver disease.
It has great significance to further clarify the substances secreted by MSCs, and to explore the definite substances contributing to immune regulation and tissue regeneration. To better investigate the mechanism involved in MSCs therapy, it is important to identify the protein, DNA and RNA released by MSCs. The proteomics and transcriptomics may play an important role in exploring the under mechanism. Cell communication with the help of EVs transfects key factors to regulate cell differentiation and induce subsequent signal transduction.
Increasing the number of cells homing to the injury site is the key point to improve the therapeutic effect of stem cells. Studying the homing characteristics of stem cells is helpful to improve the effective therapeutic quantity of stem cells. Identifying the differentiation of stem cells in vitro micro-environment gave a direction to the targeted regulation of stem cells.
Currently, there are no standardized protocols for stem cells clinical trials. To better analyze the difference between stem cells, more clinical trials should be conducted in the future. The sample size needs to be expanded and it needs to set an effective control group during the study. Therapeutic pathways and effective time points are required to be confirmed. We need to establish standardized protocols for clinical trials.
In summary, it was confirmed that MSCs has a promising therapeutic effect in liver disease therapy. More works are needed to clarify the underlying mechanism of stem cell therapeutic effects. Standardized protocols should be established according to the best time interval and reasonable therapeutic dose. Cell type, injection route and observation time points need to be better formulated in the clinical application of MSCs.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (NSFC: 81721091).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ling Lu) for the series “Stem Cell and Clinical Application” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.218). The series “Stem Cell and Clinical Application” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013;369:2525-34. [Crossref] [PubMed]
- Bernal W, Auzinger G, Dhawan A, et al. Acute liver failure. Lancet 2010;376:190-201. [Crossref] [PubMed]
- Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 2000;192:565-70. [Crossref] [PubMed]
- Fitzmaurice C, Allen C, Barber RM, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76. [Crossref] [PubMed]
- da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 2008;26:2287-99. [Crossref] [PubMed]
- Bieback K, Kern S, Kluter H, et al. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004;22:625-34. [Crossref] [PubMed]
- Miki T. Stem cell characteristics and the therapeutic potential of amniotic epithelial cells. Am J Reprod Immunol 2018;80:e13003. [Crossref] [PubMed]
- Chen L, Zhang C, Chen L, et al. Human Menstrual Blood-Derived Stem Cells Ameliorate Liver Fibrosis in Mice by Targeting Hepatic Stellate Cells via Paracrine Mediators. Stem Cells Transl Med 2017;6:272-84. [Crossref] [PubMed]
- Klingemann H, Matzilevich D, Marchand J. Mesenchymal Stem Cells - Sources and Clinical Applications. Transfus Med Hemother 2008;35:272-7. [Crossref] [PubMed]
- Liu WH, Song FQ, Ren LN, et al. The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J Cell Mol Med 2015;19:511-20. [Crossref] [PubMed]
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009;4:206-16. [Crossref] [PubMed]
- Mohammadi Gorji S, Karimpor Malekshah AA, Hashemi-Soteh MB, et al. Effect of mesenchymal stem cells on Doxorubicin-induced fibrosis. Cell J 2012;14:142-51. [PubMed]
- Liang TZ, Zhu L, Gao WL, et al. Coculture of endothelial progenitor cells and mesenchymal stem cells enhanced their proliferation and angiogenesis through PDGF and Notch signaling. Febs Open Bio 2017;7:1722-36. [Crossref] [PubMed]
- Chen LJ, Zhang CF, Chen L, et al. Human Menstrual Blood-Derived Stem Cells Ameliorate Liver Fibrosis in Mice by Targeting Hepatic Stellate Cells via Paracrine Mediators. Stem Cells Transl Med 2017;6:272-84. [Crossref] [PubMed]
- Wyke RJ, Yousif-Kadaru AG, Rajkovic IA, et al. Serum stimulatory activity and polymorphonuclear leucocyte movement in patients with fulminant hepatic failure. Clin Exp Immunol 1982;50:442-9. [PubMed]
- Possamai LA, Thursz MR, Wendon JA, et al. Modulation of monocyte/macrophage function: a therapeutic strategy in the treatment of acute liver failure. J Hepatol 2014;61:439-45. [Crossref] [PubMed]
- Luster MI, Germolec DR, Yoshida T, et al. Endotoxin-induced cytokine gene expression and excretion in the liver. Hepatology 1994;19:480-8. [Crossref] [PubMed]
- Liu Y, Ren H, Wang J, et al. Prostaglandin E2 secreted by mesenchymal stem cells protects against acute liver failure via enhancing hepatocyte proliferation. FASEB J 2019;33:2514-25. [Crossref] [PubMed]
- Salomone F, Barbagallo I, Puzzo L, et al. Efficacy of adipose tissue-mesenchymal stem cell transplantation in rats with acetaminophen liver injury. Stem Cell Res 2013;11:1037-44. [Crossref] [PubMed]
- Chen L, Xiang B, Wang X, et al. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther 2017;8:9. [Crossref] [PubMed]
- Zhu X, He B, Zhou X, et al. Effects of transplanted bone-marrow-derived mesenchymal stem cells in animal models of acute hepatitis. Cell Tissue Res 2013;351:477-86. [Crossref] [PubMed]
- Gazdic M, Markovic BS, Arsenijevic A, et al. Crosstalk between mesenchymal stem cells and T regulatory cells is crucially important for the attenuation of acute liver injury. Liver Transpl 2018;24:687-702. [Crossref] [PubMed]
- Zhang Y, Cai W, Huang Q, et al. Mesenchymal stem cells alleviate bacteria-induced liver injury in mice by inducing regulatory dendritic cells. Hepatology 2014;59:671-82. [Crossref] [PubMed]
- Milosavljevic N, Gazdic M, Simovic Markovic B, et al. Mesenchymal stem cells attenuate acute liver injury by altering ratio between interleukin 17 producing and regulatory natural killer T cells. Liver Transpl 2017;23:1040-50. [Crossref] [PubMed]
- Shi D, Zhang J, Zhou Q, et al. Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut 2017;66:955-64. [Crossref] [PubMed]
- Chitrangi S, Nair P, Khanna A. Three-dimensional polymer scaffolds for enhanced differentiation of human mesenchymal stem cells to hepatocyte-like cells: a comparative study. J Tissue Eng Regen Med 2017;11:2359-72. [Crossref] [PubMed]
- Mou XZ, Lin J, Chen JY, et al. Menstrual blood-derived mesenchymal stem cells differentiate into functional hepatocyte-like cells. J Zhejiang Univ Sci B 2013;14:961-72. [Crossref] [PubMed]
- Wang Y, Wang JL, Ma HC, et al. Mesenchymal stem cells increase heme oxygenase 1-activated autophagy in treatment of acute liver failure. Biochem Biophys Res Commun 2019;508:682-9. [Crossref] [PubMed]
- Lin BL, Chen JF, Qiu WH, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology 2017;66:209-19. [Crossref] [PubMed]
- Xie G, Diehl AM. Evidence for and against epithelial-to-mesenchymal transition in the liver. Am J Physiol Gastrointest Liver Physiol 2013;305:G881-90. [Crossref] [PubMed]
- Terai S, Ishikawa T, Omori K, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells 2006;24:2292-8. [Crossref] [PubMed]
- Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology 2011;54:820-8. [Crossref] [PubMed]
- Jang YO, Kim YJ, Baik SK, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int 2014;34:33-41. [Crossref] [PubMed]
- Suk KT, Yoon JH, Kim MY, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology 2016;64:2185-97. [Crossref] [PubMed]
- Xu L, Gong Y, Wang B, et al. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J Gastroenterol Hepatol 2014;29:1620-8. [Crossref] [PubMed]
- Mohamadnejad M, Alimoghaddam K, Bagheri M, et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int 2013;33:1490-6. [PubMed]
- Salama H, Zekri AR, Medhat E, et al. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther 2014;5:70. [Crossref] [PubMed]
- Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol 2012;27 Suppl 2:112-20. [Crossref] [PubMed]
- Shi M, Zhang Z, Xu R, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med 2012;1:725-31. [Crossref] [PubMed]
- Wang L, Li J, Liu H, et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol 2013;28 Suppl 1:85-92. [Crossref] [PubMed]
- Kayano K, Yasunaga M, Kubota M, et al. Detection of proliferating hepatocytes by immunohistochemical staining for proliferating cell nuclear antigen (PCNA) in patients with acute hepatic failure. Liver 1992;12:132-6. [Crossref] [PubMed]
- Alexander T, Thiel A, Rosen O, et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood 2009;113:214-23. [Crossref] [PubMed]
- Zhang W, Teng M, Liu B, et al. Repeated Autologous Bone Marrow Transfusion through Portal Vein for Treating Decompensated Liver Cirrhosis after Splenectomy. Gastroenterol Res Pract 2018;2018:4136082.
- Schubert T, Xhema D, Veriter S, et al. The enhanced performance of bone allografts using osteogenic-differentiated adipose-derived mesenchymal stem cells. Biomaterials 2011;32:8880-91. [Crossref] [PubMed]
- Kantarcioglu M, Demirci H, Avcu F, et al. Efficacy of autologous mesenchymal stem cell transplantation in patients with liver cirrhosis. Turk J Gastroenterol 2015;26:244-50. [Crossref] [PubMed]
- Sun LY, Wang DD, Liang J, et al. Umbilical Cord Mesenchymal Stem Cell Transplantation in Severe and Refractory Systemic Lupus Erythematosus. Arthritis and Rheumatism 2010;62:2467-75. [Crossref] [PubMed]
- Liu YY, Mu R, Wang SY, et al. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Research & Therapy 2010;12:13. [Crossref] [PubMed]
- Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007;25:1384-92. [Crossref] [PubMed]
- Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int 2012;2012:812693.
- Frese L, Dijkman PE, Hoerstrup SP. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus Med Hemother 2016;43:268-74. [Crossref] [PubMed]
- Minteer D, Marra KG, Rubin JP. Adipose-derived mesenchymal stem cells: biology and potential applications. Adv Biochem Eng Biotechnol 2013;129:59-71. [Crossref] [PubMed]
- Wu X, Luo Y, Chen J, et al. Transplantation of human menstrual blood progenitor cells improves hyperglycemia by promoting endogenous progenitor differentiation in type 1 diabetic mice. Stem Cells Dev 2014;23:1245-57. [Crossref] [PubMed]
- Borlongan CV, Kaneko Y, Maki M, et al. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev 2010;19:439-52. [Crossref] [PubMed]
- Zhang Z, Wang JA, Xu Y, et al. Menstrual blood derived mesenchymal cells ameliorate cardiac fibrosis via inhibition of endothelial to mesenchymal transition in myocardial infarction. Int J Cardiol 2013;168:1711-4. [Crossref] [PubMed]
- Rodrigues MC, Voltarelli J, Sanberg PR, et al. Recent progress in cell therapy for basal ganglia disorders with emphasis on menstrual blood transplantation in stroke. Neurosci Biobehav Rev 2012;36:177-90. [Crossref] [PubMed]
- Khanjani S, Khanmohammadi M, Zarnani AH, et al. Efficient generation of functional hepatocyte-like cells from menstrual blood-derived stem cells. J Tissue Eng Regen Med 2015;9:E124-34. [Crossref] [PubMed]
- Ong HT, Federspiel MJ, Guo CM, et al. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J Hepatol 2013;59:999-1006. [Crossref] [PubMed]
- Gardin C, Ferroni L, Bellin G, et al. Therapeutic Potential of Autologous Adipose-Derived Stem Cells for the Treatment of Liver Disease. Int J Mol Sci 2018. [Crossref] [PubMed]
- Zhao W, Ren G, Zhang L, et al. Efficacy of mesenchymal stem cells derived from human adipose tissue in inhibition of hepatocellular carcinoma cells in vitro. Cancer Biother Radiopharm 2012;27:606-13. [Crossref] [PubMed]
- Pelagalli A, Nardelli A, Fontanella R, et al. Inhibition of AQP1 Hampers Osteosarcoma and Hepatocellular Carcinoma Progression Mediated by Bone Marrow-Derived Mesenchymal Stem Cells. Int J Mol Sci 2016. [Crossref] [PubMed]
- Fontanella R, Pelagalli A, Nardelli A, et al. A novel antagonist of CXCR4 prevents bone marrow-derived mesenchymal stem cell-mediated osteosarcoma and hepatocellular carcinoma cell migration and invasion. Cancer Lett 2016;370:100-7. [Crossref] [PubMed]
- Deng Q, Zhang Z, Feng X, et al. TRAIL-secreting mesenchymal stem cells promote apoptosis in heat-shock-treated liver cancer cells and inhibit tumor growth in nude mice. Gene Ther 2014;21:317-27. [Crossref] [PubMed]
- Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005;11:367-8. [Crossref] [PubMed]
- Lv G, Zhao L, Zhang A, et al. Bioartificial liver system based on choanoid fluidized bed bioreactor improve the survival time of fulminant hepatic failure pigs. Biotechnol Bioeng 2011;108:2229-36. [Crossref] [PubMed]
- Wakitani S, Okabe T, Horibe S, et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med 2011;5:146-50. [Crossref] [PubMed]
- Chen B, Wang YH, Qian JQ, et al. Human mesenchymal stem cells for hepatitis B virus-related acute-on-chronic liver failure: a systematic review with meta-analysis. Eur J Gastroenterol Hepatol 2018;30:1224-9. [Crossref] [PubMed]
- Wang J, Liao L, Tan J. Mesenchymal-stem-cell-based experimental and clinical trials: current status and open questions. Expert Opin Biol Ther 2011;11:893-909. [Crossref] [PubMed]
- Galipeau J, Sensebe L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018;22:824-33. [Crossref] [PubMed]
- El-Shennawy SF, Abdel Aaty HE, Radwan NA, et al. Therapeutic Potential of Mesenchymal Stem Cells on Early and Late Experimental Hepatic Schistosomiasis Model. J Parasitol 2015;101:587-97. [Crossref] [PubMed]


