
Assessment of sublingual microcirculation in critically ill patients: consensus and debate
Introduction
In the intensive care unit, the main therapeutic approach to resuscitate shock patients is to normalize systemic hemodynamic parameters using mostly vasoactive agents and fluids. The expectation of improving the systemic circulation is that it results in parallel improvement of the microcirculation. However, several studies on the microcirculation in the critically ill have found that persistence of microcirculatory alterations may occur independently from systemic hemodynamic parameters and that such loss of coherence is associated with adverse outcome (1-4).
For decades, new tools and methods have been developed to assess the microcirculation in critical ill patients. However, none of these methods have been successful in discriminating between the different shock types as well as the underlying pathology of the microcirculation except monitoring the sublingual microcirculation using Hand-held vital microscopes (HVMs). In this regard, assessment of sublingual microcirculation to diagnose underlying pathology or to guide therapy deserves a special attention when considering hemodynamic monitoring of the critically ill patient. The purpose of this paper is to briefly review this topic.
What is microcirculation and why should we assess it?
It would be an oversimplification to see the cardiovascular system as bifurcating tubes which are connected in series. Large arteries and arterioles dampen the pulsatility, conduct the blood to capillaries which distribute oxygen, nutrients, hormones to the tissues in a complex and heterogeneous way. Oxygen is transported via the capillaries to the parenchymal cells to supply oxygen to mitochondria in support of oxidative phosphorylation. Blood within the capillaries with the metabolites and other waste products are then washed away into the venules, larger veins and finally into the right atrium. This process is tightly regulated by the metabolic demand of the various organs. Physical factors like shear stress and pressure exerted by the blood, humoral factors, and signals stemmed from circulating red and white blood cells constantly send signals to affect vasotone and adjust blood flow (5).
The smallest unit of the cardiovascular circulation is defined as the microcirculation. It is a network of differently sized capillaries that have a diameter less than 100 µm. Arterioles and venules (<20 µm) and capillaries (<10 µm) are the main site of oxygen transfer to the tissue. Endothelial cell lining (ECL) covers the entire lumen of the microcirculation (6). It has important roles such as maintaining hemostasis, vascular tone, barrier function and blood cell regulation such as platelet and leukocyte activation, anti-oxidant and anti-inflammatory effects. The integrity of the ECL is preserved by the endothelial cytoskeleton and connecting proteins that constitute the glycocalyx (7). The glycocalyx is a thin glycoprotein and proteoglycan layer on the apical or luminal side of EC playing role as a barrier (8), sensing mechanical stress of blood (9) and regulating vasomotor tone (10). The glycocalyx can be damaged by various factors like oxidants, cytokines and endotoxins (6,7,11). Reactive oxygen species (ROS) like hydrogen peroxide, superoxide and hydroxyl anions and other mediators like tumor necrosis factor-alpha (TNFα) and heparanase are primarily responsible for this insult (12). Furthermore, shedding of the glycocalyx exposes specific adhesion molecules associated with neutrophil activation (6), diapedesis and the release of inflammatory mediators all of which contributes to potential tissue damage. Additionally, interventions aimed at improving shock, such as fluid administration and catecholamines, may in themselves cause injury to the glycocalyx, ECL (13) and the microcirculation (14), resulting in hypoxia and tissue edema. Endothelial vascular barrier (VB) is another important structure associated with vascular integrity, cell connection, adhesion and trafficking of molecules such as integrins, gap junctions, intracellular adhesion molecules-1 (ICAM-1) and vascular cell adhesion molecules-1 (VCAM-1). An increase in levels of these endothelial vascular molecules are associated with sepsis severity, organ dysfunction and mortality (15) The resulting tissue edema is a major contributor to morbidity and mortality seen in patients in the ICU (6,11). In an experimental model of hemorrhagic shock in rats, crystalloids used for resuscitation has been found to lead to an increase in concentrations of plasma glycocalyx shedding products such as hyaluronan. These effects were found to be independent of the properties of the crystalloid solution, i.e., balanced or unbalanced (13).
Dysfunction of the microvascular perfusion is the primary result of this ECL, glycocalyx and VB dysfunction and it can lead to organ failure. Thus, assessment of the microcirculation becomes crucial to get a better insight of the underlying pathology and its resolution.
How to assess the microcirculation?
Surrogates of microcirculation and tissue perfusion can be assessed via blood samples, e.g., venoarterial CO2 gap and lactate or non-invasively through evaluating the properties of the skin. Skin circulation is the first vascular system from which blood is diverted away from vital organs during a circulatory compromise. Reduced blood flow, local endothelial dysfunction, leukocyte activation and localized vasoconstriction mediated by the sympathetic nervous system causes alterations in blood flow (16-19). Clinical evaluation of these alterations can be done by several methods, such as mottling score of the skin, capillary refill time of the index finger or skin above the patella and skin temperature gradient (20-24).
Capillary refill time (CRT) is easy and quick to assess. Prolonged CRT (>4.5 seconds on the index finger) is associated with hyperlactatemia and higher SOFA score (20). Prolonged CRT was also found to be predictive of mortality in septic shock (22). Resuscitation strategy based on normalizing CRT was found to be beneficial compared to lactate driven resuscitation strategy in septic shock patients with less organ dysfunction at day 3 (25). However, this method can suffer from individual variations amongst clinicians, although interrater agreement can be increased by proper training (22).
Mottling is a distinctive skin discoloration which primarily occurs around the skin above bone structures such as the patella. Mottling is reflective of hypoperfusion of visceral organs like liver, kidney, spleen and gut (26). A mottling score based on the extension of the mottling around the patella has been found to reliably predict organ failure severity and mortality in patients with septic shock. Mortality in septic shock patients at day 28 was 100% when mottling score was ≥4, 45% if it was ≤1 (27). Septic shock patients with an improvement in the mottling score in the first 6 hours of resuscitation was found to have a significantly better prognosis compared to those that did not (23% vs. 88%) (28).
Near infrared spectroscopy (NIRS) provides a noninvasive semiquantitative measurement of oxy- and deoxyhemoglobin saturation in a catchment volume of tissue. It has a reach of few centimeters from the applied surface, and is therefore considered representative of regional tissue oxygenation saturation. In critically patients, NIRS has been mostly applied to the thenar eminence, where there is thin fat tissue and a lower possibility of tissue edema (29). A measure of vascular reactivity using NIRS, can be achieved by applying a vascular occlusion test to the upper extremity with a pressure cuff. Such a measurement has allowed discrimination between normal and abnormal regional circulation in sepsis patients (30). However, in a general ICU population, changes in the NIRS derived parameters were found to be independent of pathophysiologic condition of the patient such as the etiology of the shock (31). NIRS measurements can also be affected from ambient temperature, other molecules such as myoglobin and skin pigmentation (31).
HVMs have been developed to directly observe and assess the functional properties of the sublingual microcirculation. This technique, now widely used, allows identification of underlying pathology in a point-of-care fashion at the bedside (1). Alterations in sublingual microcirculation have been found to correlate with microcirculatory alterations in the intestines and kidney and thus are considered as a sensitive indicator of circulatory failure (32-36). Latest generation HVMs employ Incident Dark Field (IDF) illumination technique with a high-resolution optic lens and focusing mechanism. With the improvement of the optical system and a lighter weight, IDF imaging has been shown to visualize up to 30% more capillaries and three times larger field of view than the previous generation HVM devices (37,38). These advancements make bedside monitoring of the microcirculation with HVMs easier than before. However, analysis of the videos for quantitative data with the semi-automatic software AVA® was cumbersome (39). Recently a completely automatic and faster software has been introduced, called MicroTools, which now may make point-of-care use and microcirculatory targeted therapy a reality at the bedside (40).
Analysis
The main concern in shock and resuscitation is whether the microcirculation is perfused with oxygen carrying red blood cells (RBCs) to and remove the waste products away the tissues. There are two mechanisms responsible for oxygen carrying to the tissues by the red blood cells (RBCs). These are RBC convection and oxygen diffusion from the RBCs to the tissue cells (41).
Thus, variables describing this functional state of the microcirculation are used to describe its oxygen carrying capacity to the tissues. Some of these parameters are shown below in Table 1 (1).

Full table
For a detailed analysis and description of the function of the microcirculation, dedicated image software is necessary. Vessel detection by software generates parameters which can be used to identify the presence of microcirculatory alterations and follow the progress of therapy. Additionally, analysis of the types of capillary alterations can give a better insight into the causes of underlying pathology. According to morphology and function, four different types of vessels are mostly encountered in the microcirculation (Figure 1). These are:
- Arterioles;
- Capillaries;
- Venules;
- Post capillary venules.
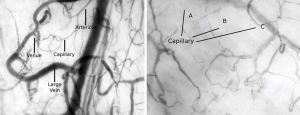
Exchange of oxygen mainly occurs at the level of the capillaries. Venules and large veins act as a conduit for RBCs. Thus, parameters concerning the capillaries are mainly used for microcirculatory analysis in the management of a critically ill patient. Total length of small vessel network is used for identification of total vessel density and those which are perfused, identify the diffusion capacity of the microcirculation. RBC flow in the capillaries can be used to identify normal and abnormal flow patterns associated with the convective capacity of the microcirculation according to the following classification:
- Capillaries with normal RBC flow;
- Capillaries with intermittent RBC flow;
- Capillaries with sluggish RBC flow;
- Capillaries with no RBC flow.
Combinations of vessel density and interpretation of flow patterns in the microcirculation and the number of flowing RBCs allow quantification of the functional capillary density and flow heterogeneity, as terms of microcirculatory tissue RBC perfusion measurement.
Proper image acquisition is necessary for analysis of the moving microcirculatory images to correctly generate functional parameters of RBC convection and oxygen diffusion (42,43). After image acquisition, analyses of the microcirculation can be done via visual observation and evaluation (eyeballing) (44), off line manual or software aided/semi-automatic image analysis software (39), or off line or online fully automatic software methods (MicroTools) (40). Eyeballing can provide quick and reliable analysis which is comparable to off line analysis (44,45). Eyeballing is mostly based on observing changes in the MFI as it is the easier to assess, even by novice users (46). MFI score also yields important clinical foresight. It has been documented that MFI score <2.6 at admission to the ICU indicates a higher ICU and in-hospital mortality (47).
Microcirculation in ICU
Microcirculatory measurements are able to allow the clinician to better interpret the patient’s condition and guide to therapy. Even though, in some cases these parameters may fail to identify the underlying pathology. A sublingual microcirculation with a sluggish but steady flow, as seen in cardiac failure, may be erroneously interpreted. Both the TVD and PVD are reduced in sepsis patients (48), neuro-ICU patients (49) and other hemodynamically compromised patients like in cardiogenic shock indicating impairment of tissue perfusion (50). Red Blood Cell velocity (RBCv) measurements should be added to the parameters to identify hypovolemia or hypotensive shock in such cases to avoid fluid overload. RBCv may be unchanged or increased (51) during hemodilution. In septic shock, however, patients frequently have slower RBC flow (52,53). Moreover, inflammatory states such as sepsis, ischemia-reperfusion and cardiac surgery results in activation and adhesion of the leukocytes to the endothelium (54). The kinetics of activated leukocytes can be quantified using space-time diagram analysis of microcirculation images (55). Thus, analysis of the sublingual microcirculation should be performed in context and it should integrate macrohemodynamic parameters for a comprehensive hemodynamic evaluation of the physiological state of the cardiovascular system (1).
Circulatory compromise associated with microcirculatory alterations can be treated by interventions such as fluid administration (56-58), blood transfusion (59-62), vasoactive agents (63,64), steroids (65), and extracorporeal membrane oxygenation.
Identification of an intact coherence between macro- and microcirculation, where changes in systemic hemodynamic variables cause a parallel improvement in microcirculatory hemodynamics, show that the vascular regulatory mechanisms are functioning in patients. In this case, targeting a macrohemodynamic parameter for therapy can be adequate to recruit the microcirculation (1). However, loss of hemodynamic coherence between the macro and microcirculation can be encountered frequently in conditions of endothelial and RBC dysfunction such as can occur in sepsis, cardiogenic shock, or any hemodynamically compromised patient (2,3,66-70). Thus, clinical evaluation of a patient where such pathological conditions with a diagnosis of some type of circulatory shock, is likely to have a loss of hemodynamic coherence and monitoring the microcirculation is advised. An uncoupling between the macro and microcirculation can occur where changes in blood viscosity, sheer stress, glycocalyx shedding, a change in erythrocyte elasticity and endothelial malfunction occur in states of disease (71). Whatever the underlying reason, during loss of hemodynamic coherence macrohemodynamic parameters does not result in a parallel improvement in the microcirculation resulting in inadequate resuscitation and the persistence of tissue hypoxia and injury (2).
The ultimate objective of resuscitation of a shock patient is to improve tissue perfusion (72), although mostly such therapies target restoring macrocirculatory parameters to baseline. However, if there is a loss of hemodynamic coherence, it renders most of the macrocirculatory targeting therapies ineffective and result into fluid overload and increased use of vasopressors, both of which can cause harm. Inadequate correction of microcirculatory variables has shown to be associated with adverse outcome in sepsis patients (48). Additionally, despite similar hemodynamic profiles, microvascular perfusion improved with treatment in the survivors, but not in the nonsurvivors of sepsis, underscoring the need to monitor the microcirculation. The severity of the microvascular dysfunction at the time of ICU admission in sepsis patients has also been found to be correlated with the development of organ dysfunction and mortality (66,73-75). Four types of microcirculatory alterations have been identified which can be associated with a loss of hemodynamic coherence. All of them result in perfusion defects, resulting in a reduced oxygen extraction capacity of the tissues (2).
Type 1 loss of hemodynamic coherence is associated with flow heterogeneity. Obstructed capillaries are next to the flowing ones and it is often associated with endothelial and RBC injury. Sepsis is a typical example of a type 1 alteration. Flow heterogeneity results in a heterogeneous perfusion and oxygen extraction deficit of the tissue. Both diffusion and convection parameters of microcirculation, i.e., TVD, FCD and MFI, are impaired (76) (Figure 2).
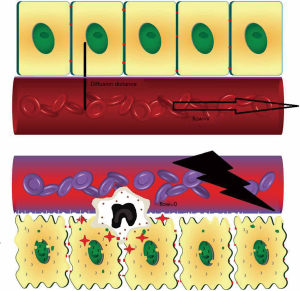
Type 2 loss of hemodynamic coherence is associated with hemodilution, where dilutional anemia reduces the number of oxygen carrying RBCs. Less RBCs flowing in the capillaries causes increased diffusion distance of oxygen to the tissue. Blood transfusions (61) and de-escalation procedures (77) may restore microcirculatory perfusion and improve oxygen carrying capacity (Figure 3).

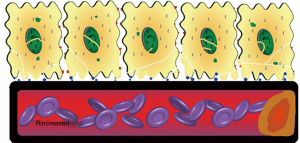
Type 3 loss of hemodynamic coherence is associated with stasis of microcirculation. Excess usage of noradrenaline may result in deteriorated microcirculation (68). A tapering of noradrenaline improved microcirculatory perfusion in early septic shock patients (78). Alternatively, an inappropriate rise in venous pressures may cause microcirculatory tamponade (79) (Figure 4).
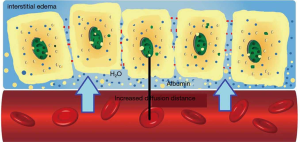
Type 4 loss of hemodynamic coherence is associated with tissue edema. Increased diffusion distance of oxygen causes impaired tissue oxygen extraction. A decreased TVD due to excess fluid was restored using diuretic therapy in post cardiac surgery patients (77) (Figure 5).
Since shock is defined as an imbalance between oxygen delivery and oxygen need at the cellular level (2), direct visualization of the microcirculation can help to diagnose microcirculatory shock (80). Any derangement in one of the constituents of the microcirculation, such as TVD, PVD, MFI, RBCs and white blood cells (WBC) or endothelium, may cause microcirculatory shock. Microcirculatory alterations will result in an inadequacy of perfusion and oxygen transport to the tissues (81).
Recruiting the microcirculation in pathologic states
Each type of microcirculatory alteration mentioned above necessitates specific types of interventions. For example, fluid resuscitation in patients with clinical signs of deteriorated organ perfusion, has been shown to benefit only those with prior microcirculation abnormalities (82). It is well known that excess fluid administration is hazardous in critically ill patients (83,84). Thus, fluid resuscitation in patients with either normal microcirculation or unresponsive microcirculation may not only be unbeneficial, but also perilous. Furthermore, it was found that early, but not in late sepsis, interventions with administration of fluids were beneficial in improving microcirculation (57). Additionally, changes in the microcirculation have been found to be independent of macrohemodynamic parameters, such as stroke volume values, emphasizing that the microcirculation can respond independently of macrohemodynamic parameters. Another result of fluid resuscitation is that, whether excessive or not, hemodilution may affect tissue oxygenation adversely even more than hemorrhage (85).
Vasoactive agents may improve tissue perfusion. Enoximone in cardiogenic shock patients improved sublingual perfused capillary density, despite unchanged cardiac index and mean arterial pressure (4). Iloprost, an analogue of prostaglandin I2 (PGI2), has vasodilatory, fibrinolytic and leukocyte inhibitory properties and was shown to improve skin mottling in patients with severe septic shock (86). The effects of dobutamine on microcirculatory perfusion has been shown to have opposing effects in patients with septic shock (63,64). An early study showed improved microcirculation accompanied by a decrease in lactate (63). However, a randomized controlled trial showed no beneficial effects of dobutamine on microcirculatory perfusion in septic shock patients despite an increase in cardiac index and left ventricular ejection fraction (64). Noradrenaline, if used inappropriately, may exert a harmful effect on microcirculation by increasing vascular resistance and impeding blood flow, resulting in a Type 3 loss of hemodynamic coherence (68). Nitroglycerine, a potent vasodilator, increased TVD in healthy subjects (87) and recruited microvascular flow in pressure guided resuscitated septic shock patients (88), although this was not confirmed in a randomized controlled trial in fluid resuscitated septic shock patients (89). Differences in study characteristics such as differences in fluid status and vasoactive usage of the patients may account for this variable result (89). Addition of a single dose of terlipressin to noradrenaline, with the expectation of tapering noradrenaline doses, improved sublingual microcirculatory flow (78,90). However, there were not any difference in restoring sublingual microcirculation between terlipressin, arginine vasopressin or noradrenaline in adequately resuscitated septic shock patients. Authors concluded that disease progression and subsequent inflammatory evolution may have affected the microcirculatory flow more than different types of vasopressors (91). However, in a catecholamine resistant septic shock, administering a bolus of terlipressin caused an immediate stasis of the microcirculation despite an increased MAP and urine production, which eventually led to the patients demise (92).
RBC transfusion has been shown to have variable effects on the microcirculation. RBC transfusion in patients with hemorrhagic shock improved in all microvascular parameters, but did not affect blood pressure, heart rate or cardiac index (59). However, it should be emphasized in early but not late sepsis blood transfusion was able to improve the microcirculation. Blood transfusion deteriorated those with preserved initial microcirculation (60,93). Blood transfusions were shown to improve specific microcirculatory indices in cardiac surgery patients (61), stable trauma patients (94) and mixed surgical patients (95). It can be concluded that, independent of the macrohemodynamic status of the patients, RBC transfusions may benefit some patients, while being ineffective in others. Importantly, systemic hemoglobin levels almost always increased, whereas effects on the microcirculations were variable (59).
Anti-inflammatory agents may also improve microcirculation. Administration of hydrocortisone in septic shock patients improved perfused vessel density and resulted in earlier shock resolution, despite insignificant changes in global hemodynamics and vasoactive agent use (65). Although corticosteroids have beneficial effects on shock duration, their effects on the outcome of septic shock patients still remain inconclusive (96). Mechanisms that lead to impairment in the microcirculation does not have a single cause.
Conclusions
Sublingual microcirculation assessment with HVMs can provide valuable pathophysiological information regarding the mechanisms underlying the type and severity of shock and provide guidance to type and amount of the therapy. It is expected that goal directed microcirculatory strategy in a point-of-care setting, with the newly introduced improved technology, may provide a next generation of hemodynamic resuscitation platform.
Acknowledgments
We would like to thank Gokhan Sert, MD for his help in the drawing the figures.
Funding: This work was supported by ‘Scientific and Technological Research Council of Turkey’ (TUBITAK Grant No:1059B191800363) to OD.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Glenn Hernández and Guo-wei Tu) for the series “Hemodynamic Monitoring in Critically Ill Patients” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.222). The series “Hemodynamic Monitoring in Critically Ill Patients” was commissioned by the editorial office without any funding or sponsorship. CI has received a grant from CytoSorb to commence a randomized controlled trial on the effect of the adsorber on the microcirculation of critically ill patients at the department of Intensive Care of the Erasmus Medical Center Rotterdam. CI and his team provide services and training with regard to clinical microcirculation. To this purpose, he runs an internet site called https://www.microcirculationacademy.org. The internet site and its activities are run by a company called Active Medical BV of which he owns shares. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ince C, Boerma EC, Cecconi M, et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European Society of Intensive Care Med. Intensive Care Med 2018;44:281-99. [Crossref] [PubMed]
- Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care 2015;19 Suppl 3:S8. [Crossref] [PubMed]
- Buijs EA, Reiss IK, Kraemer U, et al. Increasing mean arterial blood pressure and heart rate with catecholaminergic drugs does not improve the microcirculation in children with congenital diaphragmatic hernia: a prospective cohort study. Pediatr Crit Care Med 2014;15:343-54. [Crossref] [PubMed]
- Corstiaan A, Lagrand WK, van der Ent M, et al. Conventional hemodynamic resuscitation may fail to optimize tissue perfusion: an observational study on the effects of dobutamine, enoximone, and norepinephrine in patients with acute myocardial infarction complicated by cardiogenic shock. PLoS One 2014;9:e103978. [Crossref] [PubMed]
- Ait-Oufella H, Bourcier S, Lehoux S, et al. Microcirculatory disorders during septic shock. Curr Opin Crit Care 2015;21:271-5. [Crossref] [PubMed]
- Ince C, Mayeux PR, Nguyen T, et al. The endothelium in sepsis. Shock (Augusta, Ga) 2016;45:259. [Crossref] [PubMed]
- Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 2007;9:121-67. [Crossref] [PubMed]
- Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann Biomed Eng 2012;40:828-39. [Crossref] [PubMed]
- Becker BF, Chappell D, Bruegger D, et al. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res 2010;87:300-10. [Crossref] [PubMed]
- Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med 2006;259:339-50. [Crossref] [PubMed]
- Rubio-Gayosso I, Platts SH, Duling BR. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2006;290:H2247-56. [Crossref] [PubMed]
- Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 2012;18:1217-23. [Crossref] [PubMed]
- Aksu U, Bezemer R, Yavuz B, et al. Balanced vs unbalanced crystalloid resuscitation in a near-fatal model of hemorrhagic shock and the effects on renal oxygenation, oxidative stress, and inflammation. Resuscitation 2012;83:767-73. [Crossref] [PubMed]
- Marechal X, Favory R, Joulin O, et al. Endothelial glycocalyx damage during endotoxemia coincides with microcirculatory dysfunction and vascular oxidative stress. Shock 2008;29:572-6. [PubMed]
- Shapiro NI, Schuetz P, Yano K, et al. The association of endothelial cell signaling, severity of illness, and organ dysfunction in sepsis. Crit Care 2010;14:R182. [Crossref] [PubMed]
- Lima A, Bakker J. Noninvasive monitoring of peripheral perfusion. Intensive Care Med 2005;31:1316-26. [Crossref] [PubMed]
- Ait-Oufella H, Bourcier S, Alves M, et al. Alteration of skin perfusion in mottling area during septic shock. Ann Intensive Care 2013;3:31. [Crossref] [PubMed]
- Bourcier S, Joffre J, Dubée V, et al. Marked regional endothelial dysfunction in mottled skin area in patients with severe infections. Crit Care 2017;21:155. [Crossref] [PubMed]
- Becker L, Prado K, Foppa M, et al. Endothelial dysfunction assessed by brachial artery ultrasound in severe sepsis and septic shock. J Crit Care 2012;27:316.e9-14. [Crossref] [PubMed]
- Lima A, Jansen TC, van Bommel J, et al. The prognostic value of the subjective assessment of peripheral perfusion in critically ill patients. Crit Care Med 2009;37:934-8. [Crossref] [PubMed]
- Hernandez G, Pedreros C, Veas E, et al. Evolution of peripheral vs metabolic perfusion parameters during septic shock resuscitation. A clinical-physiologic study. J Crit Care 2012;27:283-8. [Crossref] [PubMed]
- Ait-Oufella H, Bige N, Boelle PY, et al. Capillary refill time exploration during septic shock. Intensive Care Med 2014;40:958-64. [Crossref] [PubMed]
- Ait-Oufella H, Bakker J. Understanding clinical signs of poor tissue perfusion during septic shock. Intensive Care Med 2016;42:2070-2. [Crossref] [PubMed]
- Dubin A, Henriquez E, Hernández G. Monitoring peripheral perfusion and microcirculation. Curr Opin Crit Care 2018;24:173-80. [Crossref] [PubMed]
- Hernández G, Ospina-Tascón GA, Damiani LP, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA 2019;321:654-64. [Crossref] [PubMed]
- Brunauer A, Koköfer A, Bataar O, et al. Changes in peripheral perfusion relate to visceral organ perfusion in early septic shock: a pilot study. J Crit Care 2016;35:105-9. [Crossref] [PubMed]
- de Moura EB, Amorim FF, da Cruz Santana AN, et al. Skin mottling score as a predictor of 28-day mortality in patients with septic shock. Intensive Care Med 2016;42:479-80. [Crossref] [PubMed]
- Ait-Oufella H, Lemoinne S, Boelle PY, et al. Mottling score predicts survival in septic shock. Intensive Care Med 2011;37:801-7. [Crossref] [PubMed]
- Lipcsey M, Woinarski NC, Bellomo R. Near infrared spectroscopy (NIRS) of the thenar eminence in anesthesia and intensive care. Ann Intensive Care 2012;2:11. [Crossref] [PubMed]
- Neto AS, Pereira VGM, Manetta JA, et al. Association between static and dynamic thenar near-infrared spectroscopy and mortality in patients with sepsis: a systematic review and meta-analysis. J Trauma Acute Care Surg 2014;76:226-33. [Crossref] [PubMed]
- Lima A, van Bommel J, Sikorska K, et al. The relation of near-infrared spectroscopy with changes in peripheral circulation in critically ill patients. Crit Care Med 2011;39:1649-54. [Crossref] [PubMed]
- Verdant CL, De Backer D, Bruhn A, et al. Evaluation of sublingual and gut mucosal microcirculation in sepsis: a quantitative analysis. Crit Care Med 2009;37:2875-81. [Crossref] [PubMed]
- Pranskunas A, Pilvinis V, Dambrauskas Z, et al. Early course of microcirculatory perfusion in eye and digestive tract during hypodynamic sepsis. Crit Care 2012;16:R83. [Crossref] [PubMed]
- Jacquet-Lagreze M, Allaouchiche B, Restagno D, et al. Gut and sublingual microvascular effect of esmolol during septic shock in a porcine model. Crit Care 2015;19:241. [Crossref] [PubMed]
- Sui F, Zheng Y, Li WX, et al. Renal circulation and microcirculation during intra-abdominal hypertension in a porcine model. Eur Rev Med Pharmacol Sci 2016;20:452-61. [PubMed]
- Lima A, van Rooij T, Ergin B, et al. Dynamic contrast-enhanced ultrasound identifies microcirculatory alterations in sepsis-induced acute kidney injury. Crit Care Med 2018;46:1284-92. [Crossref] [PubMed]
- Aykut G, Veenstra G, Scorcella C, et al. Cytocam-IDF (incident dark field illumination) imaging for bedside monitoring of the microcirculation. Intensive Care Med Exp 2015;3:40. [Crossref] [PubMed]
- Van Elteren HA, Ince C, Tibboel D, et al. Cutaneous microcirculation in preterm neonates: comparison between sidestream dark field (SDF) and incident dark field (IDF) imaging. J Clin Monit Comput 2015;29:543-8. [Crossref] [PubMed]
- Dobbe JGG, Streekstra GJ, Atasever B, et al. Measurement of functional microcirculatory geometry and velocity distributions using automated image analysis. Med Biol Eng Comput 2008;46:659. [Crossref] [PubMed]
- Hilty MP, Guerci P, Ince Y, et al. MicroTools enables automated quantification of capillary density and red blood cell velocity in handheld vital microscopy. Commun Biol 2019;2:217. [Crossref] [PubMed]
- Bateman RM, Sharpe MD, Ellis CG. Bench-to-bedside review: microvascular dysfunction in sepsis–hemodynamics, oxygen transport, and nitric oxide. Crit Care 2003;7:359. [Crossref] [PubMed]
- Massey MJ, LaRochelle E, Najarro G, et al. The microcirculation image quality score: development and preliminary evaluation of a proposed approach to grading quality of image acquisition for bedside videomicroscopy. J Crit Care 2013;28:913-7. [Crossref] [PubMed]
- Massey MJ, Shapiro NI. A guide to human in vivo microcirculatory flow image analysis. Crit Care 2016;20:35. [Crossref] [PubMed]
- Tanaka S, Harrois A, Nicolaï C, et al. Qualitative real-time analysis by nurses of sublingual microcirculation in intensive care unit: the MICRONURSE study. Crit Care 2015;19:388. [Crossref] [PubMed]
- Arnold RC, Parrillo JE, Dellinger RP, et al. Point-of-care assessment of microvascular blood flow in critically ill patients. Intensive Care Med 2009;35:1761-6. [Crossref] [PubMed]
- Cerny V, Abdo I, George RB, et al. Analysis of microcirculation measurements by novice users trained by a standardized interactive tutorial: An inter-observer variability study. Clin Hemorheol Microcirc 2016;62:123-8. [Crossref] [PubMed]
- Scorcella C, Damiani E, Domizi R, et al. MicroDAIMON study: Microcirculatory DAIly MONitoring in critically ill patients: a prospective observational study. 2018;8:64.
- De Backer D, Creteur J, Preiser JC, et al. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 2002;166:98-104. [Crossref] [PubMed]
- Pranskunas A, Tamosuitis T, Balciuniene N, et al. Alterations of conjunctival glycocalyx and microcirculation in non-septic critically ill patients. Microvasc Res 2018;118:44-8. [Crossref] [PubMed]
- Akin S, Dos Reis Miranda D, Caliskan K, et al. Functional evaluation of sublingual microcirculation indicates successful weaning from VA-ECMO in cardiogenic shock. Crit Care 2017;21:265. [Crossref] [PubMed]
- Hudetz AG, Wood JD, Biswal BB, et al. Effect of hemodilution on RBC velocity, supply rate, and hematocrit in the cerebral capillary network. J Appl Physiol (1985) 1999;87:505-9. [Crossref] [PubMed]
- Edul VSK, Ince C, Vazquez AR, et al. Similar microcirculatory alterations in patients with normodynamic and hyperdynamic septic shock. Ann Am Thorac Soc 2016;13:240-7. [PubMed]
- Edul VSK, Enrico C, Laviolle B, et al. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med 2012;40:1443-8. [Crossref] [PubMed]
- Nakagawa NK, Nogueira RA, Correia CJ, et al. Leukocyte-endothelium interactions after hemorrhagic shock/reperfusion and cecal ligation/puncture: an intravital microscopic study in rat mesentery. Shock 2006;26:180-6. [Crossref] [PubMed]
- Uz Z, van Gulik TM, Aydemirli MD, et al. Identification and quantification of human microcirculatory leukocytes using handheld video microscopes at the bedside. J Appl Physiol (1985) 2018;124:1550-7. [Crossref] [PubMed]
- Pottecher J, Deruddre S, Teboul JL, et al. Both passive leg raising and intravascular volume expansion improve sublingual microcirculatory perfusion in severe sepsis and septic shock patients. Intensive Care Med 2010;36:1867-74. [Crossref] [PubMed]
- Ospina-Tascon G, Neves AP, Occhipinti G, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med 2010;36:949-55. [Crossref] [PubMed]
- Dubin A, Pozo MO, Casabella CA, et al. Comparison of 6% hydroxyethyl starch 130/0.4 and saline solution for resuscitation of the microcirculation during the early goal-directed therapy of septic patients. J Crit Care 2010;25:659.e1-8. [Crossref] [PubMed]
- Tanaka S, Escudier E, Hamada S, et al. Effect of RBC transfusion on sublingual microcirculation in hemorrhagic shock patients: a pilot study. Crit Care Med 2017;45:e154-60. [Crossref] [PubMed]
- Sakr Y, Chierego M, Piagnerelli M, et al. Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit Care Med 2007;35:1639-44. [Crossref] [PubMed]
- Yuruk K, Almac E, Bezemer R, et al. Blood transfusions recruit the microcirculation during cardiac surgery. Transfusion 2011;51:961-7. [Crossref] [PubMed]
- Atasever B, van der Kuil M, Boer C, et al. Red blood cell transfusion compared with gelatin solution and no infusion after cardiac surgery: effect on microvascular perfusion, vascular density, hemoglobin, and oxygen saturation. Transfusion 2012;52:2452-8. [Crossref] [PubMed]
- De Backer D, Creteur J, Dubois MJ, et al. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med 2006;34:403-8. [Crossref] [PubMed]
- Hernandez G, Bruhn A, Luengo C, et al. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: a randomized, placebo-controlled, double-blind, crossover study. Intensive Care Med 2013;39:1435-43. [Crossref] [PubMed]
- Büchele GL, Silva E, Ospina-Tascon GA, et al. Effects of hydrocortisone on microcirculatory alterations in patients with septic shock. Crit Care Med 2009;37:1341-7. [Crossref] [PubMed]
- De Backer D, Donadello K, Sakr Y, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med 2013;41:791-9. [Crossref] [PubMed]
- Meinders A-J, Nieuwenhuis L, Ince C, et al. Haemodialysis impairs the human microcirculation independent from macrohemodynamic parameters. Blood Purif 2015;40:38-44. [Crossref] [PubMed]
- Dubin A, Pozo MO, Casabella CA, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care 2009;13:R92. [Crossref] [PubMed]
- Arnold RC, Dellinger RP, Parrillo JE, et al. Discordance between microcirculatory alterations and arterial pressure in patients with hemodynamic instability. J Crit Care 2012;27:531.e1-7. [Crossref] [PubMed]
- Sakr Y, Dubois MJ, De Backer D, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 2004;32:1825-31. [Crossref] [PubMed]
- De Backer D, Orbegozo Cortes D, Donadello K, et al. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence 2014;5:73-9. [Crossref] [PubMed]
- Vincent JL, De Backer D. Circulatory shock. N Engl J Med 2013;369:1726-34. [Crossref] [PubMed]
- Doerschug KC, Delsing AS, Schmidt GA, et al. Impairments in microvascular reactivity are related to organ failure in human sepsis. Am J Physiol Heart Circ Physiol 2007;293:H1065-71. [Crossref] [PubMed]
- Shapiro NI, Arnold R, Sherwin R, et al. The association of near-infrared spectroscopy-derived tissue oxygenation measurements with sepsis syndromes, organ dysfunction and mortality in emergency department patients with sepsis. Crit Care 2011;15:R223. [Crossref] [PubMed]
- Trzeciak S, McCoy JV, Dellinger RP, et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med 2008;34:2210-7. [Crossref] [PubMed]
- Shih CC, Liu CM, Chao A, et al. Matched Comparison of Microcirculation Between Healthy Volunteers and Patients with Sepsis. Asian J Anesthesiol 2018;56:14-22. [PubMed]
- Uz Z, Ince C, Guerci P, et al. Recruitment of sublingual microcirculation using handheld incident dark field imaging as a routine measurement tool during the postoperative de-escalation phase—a pilot study in post ICU cardiac surgery patients. Perioper Med (Lond) 2018;7:18. [Crossref] [PubMed]
- Nascente APM, Freitas FGR, Bakker J, et al. Microcirculation improvement after short-term infusion of vasopressin in septic shock is dependent on noradrenaline. Clinics (Sao Paulo) 2017;72:750-7. [Crossref] [PubMed]
- Vellinga NA, Ince C, Boerma EC. Elevated central venous pressure is associated with impairment of microcirculatory blood flow in sepsis: a hypothesis generating post hoc analysis. BMC Anesthesiol 2013;13:17. [Crossref] [PubMed]
- Kanoore Edul VS, Ince C, Dubin A. What is microcirculatory shock? Curr Opin Crit Care 2015;21:245-52. [Crossref] [PubMed]
- Legrand M, Ait-Oufella H, Ince C. Could resuscitation be based on microcirculation data? Yes. Intensive Care Med 2018;44:944-6. [Crossref] [PubMed]
- Pranskunas A, Koopmans M, Koetsier PM, et al. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med 2013;39:612-9. [Crossref] [PubMed]
- Malbrain MLNG, Van Regenmortel N, Saugel B, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care 2018;8:66. [Crossref] [PubMed]
- Sadaka F, Juarez M, Naydenov S, et al. Fluid resuscitation in septic shock: the effect of increasing fluid balance on mortality. J Intensive Care Med 2014;29:213-7. [Crossref] [PubMed]
- Ferrara G, Kanoore Edul VS, Martins E, et al. Intestinal and sublingual microcirculation are more severely compromised in hemodilution than in hemorrhage. J Appl Physiol (1985) 2016;120:1132-40. [PubMed]
- Dépret F, Sitbon A, Soussi S, et al. Intravenous iloprost to recruit the microcirculation in septic shock patients? Intensive Care Med 2018;44:121-2. [Crossref] [PubMed]
- Hilty MP, Pichler J, Ergin B, et al. Assessment of endothelial cell function and physiological microcirculatory reserve by video microscopy using a topical acetylcholine and nitroglycerin challenge. Intensive Care Med Exp 2017;5:26. [Crossref] [PubMed]
- Spronk PE, Ince C, Gardien MJ, et al. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet 2002;360:1395-6. [Crossref] [PubMed]
- Boerma EC, Koopmans M, Konijn A, et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med 2010;38:93-100. [Crossref] [PubMed]
- Morelli A, Donati A, Ertmer C, et al. Short-term effects of terlipressin bolus infusion on sublingual microcirculatory blood flow during septic shock. Intensive Care Med 2011;37:963-9. [Crossref] [PubMed]
- Morelli A, Donati A, Ertmer C, et al. Effects of vasopressinergic receptor agonists on sublingual microcirculation in norepinephrine-dependent septic shock. Crit Care 2011;15:R217. [Crossref] [PubMed]
- Boerma EC, van der Voort PH, Ince C. Sublingual microcirculatory flow is impaired by the vasopressin-analogue terlipressin in a patient with catecholamine-resistant septic shock. Acta Anaesthesiol Scand 2005;49:1387-90. [Crossref] [PubMed]
- Ospina-Tascón GA, Umaña M, Bermúdez WF, et al. Can venous-to-arterial carbon dioxide differences reflect microcirculatory alterations in patients with septic shock? Intensive Care Med 2016;42:211-21. [Crossref] [PubMed]
- Weinberg JA, MacLennan PA, Vandromme–Cusick MJ, et al. Microvascular response to red blood cell transfusion in trauma patients. Shock (Augusta, Ga) 2012;37:276. [Crossref] [PubMed]
- Ayhan B, Yuruk K, Koene S, et al. The effects of non-leukoreduced red blood cell transfusions on microcirculation in mixed surgical patients. Transfusion and Apheresis Science 2013;49:212-22. [Crossref] [PubMed]
- Marik PE. Steroids for sepsis: yes, no or maybe. J Thorac Dis 2018;10:S1070-3. [Crossref] [PubMed]


