
Understanding breast cancer disparities—a multi-scale challenge
Introduction
The past few decades have witnessed a steady decline in breast cancer mortality in the United States despite largely stable rates of diagnoses, a feat that is largely due to improved screening and treatment strategies in this disease (1). However, the benefits of this overall trend towards improved breast cancer-specific mortality is not equitably distributed across populations groups within the United States. Indeed, despite convergence of overall breast cancer incidence rates between European American (EA) and African American (AA) women, disparities in mortality still persist (2). While mortality hazard rates among AA women vary by breast cancer subtypes, AA women in general exhibit about 20% to 150% higher mortality relative to EA women (3). AA women are more likely to present at an earlier age, with higher-grade tumors and higher rates of triple-negative breast cancer (TNBC) (4). Accounting for age, AA women are at least 2-times more likely than their EA counterparts to be diagnosed with TNBC (3,5,6), a subtype known to be heterogeneous, aggressive and difficult to treat (7). Several studies have suggested that the racial disparities observed in mortality are due to this higher rate of TNBC in AA women (4,8,9). However, the mere increased incidence of the aggressive TNBC subtype in AA women fails to fully explain the observed disparities in outcomes, since AA women face poorer overall survival even after accounting for age and stage at diagnosis within other breast cancer subtypes as well (10,11). These results pose differing but important questions regarding the potential determinants of observed breast cancer incidence and outcome disparities across population groups.
In this review, we explore the multi-factorial nature of cancer health disparities (Figure 1). Over the course of a patient’s disease from the time of diagnosis, therapy selection to survivorship, a variety of factors potentially contribute to overall outcomes, including socioeconomic circumstance, access to care, behavioral factors, and tumor biology. While incidence rates of breast cancer are similar between AA and EA population groups (5,6), we nevertheless observe disparities between AA and EA breast cancers at the time of clinical presentation and prognosis (Figure 1) where AA women generally present with higher grade, basal-like tumors (5,6). This gap expands during the course of treatment, with AA women exhibiting higher rates of mortality that are not fully explained by medical care disparities or what is known about tumor biology. Here, we summarize current data regarding how the interplay between non-modifiable and behavioral risk factors, access to quality care, equal treatment and tumor biology may contribute to differences in breast cancer mortality rates across population groups.
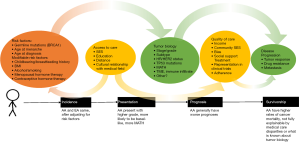
Modifiable factors that influence breast cancer risk
One of the early hypotheses proposed to explain the differential risk of TNBC in AA versus EA women involved breastfeeding practices [Figure 1, (4)]. This was motivated by the general understanding that breastfeeding, or lack thereof, was associated with increased risk of developing TNBC (12-16). Indeed, the Nurses’ Health Study found that among women with invasive breast cancer, higher parity and the absence or short duration of breastfeeding were independently associated with TNBC (17). Epidemiologic studies have noted differences in breastfeeding rates between EA and AA women, with 62% and 45%, respectively, breastfeeding at 6 months (18), thus raising the question as to whether these differences may contribute to higher incidence rates of TNBC among AA women. Indeed, the American Breast Cancer Epidemiology and Risk Consortium (AMBER) explored this association in AA women, identifying an increased risk of estrogen receptor (ER)-negative breast cancer with each additional childbirth in AA women who had not breastfed (19). Further investigating the likely biologic mechanism underlying these epidemiologic observations, Basree et al. (20) showed in mouse models and human breast tissue that abrupt involution of mammary glands following pregnancy and limited breast feeding (<6 months) results in expansion of the luminal progenitor cell compartment associated with development of basal-like tumors. Taken together, these studies suggest that the lower rates of breastfeeding by AA women may potentially contribute to higher TNBC disease burden among this group, a supposition whose validity awaits the performance of large well-controlled epidemiologic and in-depth mechanistic studies to determine the relative importance of breastfeeding as a determinant of TNBC incidence disparities across population groups.
The ability of adipocytes in obese states to secrete proinflammatory cytokines and exhibit poorer metabolic control (21) led to the hypothesis that obesity may be a major driver of aggressive TNBC biology (7) (Figure 1). Indeed, the Carolina Breast Cancer study found that women with a higher waist-to-hip ratio (WHR) had increased risk for developing TNBC (14). Likewise, a meta-analysis of eleven original articles found a significant association between obesity and TNBC in both case-case and case-control analyses (22). However, it is worth noting that this association, even across racial groups, is not definitive. The Women’s Circle of Health Study observed a significant inverse relationship between high body mass index (BMI) and hormone receptor negative breast cancer among postmenopausal women (23). Moreover, these findings were also substantiated by The Premenopausal Breast Cancer Collaborative Group who found an inverse association between BMI and cancer risk in young (18 to 24 years) women and no association across ages with TNBC (24). Regardless, the combination of a possible association between obesity and TNBC, together with a higher prevalence of obesity in AA women compared to EA women, led several groups to investigate whether increased body mass could contribute to higher rates of TNBC among AA women. The AMBER consortium showed varied trends associating obesity with breast cancer in AA women depending on the metric used, but none of which specifically linked body mass in AA women to TNBC. In fact, higher BMI correlated with increased risk for ER-positive breast cancer and decreased risk for TNBC in postmenopausal women. Additionally, high WHR in postmenopausal women was associated with increased risk of all breast cancer subtypes whereas in premenopausal women, high WHR was associated with an increased probability of ER+ tumors but not others (25). Interestingly, a study by Capers et al. (26) explored the importance of body shape in EA and AA women to predict disease associations, and found that obese AA and EA women exhibit differences in distribution of adipose tissue, insulin resistance, and lipoprotein subclasses. These results suggest that obesity in AA women might influence disease progression differently than in EA women, potentially contributing to disparities in mortality, but not to differences in TNBC incidence rates.
Smoking and alcohol consumption are mild risk factors for breast cancer incidence (27,28). However, considering that the rates of heavy smoking (29) or drinking (30) are not particularly high in the AA population compared to the EA population, and that both exposures are associated with ER-positive BC and not TNBC (31,32), it is unlikely that these behavioral risk factors have much influence on breast cancer disparities.
Biologic factors related to breast cancer incidence
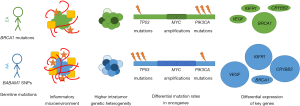
One of the major areas of research in breast cancer risk is in the heritability of the disease. The first germline mutations found to be associated with increased risk of TNBC were BRCA1/BRCA2 (33). BRCA1 is a tumor suppressor gene that plays a key role in homology-directed repair of DNA double-stranded breaks (34-36). The majority of breast cancers in women with BRCA1 mutations are TNBC (37), but despite the higher incidence rate of TNBC in AA women, several studies show the frequency of germline BRCA1 mutations is relatively low compared to the observed mutation rate in EA women (38,39). A study of 155 high-risk families evaluated at the University of Chicago found germline BRCA1 mutations in 50% of EA women with TNBC and fewer than 20% of AA women with TNBC (40), suggesting that other mechanisms may promote TNBC in AA women. For example, SNP rs8170 on the BABAM1 gene was found to be linked to TNBC in a mixed population of patients (41,42), and was associated with increased risk of TNBC in an AA population (43). This higher prevalence of a pathogenic SNP in women of African ancestry may contribute to the higher incidence rate of TNBC in AA women (Figure 2). Because these genes are insufficient to explain all inherited breast cancer risk, researchers have hypothesized that variations in combinations of several genes, known as polygenic risk models, may more accurately predict breast cancer risk (44). However, it is important to develop population-specific polygenic risk models in order to better understand the extent to which population-specific genetic factors influence racial disparities in cancer incidence (45).
Impact of access and quality of care on breast cancer outcomes
It is conceivable that in a society still recovering from hundreds of years of institutionalized discrimination, access to quality health care could be a major contributor of outcome disparities. Supporting this notion, a meta-analysis of 23 studies that examined racial patterns of care for breast cancer concluded that AA women less frequently receive radiation therapy after breast-conserving surgery, thereby suggesting that disparities in access and quality of treatment may be an underlying factor (46). Further supporting this, Pacheco et al. (9) found that race was not significantly associated with outcomes of TNBC in a single center study where patients received similar therapy and follow-up, suggesting that the observed disparities in cancer mortality may be the result of inequalities of disease management. In contrast, studies conducted in presumably equal-access health care systems such as the military health system in the United States (47) or the National Health Service in the UK (48) observed significant disparities in breast cancer outcomes between women of African versus European ancestry. Furthermore, several studies in controlled care settings have still reported differences in outcomes between racial groups. Woodward et al. (49) report that race is independently associated with overall survival in locally advanced, nonmetastatic breast cancer treated with mastectomy and doxorubicin-based chemotherapy, with AA women exhibiting poorer survival than their EA counterparts. Likewise, an analysis of 35 randomized phase III clinical trials conducted by the Southwest Oncology Group (SWOG) found that AA patients had worse survival than EA patients, despite enrollment with uniform stage, treatment and follow-up (50). Therefore, while reducing barriers to care is fundamentally important and has, in some settings, resulted in at least a 20% reduction in racial disparities in breast cancer outcomes (51), it remains unclear whether equalizing access to care alone would suffice to eradicate outcome disparities.
There is significant debate on the role of socioeconomic status (SES) in the context of cancer outcomes (52), as outlined in Table 1. Indeed, while some studies show that biological/clinical factors do not fully explain racial disparities (10,11,61-63), controlling for SES and other factors related to healthcare access do not always explain all of the racial disparities in breast cancer (53-60) (Table 1). For example, Curtis et al. (56) found that adjusting for mammography screening, tumor characteristics, biologic markers, treatment, comorbidity and SES demographics derived from the Surveillance, Epidemiology, and End Results (SEER)-Medicare data reduced the mortality difference between AA and EA women in all stages of breast cancer [HR: 1.08 (0.97–1.20)]. However, controlling for these variables did not eliminate these morality differences in women with stage II/III disease [HR: 1.30 (1.10–1.54)]. Indeed, this study used median income by zip code and “type of community”, defined as rural, less rural, urban, metropolitan, and big metropolitan, as markers of SES. A similar analysis of the SEER data by Chu et al. (64) found a significant association between race and stage-specific survival rates in younger breast cancer patients (<50 years old), a disparity that was no longer significant for patients 65 years or older due in part to access to Medicare in the older population. This suggested that uniform access to medical insurance may help to alleviate racial disparities in access to care (64). While this study did not consider SES directly, these findings still point toward discrepancies in external factors as likely being partially responsible for disparities in cancer mortality.
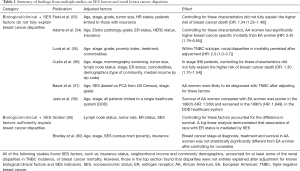
Full table
Given that racial disparities in breast cancer are not fully explained by access to care, lifestyle and socioeconomic factors alone, there has been a growing interest in exploring potential molecular differences in breast cancers across population groups to gain insights into likely biologic factors underpinning outcome disparities.
Impact of tumor biology on breast cancer disparities
While the external factors contributing to higher rates of TNBC in AA women remain uncertain, deciphering the molecular and biological drivers at the tumor level is a key step to developing treatments for aggressive disease. Multiple studies have reported relatively modest differences in somatic mutations, associated copy-number profiles, gene expression levels, tumor mutation burden and intratumor heterogeneity between breast tumor lesions from AA and EA women (Figure 2).
In an analysis of 930 patients with breast cancer, Huo et al. (42) found that tumors from women with African ancestry had a higher proportion of TP53 mutations, MYC amplifications and a lower proportion of PIK3CA mutations as compared to tumors from women of European descent; however, these molecular differences were accounted for after adjusting for intrinsic subtype (Figure 2). Keenan et al. (65) similarly studied samples from The Cancer Genome Atlas (TCGA) and found higher rates of TP53 mutations, lower rates of PIK3CA mutations and higher prevalence of the basal-like PAM50 subtype in breast cancers in AA women as compared to their EA counterparts. They also observed higher levels of intratumor heterogeneity in breast cancers in AA versus EA women, but this difference did not significantly contribute to the racial disparity in tumor recurrence (Figure 2). However, upon adjusting for TP53 mutation status, PIK3CA mutation status, as well as expression-based subtypes (PAM50) of breast cancer, almost no differences were observed in tumor recurrence hazard between AA and EA (65), thus suggesting that differences in the molecular makeup of breast cancer across populations may contribute to disparities in outcomes.
In a study focused on TNBC, comparing a total of 128 tumor samples from EA women (54%) and AA women (39%), Lindner et al. (66) found the transcriptional profiles of tumors from AA women to exhibit gene expression signatures consistent with the Basal 1 (BL1) TNBC subtype, decreased BRCA1 expression, increased activation of insulin-like growth factor 1 receptor (IGF1R) and increased expression of vascular endothelial growth factor (VEGF) activated genes (Figure 2). However, given that differential expression of IGF1R could also be associated with differences in the rates of obesity and metabolic syndrome between the AA and EA patients in this cohort, additional studies are warranted to determine whether differences in tumor molecular profiles in AA and EA patients are intrinsic to tumor biology, or merely reflective of patient comorbidities.
Another intriguing proposition entails the existence of novel subtypes of breast cancers that are unique to specific population groups considering that canonical breast cancer subtyping was derived from majority EA cohorts. Exploring this possibility, a study of 147 Ghanaian women found a counter-intuitive correlation of androgen receptor and aldehyde dehydrogenase (ALDH1) expression among TNBCs from AA women, suggesting that novel TNBC subtypes may exist among populations with African ancestry. The discovery of these novel subtypes will require large-scale profiling of AA TNBCs followed by molecular subtyping similar to the TNBC subtypes (67).
Role of the tumor microenvironment (TME) in cancer health disparities
The past decade has resulted in dramatic new advances in our understanding of the role of the TME in carcinogenesis and tumor progression. Therefore, when exploring factors contributing to aggressive cancer phenotypes in AA women and poorer outcomes, it is essential to characterize not only tumor intrinsic molecular profiles but also the TME to get a fuller picture of the disease.
Broadly, TME, which provides a favorable niche for the growth of tumor cells, is comprised of several types of stromal cells (e.g., fibroblasts, endothelial, and immune cells) and the various proteins secreted as a consequence of bi-directional tumor-stromal cross-talk. Emerging evidence suggests inherent biological differences in the TME of breast cancer patients from different racial backgrounds. Indeed, elevated levels of cytokines, including Resistin and IL-6 (68), higher vessel density (69) and increased macrophage recruitment (70) characterize the TME of breast cancers in AA women (Figure 2). These TME components render patients more susceptible to the development of aggressive tumors, faster progression of disease, and poorer patient survival (71).
It is possible that molecular drivers for higher rates of TNBC observed in AA women are differences in immune signaling. In a study of the AMBER consortium data, Hong et al. (72) chronicled genetic variations and differential activation of key immune response pathways among AA women with breast cancer. SNPs in genes involved in regulation of immune system processes, immune activation, and inflammation were associated with higher risk of breast cancer. Specifically, SNPs in the NF-κB pathway were found to associate with innate immunity and activation of the inflammatory response, leading to an increased risk of ER-positive breast cancers. Likewise, pathways associated with MAP3K1 activation were also linked to an increased risk of ER-negative cancers (72). In addition to contributing to breast cancer risk, certain variations in immune components may regulate tumor response to treatment. For example, Jenkins et al. (73) found that infiltrating carcinomas in AA women have a higher proportion of tumors that are negative for the atypical chemokine receptor 1 (DARC/ACKR1) as compared to EA women, a difference likely driven by sub-Saharan African-specific alleles in this gene. DARC/ACKR1 expression not only plays a significant role in immune regulation, but also promotes significantly enhanced survival in individuals with DARC/ACKR1-high tumors across all molecular tumor subtypes (73).
Collectively, these data suggest that genetic variations in key immune regulatory genes may underlie racial disparities in breast cancer susceptibility, as well as mortality. Nonetheless, the extent to which cancer health disparities are influenced by ancestry-related differences in the innate and adaptive immune systems remains unclear. Indeed, while germline variations could modulate immune responses across population groups, there is a growing recognition of the potential immunomodulatory role of allostatic load and/or chronic stress (74-78). As such, well-controlled longitudinal studies are warranted to explore the relative influence of germline factors or external stressors on individual patient immune responses, which in turn may contribute to outcome disparities (Figure 3).
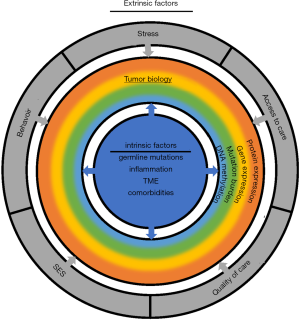
Future directions in population scale exploration of cancer health disparities
Despite the rapid drop in cost of sequencing over the past few years, large-scale molecular profiling of tumors is still an expensive proposition, thus limiting our ability to assess for differences in tumor biology at the population scale. However, recent developments in computational imaging approaches applied to routinely collected radiologic images and pathology slides have revealed previously underappreciated insights into tumor biology. Indeed, we and others have shown that tumor profiles at the radiomic and pathomic scales are associated with molecular subtypes and clinical outcomes across cancers (36,79,80). As these radiogenomic and pathomic approaches continue to be developed and validated, they will enable population-level assessments of variations in tumor biology, thus allowing the exploration of mechanisms underlying cancer health disparities at scale.
Yet another challenge in population-scale explorations of social determinants of cancer health disparities involves the assessment of SES of individual patients. Epidemiologic studies in cancer health disparities often incorporate SES status measured by education, occupation, wealth or income, but the availability and reliability of many of these measures remains a key challenge in the field (81). However, recent advancements in social science methodologies that incorporate geospatial analytics to assess economic disadvantage have enabled large-scale studies in cancer health disparities. For example, after analyzing data on Louisiana TNBC patients diagnosed in 2010–2012 with a robust measure of physical and social environment called neighborhood concentrated disadvantage index (CDI), Hossain et al. (82) found that CDI was associated with more advanced stages of TNBC at diagnosis and poor stage-specific survival. Similarly, by combining data from the US Centers for Disease Control and Prevention and the Home Mortgage Disclosure Act database, Beyer et al. (83) determined that mortgage discrimination was associated with larger racial cancer mortality disparities. In a study of 13,066 female patients, neighborhood SES (education, occupation, employment, household income, poverty, rent, and house value by census tract) and individual SES (insurance and marital status) stably explained one-half of the racial disparities in survival outcomes (84). These studies suggest that novel geospatial indices of disadvantage in combination with traditional demographic measures can provide a more comprehensive assessment of socioeconomic factors likely contributing to cancer health disparities.
Discussion
Our understanding of breast cancer disparities over the past decade has been driven by research conducted by epidemiologists, social scientists, and biologists, working largely independent of each other. However, these substantial efforts have reinforced the notion that cancer health disparities are driven by a complex interplay of factors spanning the molecular to sociologic scales. Studying differences in medical outcomes between racial groups, a socially-constructed concept, without accounting for the historical and current social environment of those groups can result in missed opportunities to improve health equity in addition to confounding our understanding of the biological factors driving disease aggressiveness. Accordingly, the next phase in breast cancer disparities research will be driven by multi-disciplinary teams of scientists who curate large-scale datasets with comprehensive tumor measurements and sociodemographic data. Indeed, advances in geospatial data collection at the sociodemographic scale coupled with innovations in radiologic/pathologic imaging and molecular profiling at the biologic scales are poised to enable such large-scale dataset curation. Additionally, recent advances in machine learning and systems biology have begun to provide the necessary methodologic frameworks to analyze such multi-scale datasets. Such a convergence of large-scale dataset curation and big data analytics is expected to better elucidate the sociologic and biologic determinants of patient outcomes, and thus enable the development of more effective interventional strategies to reduce breast cancer disparities.
Acknowledgments
Funding: This work was supported by National Cancer Institute Public Health Service awards 1P20CA233216, 1K25DK115904.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Khalid Sossey-Alaoui) for the series “Cancer Metastasis: Molecular signaling and therapeutic options” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.04.37). The series “Cancer Metastasis: Molecular signaling and therapeutic options” was commissioned by the editorial office without any funding or sponsorship. WPS reports grants from National Institutes of Health, during the conduct of the study. VV reports grants from National Institutes of Health, during the conduct of the study; grants and personal fees from Curis, Inc., grants from Philips Healthcare, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2016. Bethesda: National Cancer Institute, 2019. Available online: https://seer.cancer.gov/csr/1975_2016/
- DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin 2016;66:31-42. [Crossref] [PubMed]
- Troester MA, Sun X, Allott EH, et al. Racial differences in PAM50 subtypes in the Carolina breast cancer study. J Natl Cancer Inst 2018;110:176-82. [Crossref] [PubMed]
- Scott LC, Mobley LR, Kuo TM, et al. Update on triple-negative breast cancer disparities for the United States: a population-based study from the United States Cancer Statistics database, 2010 through 2014. Cancer 2019;125:3412-7. [Crossref] [PubMed]
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. Atlanta: American Cancer Society, Inc., 2017. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf
- American Cancer Society. Breast Cancer Facts & Figures 2019-2020. Atlanta: American Cancer Society, Inc., 2019. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf
- Siddharth S, Sharma D. Racial disparity and triple-negative breast cancer in African-American women: a multifaceted affair between obesity, biology, and socioeconomic determinants. Cancers (Basel) 2018. [Crossref] [PubMed]
- Sturtz LA, Melley J, Mamula K, et al. Outcome disparities in African American women with triple negative breast cancer: a comparison of epidemiological and molecular factors between African American and Caucasian women with triple negative breast cancer. BMC Cancer 2014;14:62. [Crossref] [PubMed]
- Pacheco JM, Gao F, Bumb C, et al. Racial differences in outcomes of triple-negative breast cancer. Breast Cancer Res Treat 2013;138:281-9. [Crossref] [PubMed]
- Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA 2006;295:2492-502. [Crossref] [PubMed]
- Boyer-Chammard A, Taylor TH, Anton-Culver H. Survival differences in breast cancer among racial/ethnic groups: a population-based study. Cancer Detect Prev 1999;23:463-73. [Crossref] [PubMed]
- Kwan ML, Kushi LH, Weltzien E, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res 2009;11:R31. [Crossref] [PubMed]
- Li CI, Beaber EF, Tang MTC, et al. Reproductive factors and risk of estrogen receptor positive, triple-negative, and HER2-neu overexpressing breast cancer among women 20-44 years of age. Breast Cancer Res Treat 2013;137:579-87. [Crossref] [PubMed]
- Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat 2008;109:123-39. [Crossref] [PubMed]
- Phipps AI, Malone KE, Porter PL, et al. Reproductive and hormonal risk factors for postmenopausal luminal, HER-2-overexpressing, and triple-negative breast cancer. Cancer 2008;113:1521-6. [Crossref] [PubMed]
- Shinde SS, Forman MR, Kuerer HM, et al. Higher parity and shorter breastfeeding duration. Cancer 2010;116:4933-43. [Crossref] [PubMed]
- Fortner RT, Sisti J, Chai B, et al. Parity, breastfeeding, and breast cancer risk by hormone receptor status and molecular phenotype: results from the Nurses’ Health Studies. Breast Cancer Research 2019;21:40. [Crossref] [PubMed]
- Beauregard JL, Hamner HC, Chen J, et al. Racial disparities in breastfeeding initiation and duration among U.S. infants born in 2015. MMWR Morb Mortal Wkly Rep 2019;68:745-8. [Crossref] [PubMed]
- Palmer JR, Viscidi E, Troester MA, et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst 2014;106:dju237. [Crossref] [PubMed]
- Basree MM, Shinde N, Koivisto C, et al. Abrupt involution induces inflammation, estrogenic signaling, and hyperplasia linking lack of breastfeeding with increased risk of breast cancer. Breast Cancer Res 2019;21:80. [Crossref] [PubMed]
- Sharma D, Davidson NE. Obesity and breast cancer: a multipartite connection. J Mammary Gland Biol Neoplasia 2013;18:253-5. [Crossref] [PubMed]
- Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat 2013;137:307-14. [Crossref] [PubMed]
- Ambrosone CB, Ciupak GL, Bandera EV, et al. Conducting molecular epidemiological research in the age of HIPAA: a multi-institutional case-control study of breast cancer in African-American and European-American women. J Oncol 2009;2009:871250. [Crossref] [PubMed]
- Premenopausal Breast Cancer Collaborative Group, Schoemaker MJ, Nichols HB, et al. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol 2018;4:e181771. [Crossref] [PubMed]
- Bandera EV, Chandran U, Hong CC, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat 2015;150:655-66. [Crossref] [PubMed]
- Capers PL, Kinsey AW, Miskell EL, et al. Visual representation of body shape in African-American and European American women: clinical considerations. Clin Med Insights Womens Health 2016;9:63-70. [PubMed]
- Kispert S, McHowat J. Recent insights into cigarette smoking as a lifestyle risk factor for breast cancer. Breast Cancer (Dove Med Press) 2017;9:127-32. [Crossref] [PubMed]
- Singletary SE. Rating the risk factors for breast cancer. Ann Surg 2003;237:474-82. [Crossref] [PubMed]
- Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults - United States, 2018. MMWR Morb Mortal Wkly Rep 2019;68:1013-9. [Crossref] [PubMed]
- Chartier K, Caetano R. Ethnicity and Health Disparities in Alcohol Research. Available online: https://pubs.niaaa.nih.gov/publications/arh40/152-160.htm
- Suzuki R, Orsini N, Mignone L, et al. Alcohol intake and risk of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis of epidemiological studies. Int J Cancer 2008;122:1832-41. [Crossref] [PubMed]
- Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol 2012;23 Suppl 6:vi7-12. [Crossref] [PubMed]
- Friedman LS, Ostermeyer EA, Szabo CI, et al. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet 1994;8:399-404. [Crossref] [PubMed]
- Hall JM, Lee MK, Newman B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science 1990;250:1684-9. [Crossref] [PubMed]
- Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci 2004;95:866-71. [Crossref] [PubMed]
- Antoniou AC, Easton DF. Models of genetic susceptibility to breast cancer. Oncogene 2006;25:5898-905. [Crossref] [PubMed]
- Mavaddat N, Barrowdale D, Andrulis IL, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev 2012;21:134-47. [Crossref] [PubMed]
- Olopade OI, Fackenthal JD, Dunston G, et al. Breast cancer genetics in African Americans. Cancer 2003;97:236-45. [Crossref] [PubMed]
- Greenup R, Buchanan A, Lorizio W, et al. Prevalence of BRCA mutations among women with triple-negative breast cancer (TNBC) in a genetic counseling cohort. Ann Surg Oncol 2013;20:3254-8. [Crossref] [PubMed]
- Nanda R, Schumm LP, Cummings S, et al. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. JAMA 2005;294:1925-33. [Crossref] [PubMed]
- Haiman CA, Chen GK, Vachon CM, et al. A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor-negative breast cancer. Nature Genetics 2011;43:1210-4. [Crossref] [PubMed]
- Huo D, Hu H, Rhie SK, et al. Comparison of breast cancer molecular features and survival by African and European Ancestry in The Cancer Genome Atlas. JAMA Oncol 2017;3:1654-62. [Crossref] [PubMed]
- Palmer JR, Ruiz-Narvaez EA, Rotimi CN, et al. Genetic susceptibility loci for subtypes of breast cancer in an African American population. Cancer Epidemiol Biomarkers Prev 2013;22:127-34. [Crossref] [PubMed]
- Mavaddat N, Michailidou K, Dennis J, et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet 2019;104:21-34. [Crossref] [PubMed]
- Wang S, Qian F, Zheng Y, et al. Genetic variants demonstrating flip-flop phenomenon and breast cancer risk prediction among women of African ancestry. Breast Cancer Res Treat 2018;168:703-12. [Crossref] [PubMed]
- Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst 2002;94:334-57. [Crossref] [PubMed]
- Andaya AA, Enewold L, Zahm SH, et al. Race and colon cancer survival in an equal-access health care system. Cancer Epidemiol Biomarkers Prev 2013;22:1030-6. [Crossref] [PubMed]
- Copson E, Maishman T, Gerty S, et al. Ethnicity and outcome of young breast cancer patients in the United Kingdom: the POSH study. Br J Cancer 2014;110:230-41. [Crossref] [PubMed]
- Woodward WA, Huang EH, McNeese MD, et al. African-American race is associated with a poorer overall survival rate for breast cancer patients treated with mastectomy and doxorubicin-based chemotherapy. Cancer 2006;107:2662-8. [Crossref] [PubMed]
- Albain KS, Unger JM, Crowley JJ, et al. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 2009;101:984-92. [Crossref] [PubMed]
- Sighoko D, Murphy AM, Irizarry B, et al. Changes in the racial disparity in breast cancer mortality in the ten US cities with the largest African American populations from 1999 to 2013: The reduction in breast cancer mortality disparity in Chicago. Cancer Causes Control 2017;28:563-8. [Crossref] [PubMed]
- Polite BN, Gluck AR, Brawley OW. Ensuring equity and justice in the care and outcomes of patients with cancer. JAMA 2019;321:1663-4. [Crossref] [PubMed]
- Field TS, Buist DS, Doubeni C, et al. Disparities and survival among breast cancer patients. J Natl Cancer Inst Monogr 2005.88-95. [Crossref] [PubMed]
- Adams SA, Butler WM, Fulton J, et al. Racial disparities in breast cancer mortality in a multiethnic cohort in the Southeast. Cancer 2012;118:2693-9. [Crossref] [PubMed]
- Lund MJ, Trivers KF, Porter PL, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat 2009;113:357-70. [Crossref] [PubMed]
- Curtis E, Quale C, Haggstrom D, et al. Racial and ethnic differences in breast cancer survival. Cancer 2008;112:171-80. [Crossref] [PubMed]
- Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 2007;109:1721-8. [Crossref] [PubMed]
- Jatoi I, Becher H, Leake CR. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the U. S. Department of Defense Healthcare system. Cancer 2003;98:894-9. [Crossref] [PubMed]
- Gordon NH. Socioeconomic factors and breast cancer in black and white Americans. Cancer Metastasis Rev 2003;22:55-65. [Crossref] [PubMed]
- Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst 2002;94:490-6. [Crossref] [PubMed]
- van Ravesteyn NT, Schechter CB, Near AM, et al. Race-specific impact of natural history, mammography screening, and adjuvant treatment on breast cancer mortality rates in the United States. Cancer Epidemiol Biomarkers Prev 2011;20:112-22. [Crossref] [PubMed]
- Joslyn SA, West MM. Racial differences in breast carcinoma survival. Cancer 2000;88:114-23. [Crossref] [PubMed]
- Porter PL, Lund MJ, Lin MG, et al. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer 2004;100:2533-42. [Crossref] [PubMed]
- Chu KC, Lamar CA, Freeman HP. Racial disparities in breast carcinoma survival rates: seperating factors that affect diagnosis from factors that affect treatment. Cancer 2003;97:2853-60. [Crossref] [PubMed]
- Keenan T, Moy B, Mroz EA, et al. Comparison of the genomic landscape between primary breast cancer in African American versus white women and the association of racial differences with tumor recurrence. J Clin Oncol 2015;33:3621-7. [Crossref] [PubMed]
- Lindner R, Sullivan C, Offor O, et al. Molecular phenotypes in triple negative breast cancer from African American patients suggest targets for therapy. PLoS One 2013;8:e71915. [Crossref] [PubMed]
- Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750-67. [Crossref] [PubMed]
- Deshmukh SK, Srivastava SK, Bhardwaj A, et al. Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget 2015;6:11231-41. [Crossref] [PubMed]
- Martin DN, Boersma BJ, Yi M, et al. Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One 2009;4:e4531. [Crossref] [PubMed]
- Mukhtar RA, Moore AP, Nseyo O, et al. Elevated PCNA+ tumor-associated macrophages in breast cancer are associated with early recurrence and non-Caucasian ethnicity. Breast Cancer Res Treat 2011;130:635-44. [Crossref] [PubMed]
- Deshmukh SK, Srivastava SK, Tyagi N, et al. Emerging evidence for the role of differential tumor microenvironment in breast cancer racial disparity: a closer look at the surroundings. Carcinogenesis 2017;38:757-65. [Crossref] [PubMed]
- Hong CC, Sucheston-Campbell LE, Liu S, et al. Genetic variants in immune-related pathways and breast cancer risk in African American women in the AMBER consortium. Cancer Epidemiol Biomarkers Prev 2018;27:321-30. [Crossref] [PubMed]
- Jenkins BD, Martini RN, Hire R, et al. Atypical chemokine receptor 1 (DARC/ACKR1) in breast tumors is associated with survival, circulating chemokines, tumor-infiltrating immune cells, and African ancestry. Cancer Epidemiol Biomarkers Prev 2019;28:690-700. [Crossref] [PubMed]
- Green McDonald P, O'Connell M, Lutgendorf SK. Psychoneuroimmunology and cancer: a decade of discovery, paradigm shifts, and methodological innovations. Brain Behav Immun 2013;30 Suppl:S1-9. [Crossref] [PubMed]
- Colditz GA, Wei EK. Preventability of cancer: the relative contributions of biologic and social and physical environmental determinants of cancer mortality. Annu Rev Public Health 2012;33:137-56. [Crossref] [PubMed]
- Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Ann N Y Acad Sci 2010;1186:5-23. [Crossref] [PubMed]
- Boyce WT, Sokolowski MB, Robinson GE. Toward a new biology of social adversity. Proc Natl Acad Sci U S A 2012;109 Suppl 2:17143-8. [Crossref] [PubMed]
- Barboza Solís C, Kelly-Irving M, Fantin R, et al. Adverse childhood experiences and physiological wear-and-tear in midlife: findings from the 1958 British birth cohort. Proc Natl Acad Sci U S A 2015;112:E738-46. [Crossref] [PubMed]
- Braman N, Prasanna P, Whitney J, et al. Association of Peritumoral Radiomics With Tumor Biology and Pathologic Response to Preoperative Targeted Therapy for HER2 (ERBB2)-Positive Breast Cancer. JAMA Netw Open 2019;2:e192561. [Crossref] [PubMed]
- Beig N, Patel J, Prasanna P, et al. Radiogenomic analysis of hypoxia pathway is predictive of overall survival in Glioblastoma. Sci Rep 2018;8:7. [Crossref] [PubMed]
- Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc 2007;99:1013-23. [PubMed]
- Hossain F, Danos D, Prakash O, et al. Neighborhood social determinants of triple negative breast cancer. Front Public Health 2019;7:18. [Crossref] [PubMed]
- Beyer KMM, Laud PW, Zhou Y, et al. Housing discrimination and racial cancer disparities among the 100 largest US metropolitan areas. Cancer 2019;125:3818-27. [Crossref] [PubMed]
- Ren JX, Gong Y, Ling H, et al. Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Cancer Res Treat 2019;173:225-37. [Crossref] [PubMed]


