
Continuous cardiac output assessment or serial echocardiography during septic shock resuscitation?
Introduction
Septic shock is the leading cause of circulatory failure in intensive care unit (ICU) patients (1). Refractory hypotension requiring vasopressors despite adequate fluid loading associated with organ dysfunction and high lactate level (i.e., tissue hypoperfusion) in a patient presenting with a suspected infection constitute the new Sepsis-3 definition of septic shock (2). Sepsis-induced organ failures are interdependent (3). This interdependence is especially evident in the presence of cardiovascular failure which reduces systemic blood flow, thus exacerbating tissue dysoxia, mitochondrial dysfunction, and ultimately resulting in further metabolic dysfunction of tissues with lactate production. In causing organ dysfunctions, sepsis-induced cardiovascular failure is life-threatening, at least in the initial phase of the disease (3).
Cardiac output is a primary component of global oxygen delivery to organs, hence a major determinant of tissue oxygen supply. Physiologically, cardiac output can vary widely and abruptly to meet the global metabolic demand of the body and its fluctuations over time (e.g., physical exercise, fever). Accordingly, measuring cardiac output in shocked patients is sound to identify low flow states and guide resuscitation. Most techniques currently used on clinical grounds provide a continuous monitoring of cardiac output (4). Critical care echocardiography (CCE), suggested as the preferred modality to initially evaluate the type of shock as opposed to more invasive technologies (5), only allows serial measurements of cardiac output (6).
We herein sought to summarize the indications for cardiac output measurement, to illustrate the respective advantages and limitations of widely spread monitoring techniques, and to discuss the relevance of continuous versus intermittent measurement of cardiac output in the specific clinical setting of septic shock.
Background: sepsis-induced cardiovascular failure
The cardiovascular failure characterizing septic shock combines various and intricate alterations of both vascular (loss of vascular tone, microvascular shunts) and cardiac function (altered systolic and diastolic ventricular properties). The combination of vasodilatation, depressed cardiac function, and compromised oxygen extraction results in tissue dysoxia and associated organ dysfunction (7). Although constantly observed in experimental settings (8), intrinsic sepsis-induced alterations of systolic and diastolic function of both cardiac ventricles have various functional consequences. The resulting hemodynamic profile depends on the degree of hypovolemia (absolute due to vascular leakage, relative due to vasoplegia), severity of cardiac failure, and potential ventriculo-arterial decoupling (9). The so-called “septic cardiomyopathy”, which currently lacks consensual definition, is commonly characterized by the presence of a left ventricular systolic dysfunction (10,11). Sepsis-induced cardiac failure typically coexist with peripheral (both arterial and venous) vasodilatation. This accounts for the absence of elevated cardiac filling pressure even in the presence of severe left ventricular systolic dysfunction (12). Importantly, the hemodynamic profile may greatly and rapidly vary according to the delay of sepsis diagnosis, the intensity of host’s pro-inflammatory response, the presence of comorbidities (e.g., underlying cardiopathy), and initial management (volume of fluid resuscitation, vasopressor support). For example, septic cardiomyopathy may be unmasked secondarily as vascular tone is restored by both the vasopressor support and control of infection (12). Accordingly, sequential evaluation of the hemodynamic status of patients with septic shock is currently suggested (5), especially when exhibiting sustained tissue hypoperfusion and associated multi-organ failure during the first days of ICU stay (13).
Why measuring cardiac output in patients with septic shock?
Cardiac output directly reflects the efficiency of the cardiovascular system to transport oxygen from the heart, the pump which provides the energy for the circulation of blood, to the entire body. As it is not as tightly regulated than blood pressure by neuro-humoral reflexes, cardiac output may abruptly drop before hypotension develops secondary to any cardiovascular disturbance (14). Similarly, the magnitude of increase of cardiac output in response to a therapeutic intervention (e.g., fluid challenge, initiation of inotropes) closely reflects its efficacy (15). Cardiac output variations can be tracked in real time, irrespective of the technique of measurement. Measuring continuously or repeatedly cardiac output provides valuable information on: (I) the stability of the hemodynamic status of a shocked patient, or (II) the development of any significant abnormality within the cardiovascular system in the presence of an unexpected acute drop, and (III) the degree of efficacy of therapeutic interventions according to the amplitude of the response obtained.
Although septic shock is a distributive shock which is typically characterized by an intense vasoplegia associated with a hyperdynamic state (i.e., elevated cardiac output), low flow states may be observed in patients with severe sepsis-induced myocardial depression (16). Interestingly, low values of both cardiac output and central venous oxygen saturation denoting inadequate oxygen delivery can be observed early in the course of septic shock in fluid-resuscitated patient under vasopressor support (17). In addition, repeated measurements of cardiac output will accurately quantify the direct effects of the chosen therapeutic interventions on the performance of the cardiovascular system. In patients sustaining septic shock, fluid resuscitation and initiation of vasopressor in the presence of refractory hypotension are the mainstays of the initial management of associated cardiovascular failure (18). Resulting modifications of cardiac loading conditions are expected to maintain adequate mean blood pressure and to restore adapted cardiac output to satisfactorily meet the global oxygen requirement.
Since the semi-quantitative clinical estimation of cardiac output has long been shown to be inaccurate in shocked patients (19), baseline and therapeutic-induced variations of cardiac output should be properly measured. In addition, tissue dysoxia may be the result of either a systemic (low cardiac output) or local mismatch between oxygen delivery and tissue demand (normal or even high cardiac output) (20). Accordingly, indirect biological markers of inadequacy between oxygen supply and metabolic needs (e.g., mixed venous or central venous oxygen saturation) remain frequently within the normal range due to impaired oxygen extraction in septic shock patients, even when associated with a severe cardiac dysfunction (21). Variations of cardiac output induced by a fluid challenge is neither accurately tracked by changes in arterial pressure (22), nor by changes in venous oxygen saturation due to their non-linear relationship (23). When oxygen delivery is below its critical level, initial increase compensates for the pre-existing oxygen debt while oxygen extraction remains maximal and venous oxygen saturation fails to change (24).
Overall, reasons for monitoring cardiac output in patients sustaining septic shock are several: (I) identifying the type of shock, (II) selecting the appropriate therapeutic intervention, and (III) evaluating patient’s response to therapy (5). Routine measurement of cardiac output is not recommended in all patients with shock (25), especially in those responding to the initial therapy (5). In contrast, the patient who fails to respond or insufficiently responds to initial therapy should be further assessed hemodynamically (5). Specifically, there is a large consensus to suggest measuring sequentially cardiac output and stroke volume in order to better evaluate the need for further fluid resuscitation or inotropes, and to track the hemodynamic response to the chosen therapeutic intervention (5).
How measure cardiac output?
The numerous techniques commercially available for measuring cardiac output have been reviewed recently (4,26). Historically, the intermittent thermodilution technique via a pulmonary artery catheter was the most frequently used technique in the 70’s to hemodynamically monitor patients with shock or acute respiratory failure (27,28). It is still widely considered the standard reference method for measuring cardiac output and has the key advantage over other “blind” systems to provide additional parameters of clinical value, including pulmonary artery pressures, right-sided and left-sided filling pressures, and mixed oxygen venous saturation (4,26). Nevertheless, its invasiveness and related complications, the need for dedicated training to obtain accurate data interpretation (29), and the absence of favorable impact on patient outcome (30), led to the restriction of its use on clinical grounds. Consequently, the pulmonary artery catheter has been supplanted by less invasive monitoring techniques over the last decade (31,32).
Methods using pulse contour analysis are most frequently used on clinical grounds to monitor continuously cardiac output. Among them, the transpulmonary thermodilution (TPT) devices are widely spread in the ICU settings. Although TPT is considered less invasive than the pulmonary artery catheter, it still requires the insertion of a central venous catheter and femoral arterial catheter. The system estimates stroke volume from the combination of arterial pressure pulse waveform analysis and TPT. It provides continuous, real-time calculation of cardiac output using proprietary algorithms based on the relationship between stroke volume and arterial pressure waveform (31). TPT provides reasonable agreement with intermittent pulmonary artery catheter measures of cardiac output in ICU patients (33). Accurate measurement of cardiac output requires the intravenous injection of three consecutive cold boluses (34). In hemodynamically unstable patients, externally calibrated systems are preferable to obtain reliable measurements of cardiac output, especially when vascular tone changes (35). Since there is a potential drift over time, recalibration is encouraged after a one-hour period (35). In patients with septic shock, hence with altered vasomotor tone, the ability to recalibrate the system on a regular basis allows to accurately and continuously track in real-time short-term variations of cardiac output during dynamic tests (e.g., passive leg raise) or therapeutic challenges (fluid loading, incremental doses of inotropes). Other monitoring systems are seldom used in the ICU settings since they are either not externally calibrated which make them unreliable in case of marked hemodynamic changes, or of limited clinical value since they fail providing other hemodynamic parameters than continuous cardiac output estimate (31).
CCE is performed and interpreted at the bedside of the ICU patients with cardiopulmonary compromise to make diagnoses and infer immediate therapeutic decisions (36). Transthoracic echocardiography is strictly noninvasive, easy to implement, highly portable in all environments and rapid to perform. Transesophageal echocardiography provides further acoustic windows to the heart and great vessels (37), with associated greater diagnostic capability (38), while being well tolerated in mechanically ventilated patients (39). This approach is widely used in the ICU to perform comprehensive hemodynamic assessment and to depict central abnormalities that are not accessible to surface ultrasound, while being less operator-dependent (37). Irrespective of the approach used, the measurement of stroke volume is based either on LV volume estimate or on Doppler method. LV end-diastolic and end-systolic volumes can be measured conventionally using two-dimensional imaging and biplane Simpson’s rule, or in real-time with three-dimensional echocardiography (40). The Doppler method combines two-dimensional imaging to measure the cross-sectional area of the cardiac orifice of interest and pulse-wave Doppler to record blood flow stroke distance at the very same location (41). For a constant flow, Doppler velocity is inversely proportional to cross-sectional area. Small variations of cross-sectional area produce large changes in Doppler velocity since it relates to the square of the vessel or orifice radius (41). Stroke volume can be virtually measured at the level of any valvular orifice or great vessel segment, providing that two-dimensional image quality and Doppler beam alignment with blood flow are adequate. The anatomical site which has been validated for stroke volume measurement with the lowest bias is the left ventricular outflow tract and the aortic valve because they are easy to clearly depict and exhibit low flow turbulence under normal physiological conditions (42). These anatomical sites of measurement are those yielding the closest agreement when compared to intermittent pulmonary artery catheter thermodilution used as reference (43). In ICU patients with sinus rhythm, transthoracic echocardiography provides a precision of 6% when performing a single measurement of left ventricular outflow tract velocity-time integral (44), and of 9% when compared to pulmonary artery catheter (6). Nevertheless, assumption of laminar flow, constant cross-sectional area, angle dependency of the Doppler beam, timing of measurement within the respiratory cycle, and operator factors may contribute to the fairly large percentage error between techniques (Table 1). Although echocardiography and thermodilution are not interchangeable for the absolute measurement of cardiac output, the Doppler approach accurately tracks directional changes in cardiac output when compared to intermittent pulmonary artery catheter thermodilution (43).

Full table
Overall, echocardiography should be considered to measure cardiac output in patients with persistent shock despite adequate fluid resuscitation (25). Although it does not provide continuous monitoring, CCE can be used for the sequential evaluation of cardiac function in patients with shock (5). Routine use of the pulmonary artery catheter is not recommended for patients with shock (5,25). It remains suggested in patients with refractory shock and right ventricular dysfunction, especially in the presence of associated acute respiratory distress syndrome (5).
How interpret cardiac output values?
Irrespective of its origin, a markedly decreased cardiac output is consistently detrimental (15). Septic shock patients with low oxygen delivery despite aggressive therapy have a markedly high mortality rate (45). Accordingly, low cardiac output value must trigger additional diagnostic work-up to promptly identify the underlying leading mechanism of cardiovascular failure and guide therapeutic intervention. The key value of TPT and CCE is that they both provide additional hemodynamic parameters which have been previously validated to distinguish a persistent fluid responsiveness from an overt cardiac dysfunction at the origin the low flow state.
There is currently no consensus to define a clinically relevant change in cardiac output (15), whether it is observed during the course of the septic shock or in response to a specific therapeutic intervention (e.g., fluid challenge, inotrope). To take into account the reproducibility of measurements, their potential technical limitations and the physiological relevance of observed variations, most studies assessing the clinical value of hemodynamic parameters to predict fluid responsiveness use a 15% threshold of increase in cardiac index (46). Such assumption is not necessarily correct (15). For example, a 10% increase of cardiac output after a 250-mL fluid challenge may significantly improve the cardiovascular failure of a patient with severe septic shock.
It is challenging to determine what would be the optimal value of cardiac output for a given patient at a given point in time (15), and multiple clinical and biological factors should be considered (26). Randomized controlled trials assessing a goal-directed strategy to supranormal oxygen delivery compared to standard of care provided negative results (47). Moreover, aggressive efforts to increase oxygen delivery were found to be deleterious with higher associated hospital mortality in the interventional than in the control arm (48). Detrimental side effects of high doses of catecholamines required to reach supranormal oxygen delivery presumably played a determinant role in the observed negative results on outcome. Accordingly, it is currently recommended not to target absolute values (especially supranormal values) of oxygen delivery in patients with shock (5,25).
Which technique to use?
The cardiovascular failure associated with septic shock frequently results from intricate mechanisms, all of them potentially leading to a decreased cardiac output: hypovolemia (decreased venous return, hence preload), cardiac dysfunction (septic cardiomyopathy), vasoplegia (decreased venous return and afterload) with potential dynamic left ventricular obstruction (49) or ventriculo-arterial decoupling (9). Other causes of inadequate or low cardiac output such as purulent tamponade, severe left-sided valvular regurgitation or central anatomical shunts related to acute infective endocarditis are rare. Overall, the technique used for the hemodynamic assessment of septic shock patients—irrespective of its continuous or discontinuous nature—should provide the leading mechanism of low or inadequate cardiac output in addition to its absolute value, to best guide therapy. Accordingly, monitoring systems without external calibration or providing little hemodynamic information other than cardiac output determination should be discouraged (31).
TPT and CCE have their respective advantages and limitations (Table 1). In 137 ventilated patients with septic shock who were successively assessed using both the TPT and transesophageal echocardiography in random order, the independent interpretation of hemodynamic profile at the bedside was discordant in 66% of the cases (50). When reviewed by experts, hemodynamic data provided by both approaches were concordant in 100/129 patients (77.5%). Potential sources of discrepancy between TPT and CCE were identified in 16 of 37 patients (43%), one-half being related to the echocardiographic identification of acute cor pulmonale (50). Indeed, TPT fails to accurately identify right ventricular failure (51), acute cor pulmonale constituting its most severe presentation (52). Pneumonia is the most frequent source of infection responsible for septic shock (20), and is a risk factor for the development of acute cor pulmonale when responsible for the acute respiratory distress syndrome (53). In a recent multicenter cohort of ventilated patients who were hemodynamically assessed using transesophageal echocardiography for shock, 8% of the study population exhibited acute cor pulmonale (54). In ventilated patients with right ventricular failure, increased pulse pressure variation or stroke volume variation measured by TPT may erroneously trigger fluid loading (55). A significant pulse pressure variation (or stroke volume variation) is less likely to predict fluid responsiveness in ventilated patients with right ventricular dilatation, and this is even more pronounced when the dilatation increased. In this case, respiratory variation in pulse pressure or left ventricular stroke volume is mostly due to a cyclic afterloading of the failing (hence dilated) right ventricle at each mechanical insufflation rather than secondary to the reduction of right ventricular preload—hence stroke volume of a preload-dependent heart—by the tidal ventilation (56). As such, it is not associated with fluid responsiveness and should be considered as a false-positive result of these widely used indices to guide fluid resuscitation (36). Overall, although its prognostic impact in sepsis remains debated (57,58), early identification of right ventricular failure in patients with septic shock is clinically relevant for guiding therapy.
CCE is ideally suited to depict other sources of inaccuracy of the TPT (50). CCE allows expedite assessment of left-sided valvulopathies (59). Severe aortic or mitral regurgitation may adversely interfer with the internal algorithm of TPT for the measurement of cardiac output and associated hemodynamic parameters (60). In patients with markedly low flow state, the cardiac output may be overestimated by the thermodilution technique because of the loss of thermal indicator (61). Similarly, a high degree of tricuspid regurgitation (or intracardiac shunts) may result in underestimated cardiac output in ventilated patients because of the recycling of the indicator fluid across the triscupid valve (62). The prevalence of tricuspid regurgitation is high in patients under positive-pressure ventilation (63). Moderate-to-severe tricuspid regurgitation which could potentially alters the accuracy of thermodilution involves a large proportion of patients (63), and the regurgitant volume increases with the level of positive end-expiratory pressure (64). Finally, dynamic left ventricular outflow tract obstruction is commonly depicted by CCE in the early course of septic shock (49), whereas it remains outside the diagnostic field of TPT (50). In these patients, pulmonary artery catheter discloses a reduced cardiac output with elevated left filling pressures which may be erroneously interpreted as cardiac failure (65). In contrast, CCE easily identifies and quantifies both the pressure gradient induced by the dynamic obstruction and frequently associated eccentric mitral regurgitation in the presence of an underlying systolic anterior mitral motion (66).
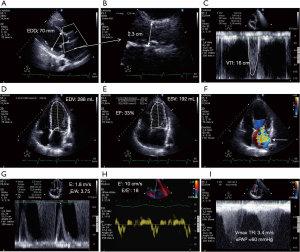
CCE is an unparalleled technique which provides a comprehensive hemodynamic assessment for expedite diagnostic work-up and prompt direct therapeutic impact (32,36,59). In addition to clear depiction of distinct cardiovascular phenotypes in septic shock patients (67), CCE has the unique advantage of depicting the distinct components generating left ventricular stroke volume (Figure 1). In visualizing cardiac structures in real time, one can readily assess if the stroke volume is mostly generated by a preserved myocardial contractility or by an enlarged, yet severely depressed ventricle (68) (Figure 2). Left cardiac cavities cannot dilate acutely, including in septic patients (69). Accordingly, the size of both the left atrium and ventricle provide information on the duration of contractile impairment, with actual dilatation suggesting an underlying chronic cardiac disease (59). Finally, CCE provides valuable information on both left ventricular diastolic properties and filling pressures (70,71). This allows assessing the tolerance of fluid resuscitation, especially in patients presenting with septic shock and acute respiratory failure (72). This information is clinically relevant since sepsis-induced left ventricular diastolic dysfunction appears prognostic (73), and positive fluid balance is associated with poor outcome in patients with septic shock (74). Finally, CCE provides a reliable estimate of systolic pulmonary artery pressure based on the measurement of the systolic gradient between the right atrium and ventricle (75). For this assessment to be accurate, the value of central venous pressure which is added to this gradient to obtain the corresponding systolic pulmonary artery pressure must be measured invasively through the central venous catheter (76), rather than estimated on both inferior vena cava diameter and inspiratory collapse in spontaneously breathing patients (77).
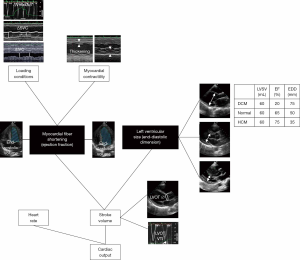
Overall, experts widely advocate using echocardiography as a first-line modality to initially evaluate the type of shock, as opposed to more invasive techniques (5,26). This suggestion relies on the unique information provided by CCE on: (I) the hemodynamic profile of septic shock at a precise time point; (II) the resulting selection of most appropriate therapeutic options (e.g., volume loading, inotropes, ultrafiltration); and (III) the (repeated) assessment of both the efficacy (amplitude of the positive response) and tolerance (absence of side-effect) of therapy (5).
Which timing of hemodynamic assessment: continuous vs. serial?
Although CCE cannot provide continuous hemodynamic monitoring, it is the best bedside method to assess cardiac function repeatedly (5) and it provides invaluable information on the causative mechanisms of cardiovascular failure associated with shock (32). Serial CCE assessments of patients hospitalized in the ICU have shown that the hemodynamic profile of septic shock may change over time, according to the evolution of the disease and response to therapy. For example, sepsis-induced left ventricular systolic dysfunction is predominantly diagnosed when CCE is performed within the first 12 hours following ICU admission (21,78-80). Nevertheless, a left or right ventricular dysfunction has recently been identified in one-third of septic patients in the Emergency Department who underwent early echocardiographic assessment after a median fluid resuscitation of only 500 mL (IQR: 187–1,500 mL) (81). In contrast, left ventricular systolic function may only be impaired on day 2 or even day 3 of ICU stay, while being considered normal on initial CCE assessment (79,80). The development of delayed cardiac dysfunction has been ascribed to the restoration of vasomotor tone (control of sepsis, vasopressor support) which unmasks sepsis-induced intrinsic contractile dysfunction (12). Similarly, right ventricular failure or acute cor pulmonale may develop during the ICU stay in patients under protective ventilation for moderate-to-severe acute respiratory distress syndrome which is of infectious origin in up to 60% of the cases (82,83). Finally, initial fluid resuscitation is best driven when sequentially assessed, even in the early phase of septic shock (81). For all these reasons, CCE should be repeated during the initial course of septic shock, with at least a daily hemodynamic assessment (84), since it is ideally suited for sequential evaluations (85).
Then, what would be the superiority of a continuous hemodynamic monitoring using alternative “blind” (i.e., without direct visualization of heart and great vessels) techniques (e.g., TPT)? There is a paucity of evidence demonstrating the superiority of continuous monitoring over intermittent cardiac output measurement (26). Continuous measurement of cardiac output and stroke volume is undoubtedly of major clinical value when predicting fluid responsiveness using either a so-called “dynamic parameter” (e.g., stroke volume variations, end-expiratory occlusion test) or a passive leg raise (86). Continuous monitoring of cardiac output may also be used for early detection of any cardiovascular event before hypotension occurs. Using this approach, any unexpected drop in cardiac output triggers further diagnostic work-up using the full set of hemodynamic parameters provided by the TPT after the same injection of cold boluses (87). Alternatively, the occurrence of hypotension in the absence of associated relevant decrease in cardiac output can be ascribed to a decrease of systemic vascular resistance. Nevertheless, continuous cardiac output monitoring using TPT is cumbersome since it requires regular (hourly) external recalibration for accurate tracking of cardiac output variations (35). In addition, it may lead to overtreatment if variations of cardiac output are not associated with worsening of tissue perfusion but still result in undue therapeutic interventions. In this case, unnecessary therapy (e.g., excessive fluid loading or inotrope administration) may result in deleterious side effects (48). Indeed, fluid resuscitation should only be performed in a fluid-responder patient when his cardiac output remains inadequate as reflected by persisting tissue hypoperfusion (e.g., high lactate, low central venous oxygen saturation) (88). Similarly, inotropic agents should be given only in patients with cardiac dysfunction when the low or inadequate cardiac output is associated with signs of tissue hypoperfusion (5). Indeed, the use of Dobutamine is suggested in patients with evidence of persistent hypoperfusion despite adequate fluid loading and the use of vasopressor agents (18). In contrast, inotropes should not be administered for isolated impaired cardiac function (5). No inotropic treatment has shown a positive impact on patient-centered outcomes in septic shock (18). Mortality in patients randomized to Dobutamine added to norepinephrine was no different compared to epinephrine alone (89). No randomized controlled trials have yet compared the effects of Dobutamine versus placebo on clinical outcomes. The ADAPT randomized controlled trial will compare the effects of Dobutamine versus placebo in adjunct to standard of care in septic shock patients with CCE documented septic cardiomyopathy (NCT04166331). In the LeoPARDS study, adult patients who presented with septic shock and had received vasopressors for at least 4 hours were eligible for being allocated to receive either a blinded 24-h infusion of Levosimendan or placebo in addition to standard of care (90). The addition of Levosimendan to standard treatment was not associated with less severe organ dysfunction or lower mortality. Furthermore, the administration of Levosimendan was associated with a lower likelihood of successful weaning from mechanical ventilation and a higher risk of supraventricular tachyarrhythmia (90). Interestingly, no hemodynamic assessment or monitoring was required prior enrollment into this trial. Since the initiation of inotropes may be deleterious in the presence of persisting hypovolemia or left ventricular dynamic obstruction, fluid responsiveness must be confidently excluded beforehand and CCE should ideally rule out a turbulent ejection pattern through left ventricular outflow tract which may be worsened by inadvertent inotropic stimulation (49,65). In the Hemosepsis study, adverse effects potentially related to changes in acute therapy were scarcely observed and with a similar frequency, whether hemodynamic assessments obtained by TPT and CCE yielded concordant results or not (50). Finally, the invasiveness of routinely used technique of monitoring is relevant to consider in order to limit care-related complications (30). When respecting respective contraindications, both TPT and transesophageal echocardiography can be safely used on clinical grounds with a low complication rate (91,92). In our experience, less than 10% of ICU patients eligible for hemodynamic monitoring present with a contra-indication for a given technique (50).
Another relevant reason for choosing a continuous versus intermittent measurement of cardiac output would be a potential impact of hemodynamic monitoring on the prognosis of septic shock patients. When compared with the sole clinical assessment, hemodynamic monitoring provides relevant additional information with a potential impact on therapeutic decisions (93). Unfortunately, the use of advanced hemodynamic monitors to guide resuscitation has failed improved patient-centered outcomes (20). Indeed, if obtained data are interpreted or applied inappropriately, resulting therapeutic interventions are ineffective or harmful, and the continuous monitoring will not improve outcome or even may be deleterious (26). A randomized controlled trial comparing the systematic monitoring of patients with septic shock and/or acute respiratory distress syndrome using TPT versus standard of care was interrupted prematurely for futility (94). In the Hemosepsis study, therapeutic guidance based on hemodynamic monitoring had no significant impact on lactate clearance and prognosis, whether TPT and CCE provided concordant data and therapeutic suggestions or not (50). As previously mentioned, late goal-directed strategy based on pulmonary artery catheter monitoring which aimed at systematically increasing oxygen delivery in ICU patients were either neutral (95) or detrimental on mortality (48). Even in the absence of goal-oriented strategy, hemodynamic monitoring with pulmonary artery catheter fails to increase or confer benefit on overall mortality (96).
Future perspectives
Miniaturized transesophageal echocardiography probes have emerged that facilitate prolonged esophageal insertion for serial hemodynamic assessment of unstable ICU patients (97,98). Future monitoring systems will presumably become less invasive and incorporate latest technological and electronic innovations, including artificial intelligence and telemetry (31). Because of frequent dissociation between the macro- and microcirculation, continuous monitoring of the microcirculation would be promising (99). Beside the improvement of monitoring devices, a better characterization of the cardiovascular phenotypes of septic shock patients will undoubtedly allow selecting more adequately those who could benefit from a specific therapeutic intervention (68). In addition, the paradigm shifts currently operating on the choice of primary outcome in most randomized controlled trials evaluating new therapeutic strategies in septic shock promises to best identify positive effects of monitor-guided interventions on patient-centered outcomes, such as organ failure rather than mortality (100). CCE will undoubtedly continue to play a pivotal role in the hemodynamic assessment of patients with septic shock, since it provides unique insights into the evolving mechanisms of cardiovascular failure during resuscitation and patient course, allowing a tailoring approach over time (36). In addition, the use of CCE will progressively shift from punctual diagnostic evaluations towards serial assessments with time windows width adapted to both the clinical presentation and therapeutic interventions, not only for the management of cardiovascular failure, but also for that of acute respiratory failure (72,101).
Conclusions
The measurement and monitoring of cardiac output remains key for diagnostic and therapeutic purposes. Since continuous monitoring of cardiac output is only provided by “blind” techniques, the system used should have external calibration for data accuracy and offer additional hemodynamic indices of preload-responsiveness. Significant variation of cardiac output should be interpreted in light of both its direction and magnitude, when taking into account the clinical presentation. Since inotrope may have deleterious effects if misused, their initiation should be based on the documentation of a cardiac dysfunction at the origin of the low flow state by CCE. Among the numerous advantages of CCE over continuous monitors, the early identification of potential source of imprecision of thermodilution accounts for its clinical value in initial hemodynamic assessment of shocked patients. Overall, TPT allowing continuous measurement of cardiac output and CCE appear complementary rather than mutually exclusive in patients with septic shock who require advanced hemodynamic monitoring.
Acknowledgments
The author gratefully thanks Dr. Marine Goudelin and Dr. Bruno Evrard for their help in data collection and bibliographic research.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Glenn Hernández and Guo-wei Tu) for the series “Hemodynamic Monitoring in Critically Ill Patients” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.04.11). The series “Hemodynamic Monitoring in Critically Ill Patients” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362:779-89. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med 2007;35:2408-16. [Crossref] [PubMed]
- Thiele RH, Bartels K, Gan TJ. Cardiac output monitoring: a contemporary assessment and review. Crit Care Med 2015;43:177-85. [Crossref] [PubMed]
- Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1795-815. [Crossref] [PubMed]
- Mercado P, Maizel J, Beyls C, et al. Transthoracic echocardiography: an accurate and precise method for estimating cardiac output in the critically ill patient. Critical Care 2017;21:136. [Crossref] [PubMed]
- Hochstadt A, Meroz Y, Landesberg G. Myocardial dysfunction in severe sepsis and septic shock: more questions than answers? J Cardiothorac Vasc Anesth 2011;25:526-35. [Crossref] [PubMed]
- Joulin O, Marechaux S, Hassoun S, et al. Cardiac force-frequency relationship and frequency-dependent acceleration of relaxation are impaired in LPS-treated rats. Crit Care 2009;13:R14. [Crossref] [PubMed]
- Guarracino F, Ferro B, Morelli A, et al. Ventriculoarterial decoupling in human septic shock. Crit Care 2014;18:R80. [Crossref] [PubMed]
- Beesley SJ, Weber G, Sarge T, et al. Septic Cardiomyopathy. Crit Care Med 2018;46:625-34. [Crossref] [PubMed]
- Martin L, Derwall M, Al Zoubi S, et al. The Septic Heart: Current Understanding of Molecular Mechanisms and Clinical Implications. Chest 2019;155:427-37. [Crossref] [PubMed]
- Vieillard-Baron A, Cecconi M. Understanding cardiac failure in sepsis. Intensive Care Med 2014;40:1560-3. [Crossref] [PubMed]
- Seymour CW, Rosengart MR. Septic Shock: Advances in Diagnosis and Treatment. JAMA 2015;314:708-17. [Crossref] [PubMed]
- Cholley B. Echocardiography in the intensive care unit: beyond “eyeballing”. A plea for the broader use of the aortic velocity-time integral measurement. Intensive Care Med 2019;45:898-901. [Crossref] [PubMed]
- Pinsky MR. Why measure cardiac output? Crit Care 2003;7:114-6. [Crossref] [PubMed]
- Vincent JL, De Backer D. Circulatory shock. New Engl J Med 2013;369:1726-34. [Crossref] [PubMed]
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Hiemstra B, Eck RJ, Keus F, et al. Clinical examination for diagnosing circulatory shock. Curr Opin Crit Care 2017;23:293-301. [Crossref] [PubMed]
- Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet 2018;392:75-87. [Crossref] [PubMed]
- Bouferrache K, Amiel JB, Chimot L, et al. Initial resuscitation guided by the Surviving Sepsis Campaign recommendations and early echocardiographic assessment of hemodynamics in intensive care unit septic patients: a pilot study. Crit Care Med 2012;40:2821-7. [Crossref] [PubMed]
- Pierrakos C, Velissaris D, Scolletta S, et al. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med 2012;38:422-8. [Crossref] [PubMed]
- Jain A, Shroff S, Janicki JS, Reddy HK, Weber KT. Relation vetween mixed venous oxygen saturation and cardiac index. Nonlinearity and normalization for oxygen uptake and hemoglobin. Chest 1991;99:1403-9. [Crossref] [PubMed]
- Teboul JL, Hamzaoui O, Monnet X. SvO2 to monitor resuscitation of septic patients: let's just understand the basic physiology. Crit Care 2011;15:1005. [Crossref] [PubMed]
- Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, 27-28 April 2006. Intensive Care Med 2007;33:575-90. [Crossref] [PubMed]
- Vincent JL, Rhodes A, Perel A, et al. Clinical review: Update on hemodynamic monitoring--a consensus of 16. Crit Care 2011;15:229. [Crossref] [PubMed]
- Swan HJ, Ganz W, Forrester J, et al. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. New Engl J Med 1970;283:447-51. [Crossref] [PubMed]
- Ganz W, Donoso R, Marcus HS, et al. A new technique for measurement of cardiac output by thermodilution in man. Am J Cardiol 1971;27:392-6. [Crossref] [PubMed]
- Gnaegi A, Feihl F, Perret C. Intensive care physicians' insufficient knowledge of right-heart catheterization at the bedside: time to act? Crit Care Med 1997;25:213-20. [Crossref] [PubMed]
- Connors AF Jr, Speroff T, Dawson NV, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA 1996;276:889-97. [Crossref] [PubMed]
- Teboul JL, Saugel B, Cecconi M, et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med 2016;42:1350-9. [Crossref] [PubMed]
- De Backer D, Bakker J, Cecconi M, et al. Alternatives to the Swan-Ganz catheter. Intensive Care Med 2018;44:730-41. [Crossref] [PubMed]
- Sakka SG, Reinhart K, Meier-Hellmann A. Comparison of pulmonary artery and arterial thermodilution cardiac output in critically ill patients. Intensive Care Med 1999;25:843-6. [Crossref] [PubMed]
- Monnet X, Persichini R, Ktari M, et al. Precision of the transpulmonary thermodilution measurements. Crit Care 2011;15:R204. [Crossref] [PubMed]
- Hamzaoui O, Monnet X, Richard C, et al. Effects of changes in vascular tone on the agreement between pulse contour and transpulmonary thermodilution cardiac output measurements within an up to 6-hour calibration-free period. Crit Care Med 2008;36:434-40. [Crossref] [PubMed]
- Vignon P. What is new in critical care echocardiography? Crit Care 2018;22:40. [Crossref] [PubMed]
- Vignon P, Merz TM, Vieillard-Baron A. Ten reasons for performing hemodynamic monitoring using transesophageal echocardiography. Intensive Care Med 2017;43:1048-51. [Crossref] [PubMed]
- Vignon P, Mentec H, Terré S, et al. Diagnostic accuracy and therapeutic impact of transthoracic and transesophageal echocardiography in mechanically ventilated patients in the ICU. Chest 1994;106:1829-34. [Crossref] [PubMed]
- Vignon P, Mayo P. Echocardiography in the critically ill: an overview. In: de Backer D, Cholley BP, Slama M, et al. editors. Hemodynamic monitoring using echocardiography in the critically ill. Berlin: Springer, 2011:1-9.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Zoghbi WA, Quinones MA. Determination of cardiac output by Doppler echocardiography: a critical appraisal. Herz 1986;11:258-68. [PubMed]
- Quiñones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 2002;15:167-84. [Crossref] [PubMed]
- Wetterslev M, Møller-Sørensen H, Johansen RR, et al. Systematic review of cardiac output measurements by echocardiography vs. thermodilution: the techniques are not interchangeable. Intensive Care Med 2016;42:1223-33. [Crossref] [PubMed]
- Jozwiak M, Mercado P, Teboul JL, et al. What is the lowest change in cardiac output that transthoracic echocardiography can detect? Crit Care 2019;23:116. [Crossref] [PubMed]
- Tuchschmidt J, Fried J, Astiz M, et al. Elevation of cardiac output and oxygen delivery improves outcome in septic shock. Chest 1992;102:216-20. [Crossref] [PubMed]
- Ansari BM, Zochios V, Falter F, et al. Physiological controversies and methods used to determine fluid responsiveness: a qualitative systematic review. Anaesthesia 2016;71:94-105. [Crossref] [PubMed]
- Poeze M, Greve JW, Ramsay G. Meta-analysis of hemodynamic optimization: relationship to methodological quality. Crit Care 2005;9:R771-9. [Crossref] [PubMed]
- Hayes MA, Timmins AC, Yau EH, et al. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 1994;330:1717-22. [Crossref] [PubMed]
- Chauvet JL, El-Dash S, Delastre O, et al. Early dynamic left intraventricular obstruction is associated with hypovolemia and high mortality in septic shock patients. Crit Care 2015;19:262. [Crossref] [PubMed]
- Vignon P, Begot E, Mari A, et al. Hemodynamic Assessment of Patients With Septic Shock Using Transpulmonary Thermodilution and Critical Care Echocardiography: A Comparative Study. Chest 2018;153:55-64. [Crossref] [PubMed]
- Combes A, Berneau JB, Luyt CE, et al. Estimation of left ventricular systolic function by single transpulmonary thermodilution. Intensive Care Med 2004;30:1377-83. [Crossref] [PubMed]
- Jardin F, Dubourg O, Bourdarias JP. Echocardiographic pattern of acute cor pulmonale. Chest 1997;111:209-17. [Crossref] [PubMed]
- Mekontso Dessap A, Boissier F, Charron C, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 2016;42:862-70. [Crossref] [PubMed]
- Vignon P, Repessé X, Bégot E, et al. Comparison of Echocardiographic Indices Used to Predict Fluid Responsiveness in Ventilated Patients. Am J Respir Crit Care Med 2017;195:1022-32. [Crossref] [PubMed]
- Mahjoub Y, Pila C, Friggeri A, et al. Assessing fluid responsiveness in critically ill patients: False-positive pulse pressure variation is detected by Doppler echocardiographic evaluation of the right ventricle. Crit Care Med 2009;37:2570-5. [Crossref] [PubMed]
- Vieillard-Baron A, Matthay M, Teboul JL, et al. Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med 2016;42:739-49. [Crossref] [PubMed]
- Vallabhajosyula S, Kuma M, Pandompatam G, et al. Prognostic impact of right ventricular dysfunction in sepsis ans septic shock: an 8-year historical cohort study. Ann Intensive Care 2017;7:94. [Crossref] [PubMed]
- Huang SJ, Nalos M, McLean A. Is early ventricular dysfunction or dilatation associated with lower mortality rate in adult severe sepsis and septic shock? A meta-analysis. Crit Care 2013;17:R96. [Crossref] [PubMed]
- McLean AS. Echocardiography in shock management. Crit Care 2016;20:275. [Crossref] [PubMed]
- Nishikawa T, Dohi S. Errors in measurement of cardiac output by thermodilution. Can J Anaesth 1993;40:142-53. [Crossref] [PubMed]
- van Grondelle A, Ditchey RV, Groves BM, et al. Thermodilution method overestimates low cardiac output in humans. Am J Physiol 1983;245:H690-2. [PubMed]
- Balik M, Pachl J, Hendl J. Effect of the degree of tricuspid regurgitation on cardiac output measurements by thermodilution. Intensive Care Med 2002;28:1117-21. [Crossref] [PubMed]
- Jullien T, Valtier B, Hongnat JM, et al. Incidence of tricuspid regurgitation and vena caval backward flow in mechanically ventilated patients. A color Doppler and contrast echocardiographic study. Chest 1995;107:488-93. [Crossref] [PubMed]
- Artucio H, Hurtado J, Zimet L, et al. PEEP-induced tricuspid regurgitation. Intensive Care Med 1997;23:836-40. [Crossref] [PubMed]
- Adamopoulos C, Tsagourias M, Arvaniti K, et al. Weaning failure from mechanical ventilation due to hypertrophic obstructive cardiomyopathy. Intensive Care Med 2005;31:734-7. [Crossref] [PubMed]
- Vignon P. Cardiovascular failure and weaning. Ann Transl Med 2018;6:354. [Crossref] [PubMed]
- Géri G, Vignon P, Aubry A, et al. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: a post hoc analysis. Intensive Care Med 2019;45:657-67. [Crossref] [PubMed]
- Vignon P. Hemodynamic assessment of critically ill patients using echocardiography Doppler. Curr Opin Crit Care 2005;11:227-34. [Crossref] [PubMed]
- Vieillard Baron A, Schmitt JM, Beauchet A, et al. Early preload adaptation in septic shock? A transesophageal echocardiographic study. Anesthesiology 2001;94:400-6. [Crossref] [PubMed]
- Vignon P. Ventricular diastolic abnormalities in the critically ill. Curr Opin Crit Care 2013;19:242-9. [Crossref] [PubMed]
- Vignon P. Echocardiographic assessment of pulmonary artery occlusion pressure in ventilated patients: a transoesophageal study. Crit Care 2008;12:R18. [Crossref] [PubMed]
- Vignon P, Repessé X, Vieillard-Baron A, et al. Critical care ultrasonography in acute respiratory failure. Crit Care 2016;20:228. [Crossref] [PubMed]
- Sanfilippo F, Corredor C, Fletcher N, et al. Diastolic dysfunction and mortality in septic patients: a systematic review and meta-analysis. Intensive Care Med 2015;41:1004-13. [Crossref] [PubMed]
- Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011;39:259-65. [Crossref] [PubMed]
- Vignon P. Assessment of pulmonary arterial pressure using critical care echocardiography: dealing with the Yin and the Yang? Crit Care Med 2019;47:126-8. [Crossref] [PubMed]
- Mercado P, Maizel J, Beyls C, et al. Reassessment of the accuracy of cardiac Doppler pulmonary artery pressure measurements in ventilated ICU patients: a simultaneous Doppler-catheterization study. Crit Care Med 2019;47:41-8. [Crossref] [PubMed]
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009;179:615-21. [Crossref] [PubMed]
- Etchecopar-Chevreuil C, François B, Clavel M, et al. Cardiac morphological and functional changes during early septic shock: a transesophageal echocardiographic study. Intensive Care Med 2008;34:250-6. [Crossref] [PubMed]
- Vieillard-Baron A, Caille V, Charron C, et al. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 2008;36:1701-6. [Crossref] [PubMed]
- Boissier F, Razazi K, Seemann A, et al. Left ventricular systolic dysfunction during septic shock: the role of loading conditions. Intensive Care Med 2017;43:633-42. [Crossref] [PubMed]
- Lafon T, Appert A, Hadj M, et al. Comparative Early Haemodynamic Profiles in Patients Presenting to the Emergency Department With Septic and Non-Septic Acute Circulatory Failure Using Focused Echocardiography. Shock 2020;53:695-700. [Crossref] [PubMed]
- Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med 2013;39:1725-33. [Crossref] [PubMed]
- Lhéritier G, Legras A, Caille A, et al. Prevalence and prognostic value of acute cor pulmonale and patent foramen ovale in ventilated patients with early acute respiratory distress syndrome: a multicenter study. Intensive Care Med 2013;39:1734-42. [Crossref] [PubMed]
- Jardin F, Fourme T, Page B, et al. Persistent preload defect in severe sepsis despite fluid loading: A longitudinal echocardiographic study in patients with septic shock. Chest 1999;116:1354-9. [Crossref] [PubMed]
- Belcour D, Jabot J, Grard B, et al. Prevalence and risk factors of stress cardiomyopathy after convulsive status epilepticus in ICU Patients. Crit Care Med 2015;43:2164-70. [Crossref] [PubMed]
- Monnet X, Marik PE, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care 2016;6:111. [Crossref] [PubMed]
- Sakka SG, Reuter DA, Perel A. The transpulmonary thermodilution technique. J Clin Monit Comput 2012;26:347-53. [Crossref] [PubMed]
- Vignon P. Evaluation of fluid-responsiveness: back to venous return. Intensive Care Med 2004;30:1699-701. [Crossref] [PubMed]
- Annane D, Vignon P, Renault A, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet 2007;370:676-84. [Crossref] [PubMed]
- Gordon AC, Perkins GD, Singer M, et al. Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis. N Engl J Med 2016;375:1638-48. [Crossref] [PubMed]
- Belda FJ, Aguilar G, Teboul JL, et al. Complications related to less-invasive haemodynamic monitoring. Br J Anaesth 2011;106:482-6. [Crossref] [PubMed]
- Hüttemann E, Schelenz C, Kara F, et al. The use and safety of transesophageal echocardiography in the general ICU. A minireview. Acta Anaesthesiol Scand 2004;48:827-36. [Crossref] [PubMed]
- Perel A, Saugel B, Teboul JL, et al. The effects of advanced monitoring on hemodynamic management in critically ill patients: a pre and post questionnaire study. J Clin Monit Comput 2016;30:511-8. [Crossref] [PubMed]
- Zhang Z, Ni H, Qian Z. Effectiveness of treatment based on PiCCO parameters in critically ill patients with septic shock and/or acute respiratory distress syndrome: a randomized controlled trial. Intensive Care Med 2015;41:444-51. [Crossref] [PubMed]
- Gattinoni L, Brazzi L, Pelosi P, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med 1995;333:1025-32. [Crossref] [PubMed]
- Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA 2005;294:1664-70. [Crossref] [PubMed]
- Vieillard-Baron A, Slama M, Mayo P, et al. A pilot study on safety and clinical utility of a single-use 72-hour indwelling transesophageal echocardiography probe. Intensive Care Med 2013;39:629-35. [Crossref] [PubMed]
- Begot E, Dalmay F, Etchecopar C, et al. Hemodynamic assessment of ventilated ICU patients with cardiorespiratory failure using a miniaturized multiplane transesophageal echocardiography probe. Intensive Care Med 2015;41:1886-94. [Crossref] [PubMed]
- De Backer D, Donadello K, Sakr Y, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med 2013;41:791-9. [Crossref] [PubMed]
- Lambden S, Laterre PF, Levy MM, et al. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care 2019;23:374. [Crossref] [PubMed]
- Goudelin M, Champy P, Amiel JB, et al. Left ventricular overload identified by critical care echocardiography is key in weaning-induced pulmonary edema. Intensive Care Med 2020. [Crossref] [PubMed]


