
Fluid administration for acute circulatory dysfunction using basic monitoring
Introduction
Fluids administration and acute circulatory dysfunction: an old and steady marriage
The decision to infuse fluid to revert acute circulatory dysfunction derives from the basic physiological concept that a fluid loss (absolute or relative) should be treated with fluid replacement. This self-evident assumption has been firstly tested by Dr. Thomas Latta about 200 years ago to treat a cholera-related hypovolemic shock in an elderly woman (1). He decided to inject small and repeated fluid boluses of a crystalloid solution, probably anticipating the definition of a fluid challenge (FC). Interestingly, the first bolus did not have any visible effect, but after multiple FCs (overall 2.8 litres) “soon the sharpened features, and sunken eye, and fallen jaw, pale and cold, bearing the manifest imprint of death’s signet, began to glow with returning animation; the pulse returned to the wrist”. Dr. Latta showed that giving fluids during acute circulatory dysfunction and titrating fluid administration on the clinical response of the patient are quite reasonable ideas, since 1831.
Usually an episode of acute circulatory failure leads to a complex clinical scenario called shock, which is characterized by the unbalanced relationship between the oxygen delivery (DO2), provided by the cardiac function and associated to the oxygen blood content, and the systemic oxygen request. In this scenario, the inadequate cellular oxygen utilization may be due to either oxygen request exceeding DO2 supply, or to the cellular inability of using O2 because of mitochondrial dysfunction (2).
On the one hand, an aggressive and prompt fluid resuscitation in the early phase of acute circulatory failure is a key and recommended intervention (3,4), on the other hand the hemodynamic targets and the safety limits indicating whether or not stopping this treatment in already resuscitated patients are still undefined (3,5). However, a targeted fluid management is of pivotal importance to improve the outcome of hemodynamically unstable intensive care unit (ICU) patients, since both hypovolemia and hypervolemia are harmful (6).
Basically, the only physiological reason to administer fluids during an episode of acute circulatory shock is to increase the stroke volume (SV) by increasing the heart preload, a physiological response called fluid responsiveness. The ability of the heart function to provide a constant systemic blood flow is the final effect of the interactions between cardiac function, venous return and systemic vascular impedance. The first variable has been originally described by Otto Frank and Ernest Starling more than 100 years ago, whereas and our knowledge regarding venous return is primarily based on Arthur Guyton’s studies on the relationship between the elastic recoil of venous capacitance vessels, the volume stretching the veins and the compliance of the veins and the resistance of venous system. Accordingly, an acute circulatory dysfunction may be due to a decreased cardiac performance or to an inadequate preload.
In this context, the decision to administer fluids presumes that the plateau of cardiac function is not reached and that, accordingly, an increase in preload would be associated to a concomitant increase in cardiac output (CO). However, clinical assessment of Frank-Starling curve’s ventricle position is complex and the bedside and the prediction of fluid responsiveness in ICU patients is still challenging and not routinely used (7,8).
In fact, systemic pressure is usually the first trigger to give a FC and, moreover, often the clinical goal at the bedside is to increase blood pressure by infusing fluids (8). However, the use of blood pressure as surrogate of CO can be misleading, since the increase in arterial pressure during an FC is quite unpredictable and is closely related to the systemic vascular tone and arterial elastance (8). In fact, the assumption that hypotension and shock are synonymous could be misleading, since restoring mean arterial pressure (MAP) above predetermined values is not necessarily associated to shock reversal whereas MAP values below those indicated by international guidelines does not always indicate a shock, needing a titration on the single patient (6). Moreover, the physiological relationship between changes in systemic pressures and SV becomes weak in previously resuscitated ICU patients, especially during an episode of septic shock (9-11). Since, in the context of an acute circulatory dysfunction, nor the systemic pressure neither the simple CO value are completely informative, the decision to give fluids should be also based on other simple, reliable and repeatable clinical and non-clinical parameters coupling the signals derived from cardiac function and those related to systemic response (Figure 1).
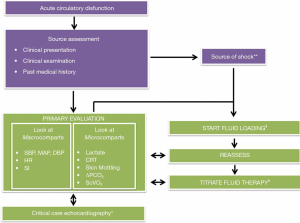
How to manage the early phase of acute circulatory dysfunction: general considerations
Acute circulatory dysfunction assessment and treatment is often a challenge clinical scenario because of two main reasons, often associated. First of all, this is a time-related situation, which should be promptly faced to avoid multi organ dysfunction. For this purpose, the recognition of clinical signs of acute circulatory dysfunction is of pivotal importance. As second, and according to the first point, this syndrome needs a prompt treatment that should be titrated on the physiological response of the patients by assessing simple and reliable parameters. This is particularly true for the great majority of cases initially managed outside the ICU. In fact, there is a large variability in the initial assessment and treatment of this syndrome, due to different backgrounds of professionals involved in initial resuscitation, especially in resource-limited settings including low-income countries and settings (14).
The source of hemodynamic instability could primarily be assumed on the basis of data reported in the literature for patients admitted to the ICU. For instance, a large trial on 1,600 patients demonstrated that the acute circulatory dysfunction is related to septic shock in the vast majority of ICU patients (62%), while cardiogenic shock (16%), hypovolemic shock (16%) and other types of distributive (4%) or obstructive (2%) shock are less frequent (13). Considering this prevalence, a de novo acute circulatory failure presented in the emergency department (ED) should be primarily considered as related to a septic event, and accordingly treated, in absence of any evident clinical signs of a different pattern (i.e., an evident fluid loss, signs of severe right ventricle dysfunction etc.).
How to manage the early phase of acute circulatory dysfunction: clinical variables used in the decision making-process of fluid administration
An acute circulatory dysfunction is often approached by using an aggressive fluid resuscitation (3). The physiological purpose of this strategy is to optimize the CO to improve the DO2. However, a single physiological or biochemical parameter able to define the balance between the changes in CO and in DO2 (coupling “macro” and “micro” circulation) is still not available.
At the bedside, the ability of ICU physicians in estimating the exact CO value based on clinical examinations is rather low (i.e., 42–62% of the cases), often leading to incongruent evaluations (meaning that the estimated CO was increased whereas the real CO was decreased, or vice versa) (18). However, at the bedside the diagnosis of an acute circulatory dysfunction is primarily clinical. In fact, a very low CO could be harmful, since it is a primary determinant of peripheral oxygen supply, however there is not a mathematical correlation between the CO measurement and the adequacy of peripheral blood flow. In other words, normal or even high values or CO could be insufficient, if metabolic demand is not adequately supplied.
For all these reasons, the basic monitoring an acute circulatory dysfunction should be focused of that parameters coupling the “macro” and “micro” response to the shock and tracking the changes of the two systems in response to the therapy (see Table 1 and Figure 1).
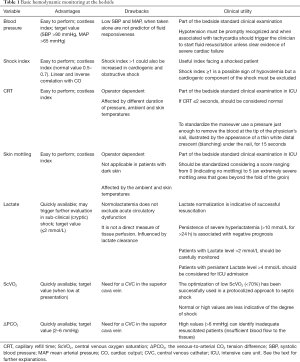
Full table
Role of basic numbers: systemic pressures and renal function
Arterial blood pressure (ABP) depends on several factors: the amount of blood ejected by the heart, the arterial compliance and the systemic vascular resistance and the arterial system modulates vessels tone in order to keep perfusion pressure, i.e., MAP, constant.
Although arterial hypotension is not per se a sign of acute circulatory dysfunction, a systolic ABP lower than 90 mmHg (or less than 40 mmHg in previously hypertensive patients) or a MAP less than 65 mmHg should be promptly recognized. Facing a shocked patient in the first minutes, one of the fundamental questions should be if she/he would benefit from a fluid therapy. The MAP value alone is not sufficient to trigger a fluid resuscitation: a low MAP can be not associated with hypovolemic or septic shock or, in contrast, might be maintained adequate thanks to compensatory mechanism like an increase of vascular resistances (6). Moreover, low systolic ABP could be associated with either a normal diastolic ABP (i.e., 70 mmHg) or lower value (i.e., 40 mmHg). As one of the main determinants of diastolic ABP is the arteriolar tone, a low systolic and diastolic ABP, suggest a low vascular tone, especially in the presence of tachycardia and therefore the need of early vasopressor (14).
Tachycardia is an important early sign of shock but obviously it could be also due to pain, anxiety, fever, anemia, inflammation. For these reasons it should not be used alone as a predictor of fluid responsiveness (6,19). Basically, in shocked patient, both hypotension and tachycardia should trigger the clinician to start fluid resuscitation unless clear evidence of severe cardiac failure.
A useful and easy indicator of hypovolemia is the “shock index” (SI), i.e., the ratio of heart rate (HR) divided by systolic ABP (HR/SBP). This index was originally described in trauma patients but its relevance has also been demonstrated in septic patients (20). The SI has a linear and inverse correlation with the CO and in healthy adults its normal range is 0.5–0.7. A value ≥1 is related with the extent of hypovolemia but it is important to underline that it could also be increased in cardiogenic and obstructive shock. Therefore, with a SI ≥1 a fluid therapy should start always checking a possible cardiac component of the shock (14).
An attractive method to investigate a hypovolemic status is the passive leg raising (PLR) test. It can be considered a brief and completely reversible “self volume-challenge” because of the shift of around 300 mL of blood from the legs to the intra-thoracic compartment avoiding the risk of fluid over load. Of course, the effect of PLR is time limited with the apex of the increasing of the CO 1 min after starting the manoeuvre (21,22). The reliability of the PLR is known to be significant when a direct measurement of the CO is available. However, the changes in the pulse pressure after he PLR (i.e., the difference between systolic and diastolic pressure) could be useful in the assessment of fluid responsiveness, despite a lower sensitivity and specificity as compared to the changes in CO (23).
Another insidious marker of possible hypoperfusion is the urinary output (14). Oliguria is a non-specific symptom and could be already presents in mild dehydration. Moreover, urinary output may not reflect a systemic hypoperfusion during early circulatory dysfunction: some neurohormonal compensatory mechanisms could be responsible of a preservation and sometimes even an increase renal blood flow and in this case extra fluids could alter renal perfusion by increasing venous congestion. In synthesis fluid administration does not necessary lead to a restoration of normal diuresis and the oliguria could be the results of profound intra-renal microcirculatory abnormalities that are not related to hypoperfusion (24).
Peripheral perfusion assessed by skin functional and clinical assessment
Role of skin mottling and capillary refill time (CRT)
The systemic response to between O2 delivery and consumption is focused on the redistribution of the blood from non-vital to vital organs. In this condition, the skin is one of the non-vital organs receiving a reduction of systemic flow, however clinical assessment of skin perfusion is not routinely used by the physicians as trigger to guide fluid resuscitation, as confirmed by the results of the FENICE study (8). For sure, peripheral perfusion can be influenced by ambient temperature, skin color and inter-observer variability. However, these limitations are balanced by the advantages of using inexpensive, non-invasive and easily accessible parameters as a surrogate of more invasive and costly monitors, at least during the initial phase of resuscitation (14). As confirm, the progression of skin mottling is associated to lactate levels and urinary output, but not with CO values, confirming the functional decoupling between cardiac function and progression of shock (25,26).
The assessment of mottling skin as a semi-quantitative approach based on mottling extension around the knee has been recently proposed Ait-Oufella et al. (25). This score ranges from 0 (no mottling) to 5 (an extremely severe mottling area that goes beyond the fold of the groin): a score ≥4 and persistence of high values during the first 6 h from the ICU admission were both associated with worst outcomes. Skin temperature could also be integrated in this setting. In fact, a recent prospective observational study showed that toe-to-room temperature gradient reflects tissue perfusion and correlates with prognosis in ICU patients with severe infections (27). The CRT is a very attractive tool because is easy to learn, inexpensive, repeatable and can be in pre-ICU, ICU, and resource-limited settings. This tool measures the time required to recolor the tip of a finger after the application of a pressure to cause blanching.
For sure the standardization of the manoeuvre is the main limitation of the CRT, since this manoeuvre depends on the extent and the modality of the applied pressure. CRT was calculated by applying for 15 seconds enough pressure to remove the blood at the tip of the physician’s nail illustrated by appearance of a thin white distal crescent under the nail (28). In this prospective observational study on 59 patients, CRT at 6 h after ICU admission was strongly predictive of 14-day mortality [area under the curve 0.84 (0.75–0.94)]. This finding has been confirmed in a prospective large cohort study on patients hospitalized in the emergency room for hypotension, showing a strong association between CRT and in-hospital mortality (29). In another study, Hernandez et al. authors reported that CRT <4 seconds, 6 h after resuscitation was associated with resuscitation success, with normalization of lactate levels 24 h after the occurrence of severe sepsis/septic shock (30).
More recently, the ANDROMEDA-SHOCK (a multicenter, randomized trial conducted at 28 ICUs in 5 countries) assessed whether or not a normalized CRT could be superior to lactate as a target for early septic shock resuscitation. The normalized CRT was assessed by applying a fixed pressure to ventral surface of right index finger distal phalanx until skin was blanched and then maintained for 10 seconds, by using a glass microscope slide, The time for return for normal skin color was registered with a chronometer, assessed every 30 minutes and considered abnormal for a time greater than 3 seconds. The resuscitation strategy targeting normalization of CRT, compared with a strategy targeting serum lactate levels, did not reduce all-cause 28-day mortality. However, peripheral perfusion–targeted resuscitation was associated with beneficial effects on the secondary outcome of sequential organ failure assessment (SOFA) score at 72 h and lower 28-day mortality in the predefined subgroup of patients with less severe organ dysfunction at baseline (31).
Role of lactate levels
Since early studies of 1960’s and 1970’s blood lactate concentrations have been used extensively as a biochemical marker of unbalanced tissue perfusion in ICU patients (32,33). Regardless of the mechanism related to the occurrence of hyperlactatemia, and especially the persistence of hyperlactatemia during the ICU stay, remains a major negative prognostic factor in critically ill patients. In fact, a lactate level of >2 mmol/L at ICU admission or during ICU stay was associated with mortality rates of up to 40% (34,35), while a value exceeding 10 mmol/L are associated with high mortality rate of about 80% or more (36-38). The Surviving Sepsis Campaign guideline suggests guiding resuscitation to normalize lactate in patients with elevated levels (3) and lactate-guided resuscitation significantly reduced mortality as compared to resuscitation without lactate monitoring (3).
Measuring the absolute value of lactate level and the tracking its changes during the resuscitation is key. Whenever possible, blood lactate concentrations should be measured and the obtained values should be integrating with clinical examination (14). For sure the availability of lactate values is not always ensured, especially in health care facilities outside the ICU. However, several point-of-care capillary lactate measurement devices are becoming available in the marker and could help in routinely assessment of this parameter.
How to manage the early phase of acute circulatory dysfunction: role of central venous catheter (CVC) placement and critical care echocardiography (CCE)
CVC insertion and use
The role of CVC placement in the early phase of management of acute circulatory dysfunction is controversial. On the one hand, the insertion of a CVC for the measurement of central venous pressure (CVP) to guide fluid resuscitation is not recommended (14). In fact, CVP cannot be used as a predictor of fluid responsiveness and, accordingly, CVP placement with the only purpose of obtaining this number is unessential. However, as a matter of fact, the number of CVC inserted in the ED is increased. For instance, a large retrospective analysis found that the 25% of all ED admissions between 2003 and 2006 in 310 hospitals in California, underwent CVC placement, with absolute numbers more than doubling from 2,957 to 6,290 over the 4 years of the study (39). This escalation in CVC placement in the ED could reflect an increasing acceptance of Early Goal Directed Therapy to manage the early phase of sepsis, as firstly proposed by Rivers et al. (40). Pragmatically, recent expert panel recommendations suggest that if the patient has a CVC in place, it could be used to optimize fluid resuscitation also on the basis of CVP measurements. In fact, CVP could be used as a safety limit/endpoint, since its increase during a FC could reflect the absence of fluid responsiveness (14).
The insertion of a CVC could also be useful to assess two other clinical parameters, which could provide adjunctive relevant clinical information: the venous-to-arterial CO2 tension difference (ΔPCO2) and central venous oxygen saturation (ScVO2).
ΔPCO2 reflects the balance between its production by the tissues and its elimination through the lungs and could be considered as a marker of the adequacy of CO to the global metabolic conditions. In fact, a value >6 mmHg suggests that CO values is not high enough with respect to systemic metabolic requests (41).
ScvO2 is a surrogate of mixed venous oxygen saturation (normally the ScvO2 is 2% to 3% lower than SvO2) and reflects the balance between the DO2 and consumption, being a surrogate value (42). A low ScvO2 has been previously considered as a therapeutic target in the management of early phases of septic shock (40,43,44) and this approach was effective in reducing the mortality of septic shock (40). However, less severe critically ill patients presenting in the ED with higher ScvO2 values would probably benefit less of the optimization of this parameter (45-47). Moreover, the persistence of high blood values of ScvO2 is associated with mortality in septic shock patients, probably indicating an irreversible impairment of the oxygen extraction by the cells (48).
CCE: a “basic” monitoring?
The role of the echocardiography in the management of critically ill patients has changed in the last decades, becoming an oriented and focused exam performed and interpreted at the bedside by the intensivists to customize the therapy and to reassess the effects of the strategies adopted (49). For decades, the echocardiography assessment has been performed with big devices, limiting the broad applicability of this technique. However, the miniaturization of medical devices has boosted the echocardiography as part of the daily clinical assessment of patient, inside and outside the ICUs. In fact, the design of pocket-sized equipment continues to evolve. Recently, mobile application–based ultrasound systems have emerged wherein a smartphone or tablet can turn into a handheld ultrasound simply by plugging in a transducer or connecting wirelessly. The scope of the goal-directed CCE is to provide images instead of numbers, and a qualitative evaluation of cardiac structure and function. For this reason, the key challenge of CCE is obtaining the most informative pictures, using very few, echocardiographic views in very complex clinical scenarios, when the need of obtaining immediate information is reduced, for example, by positioning limitations, lung interference, and patient’s agitation (50). Whenever available, the CCE should now be considered as a part of the routinely assessment of patients with an acute hemodynamic instability, since the qualitative assessment of cardiac function plays a central role in therapy.
Conclusions
Fluid administration for acute circulatory dysfunction is challenging, especially resource-limited settings. The assessment of the physiological changes in basic hemodynamic parameters is of pivotal importance to guide fluid resuscitation, which is considered the first step to face hemodynamic instability. During acute circulatory failure, the blood flow is redistributed from non-vital to vital organs. For this reason, clinical assessment of skin perfusion could be useful to guide fluid resuscitation, as long as basic numbers related to systemic pressures and renal function. CVC insertion and CCE could add additional important information to optimize the therapy and to titrate the response.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Glenn Hernández and Guo-Wei Tu) for the series “Hemodynamic monitoring in critically ill patients” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.04.14). The series “Hemodynamic Monitoring in Critically Ill Patients” was commissioned by the editorial office without any funding or sponsorship. Dr. Messina received travel expenses and registration for meetings, congresses, and courses and lecture fees from Vygon. Prof. Cecconi received Honoraria and/or Travel Expenses from Edwards Lifesciences. Prof. Cecconi is a consultant for Edwards Lifesciences, LiDCO and Cheetah Medical. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Latta T. Relative to the treatment of cholera by the copious injection of aqueous and saline fluid into the veins. Lancet 1832;2:274-77.
- Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002;360:219-23. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med 2013;369:1243-51. [Crossref] [PubMed]
- Hjortrup PB, Haase N, Bundgaard H, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med 2016;42:1695-705. [Crossref] [PubMed]
- Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1795-815. [Crossref] [PubMed]
- Monnet X, Marik PE, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care 2016;6:111. [Crossref] [PubMed]
- Cecconi M, Hofer C, Teboul JL, et al. Fluid challenges in intensive care: the FENICE study: A global inception cohort study. Intensive Care Med 2015;41:1529-37. [Crossref] [PubMed]
- Lakhal K, Ehrmann S, Perrotin D, et al. Fluid challenge: tracking changes in cardiac output with blood pressure monitoring (invasive or non-invasive). Intensive Care Med 2013;39:1953-62. [Crossref] [PubMed]
- Dufour N, Chemla D, Teboul JL, et al. Changes in pulse pressure following fluid loading: a comparison between aortic root (non-invasive tonometry) and femoral artery (invasive recordings). Intensive Care Med 2011;37:942-9. [Crossref] [PubMed]
- Pierrakos C, Velissaris D, Scolletta S, et al. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med 2012;38:422-8. [Crossref] [PubMed]
- Messina A, Greco M, Cecconi M. What should I use next if clinical evaluation and echocardiographic haemodynamic assessment is not enough?. Curr Opin Crit Care 2019;25:259-65. [Crossref] [PubMed]
- De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010;362:779-89. [Crossref] [PubMed]
- Cecconi M, Hernandez G, Dunser M, et al. Fluid administration for acute circulatory dysfunction using basic monitoring: narrative review and expert panel recommendations from an ESICM task force. Intensive Care Med 2019;45:21-32. [Crossref] [PubMed]
- Cecconi M, Parsons AK, Rhodes A. What is a fluid challenge? Curr Opin Crit Care 2011;17:290-5. [Crossref] [PubMed]
- Messina A, Pelaia C, Bruni A, et al. Fluid Challenge During Anesthesia: A Systematic Review and Meta-analysis. Anesth Analg 2018;127:1353-64. [Crossref] [PubMed]
- Messina A, Longhini F, Coppo C, et al. Use of the Fluid Challenge in Critically Ill Adult Patients: A Systematic Review. Anesth Analg 2017;125:1532-43. [Crossref] [PubMed]
- Hiemstra B, Eck RJ, Keus F, et al. Clinical examination for diagnosing circulatory shock. Curr Opin Crit Care 2017;23:293-301. [Crossref] [PubMed]
- Vincent JL, De Backer D. Circulatory shock. N Engl J Med 2013;369:1726-34. [Crossref] [PubMed]
- Berger T, Green J, Horeczko T, et al. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med 2013;14:168-74. [Crossref] [PubMed]
- Monnet X, Marik P, Teboul JL. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med 2016;42:1935-47. [Crossref] [PubMed]
- Monnet X, Teboul JL. Passive leg raising: five rules, not a drop of fluid! Crit Care 2015;19:18. [Crossref] [PubMed]
- Cavallaro F, Sandroni C, Marano C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med 2010;36:1475-83. [Crossref] [PubMed]
- Prowle J, Bagshaw SM, Bellomo R. Renal blood flow, fractional excretion of sodium and acute kidney injury: time for a new paradigm? Curr Opin Crit Care 2012;18:585-92. [Crossref] [PubMed]
- Ait-Oufella H, Lemoinne S, Boelle PY, et al. Mottling score predicts survival in septic shock. Intensive Care Med 2011;37:801-7. [Crossref] [PubMed]
- Jouffroy R, Saade A, Tourtier JP, et al. Skin mottling score and capillary refill time to assess mortality of septic shock since pre-hospital setting. Am J Emerg Med 2019;37:664-71. [Crossref] [PubMed]
- Bourcier S, Pichereau C, Boelle PY, et al. Toe-to-room temperature gradient correlates with tissue perfusion and predicts outcome in selected critically ill patients with severe infections. Ann Intensive Care 2016;6:63. [Crossref] [PubMed]
- Ait-Oufella H, Bige N, Boelle PY, et al. Capillary refill time exploration during septic shock. Intensive Care Med 2014;40:958-64. [Crossref] [PubMed]
- Londono J, Nino C, Diaz J, et al. Association of Clinical Hypoperfusion Variables With Lactate Clearance and Hospital Mortality. Shock 2018;50:286-92. [Crossref] [PubMed]
- Hernandez G, Pedreros C, Veas E, et al. Evolution of peripheral vs metabolic perfusion parameters during septic shock resuscitation. A clinical-physiologic study. J Crit Care 2012;27:283-8. [Crossref] [PubMed]
- Hernandez G, Ospina-Tascon GA, Damiani LP, et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019;321:654-64. [Crossref] [PubMed]
- Broder G, Weil MH. Excess Lactate: An Index of Reversibility of Shock in Human Patients. Science 1964;143:1457-9. [Crossref] [PubMed]
- Weil MH, Afifi AA. Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock). Circulation 1970;41:989-1001. [Crossref] [PubMed]
- Khosravani H, Shahpori R, Stelfox HT, et al. Occurrence and adverse effect on outcome of hyperlactatemia in the critically ill. Crit Care 2009;13:R90. [Crossref] [PubMed]
- Juneja D, Singh O, Dang R. Admission hyperlactatemia: causes, incidence, and impact on outcome of patients admitted in a general medical intensive care unit. J Crit Care 2011;26:316-20. [Crossref] [PubMed]
- Peretz DI, Scott HM, Duff J, et al. The significance of lacticacidemia in the shock syndrome. Ann N Y Acad Sci 1965;119:1133-41. [Crossref] [PubMed]
- Nichol AD, Egi M, Pettila V, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care 2010;14:R25. [Crossref] [PubMed]
- Haas SA, Lange T, Saugel B, et al. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med 2016;42:202-10. [Crossref] [PubMed]
- Theodoro D, Owens PL, Olsen MA, et al. Rates and timing of central venous cannulation among patients with sepsis and respiratory arrest admitted by the emergency department*. Crit Care Med 2014;42:554-64. [Crossref] [PubMed]
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368-77. [Crossref] [PubMed]
- Lamia B, Monnet X, Teboul JL. Meaning of arterio-venous PCO2 difference in circulatory shock. Minerva Anestesiol 2006;72:597-604. [PubMed]
- Bloos F, Reinhart K. Venous oximetry. Intensive Care Med 2005;31:911-3. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 2008;34:17-60. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. [Crossref] [PubMed]
- Investigators A, Group ACT, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496-506. [Crossref] [PubMed]
- Pro CI, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014;370:1683-93. [Crossref] [PubMed]
- Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015;372:1301-11. [Crossref] [PubMed]
- Textoris J, Fouche L, Wiramus S, et al. High central venous oxygen saturation in the latter stages of septic shock is associated with increased mortality. Crit Care 2011;15:R176. [Crossref] [PubMed]
- Vignon P, Begot E, Mari A, et al. Hemodynamic Assessment of Patients With Septic Shock Using Transpulmonary Thermodilution and Critical Care Echocardiography: A Comparative Study. Chest 2018;153:55-64. [Crossref] [PubMed]
- Walley PE, Walley KR, Goodgame B, et al. A practical approach to goal-directed echocardiography in the critical care setting. Crit Care 2014;18:681. [Crossref] [PubMed]


