
Glucosamine potentiates the differentiation of adipose-derived stem cells into glucose-responsive insulin-producing cells
Introduction
Diabetes mellitus is a metabolic disease associated with various complications, including diabetic nephropathy, diabetic retinopathy, stroke, and cardiovascular disease (1-3). Type 1 diabetes (T1D) is an autoimmune disease, which results from the destruction of pancreatic beta-cells and results in defective insulin production. Type 2 diabetes results from the relative deficiency of insulin secretion due to the failure of compensation of insulin resistance. Therefore, supplementation of insulin is one strategy that is used to treat both types of diabetes. Currently, the therapy of T1D includes insulin injection, however, it has disadvantages such as the need for daily injections and pain at the injection site (4). Islet transplantation is another effective strategy for restoration of insulin secretion. However, the scarcity of islet sources or pancreas donors poses an obstacle for islet transplantation (5-7). In order to address these concerns, studies have been performed to develop a method for the differentiation of mesenchymal stem cells (MSCs) into insulin secreting cells (8-10).
MSCs, including bone marrow stem cells and adipose derived stem cells (ADSCs), are known to differentiate into insulin-producing cells (IPCs) (11-13). Several studies have reported protocols for differentiation of MSCs into IPCs (14,15). However, it is necessary to improve the methods of differentiation of ADSCs into IPCs for clinical use.
Glucosamine is known to be a structural polysaccharide found in chitosan or chitin in the exoskeleton of crustaceans and arthropods, and glucosamine sulphate is the sulphate derivative of the natural amino monosaccharide glucosamine. Glucosamine plays a role in the formation and function of tendon, skin, bone, heart valves, blood vessels, articular surfaces, and mucus secretions of the digestive and respiratory tracts (16). In addition, glucosamine has been reported to increase migration and proliferation of stem cells. Glucosamine enhances the differentiation of cartilage from stem cells (17,18). In this study, we investigated whether addition of glucosamine into the differentiation medium of hADSCs for differentiation into IPCs is beneficial for improvement of generation of functional IPCs.
Methods
Culture of human adipose-derived stem cells (hADSCs)
hADSCs (Thermo Fisher Scientific, MA, USA) were grown on cell culture plates in growth medium (MesenPRO RSTM Medium kit; Thermo Fisher Scientific) containing 1% penicillin-streptomycin (Welgene, Deagu, Korea) and 1% GlutaMax (Gibco, Life Technologies, Grand Island, NY, USA) for 3–4 days. hADSCs between passages 3–6 were seeded in 12-well plates (5×105 cells/well) and used for differentiation.
Differentiation of hADSCs into IPCs
hADSCs (5×105 cells/well) were seeded into 12-well plates and then grown in the differentiation medium following the procedure for differentiation as shown Figure 1A.
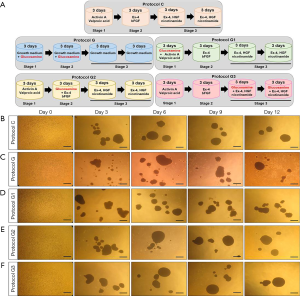
As illustrated in Figure 1A, five differentiation protocols were used: (I) protocol C, which involved 3 days of incubation in basic medium [serum-free DMEM/F12 (1:1) medium supplemented with 1% B27 serum-free supplement (Thermo Fisher Scientific), 1% N2 supplement (Thermo Fisher Scientific), and 1% penicillin/streptomycin (Gibco)] plus 50 ng/mL activin A (PeproTech, NJ, USA) and 2 mM valproic acid (Sigma-Aldrich, MO, USA), 3 days of incubation in basic medium plus 10 nM exendin-4 (Ex-4; Sigma-Aldrich) and 10 ng/mL fibroblast growth factor-basic (bFGF; Sigma-Aldrich), 6 days of incubation in basic medium plus 10 nM Ex-4 (Sigma-Aldrich), 50 ng/mL human growth factor (HGF; PeproTech) and 10 mM nicotinamide (Sigma-Aldrich), (II) protocol G, which involved only 6 days of incubation in growth medium (low glucose DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin) plus 10 mM glucosamine (Sigma-Aldrich), 6 days of incubation in growth medium (DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin); (III) protocol G1, the same as Protocol C except that 10 mM glucosamine is added for the first 3 days; (IV) protocol G2, the same as Protocol C except that 10 mM glucosamine is added for the second 3 days; (V) protocol G3, the same as Protocol C except that 10 mM glucosamine is added for the last 6 days. After differentiation, samples were collected for various experiments.
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was isolated from cells using Trizol reagent (TaKaRa, Shiga, Japan) according to the manufacturer’s protocol. RNA was treated with DNase to remove genomic DNA. cDNA was synthesized using 2 µg RNA using a PrimScriptTM 1st strand cDNA synthesis kit (TaKaRa). PCR was performed in duplicates and the reaction mixture contained the SYBR Green mixture (TaKaRa), 100 pmol of each, forward and reverse primers, and 50 ng cDNA template. PCR amplification was carried out using the CFX96 Touch™ Real-Time PCR Detection System and completed in 40 cycles. Relative gene expression levels were normalized with those of human cyclophilin using the comparative CT method. Primers were designed to amplify the following gene sequences: Cyclophilin F; TGCCATCGCCAAGGAGTAG, R; TGCACAGACGGTCACTCAAA, insulin F; GCAGCCTTTGTGAACCAACA, R; TTCCCCGCACACTAGGTAGAGA, pancreatic and duodenal homeobox1 (PDX1) F; GAACTTGACCGAGAGACACATCAA, R; TTGTCCTCCTCCTTTTTCCACTT, neurogenin 3 (NGN3), F; CGGAGTCGGCGAAAGAAG, R; CGTCCAGTGCCGAGTTGAG, Paired box protein Pax-6 (PAX6) F; TGCGACATTTCCCGAATTCT, R; GATGGAGCCAGTCTCGTAATACCT synaptotagmin-4 (Syt4), F; ACCTCACATAAGAACCAT, R; TTATTCACTAACTAATTCAACAAT, glucokinase (Gck), F; GGACTTTGAAATGGATGT, R; TGATGGTCTTCGTAGTAG, and glucose transporter 2 (Glut2) F; TACAATGACAGAAGATAA, R; ATAGTGAGATATTATTACCT, forward (F) 5'-3' and reverse (R) 5'-3'.
Glucose stimulated insulin secretion (GSIS)
Insulin release from insulin secretory cells was measured using the Krebs Ringer bicarbonate (KRB) buffer. hADSCs were preincubated for 2 hours in the KRB buffer containing 0.2 mM glucose (Gibco). For the first experiment, hADSCs were incubated for 2 hours in KRB buffer containing 3 or 20 mM glucose (Sigma-Aldrich). For the second experiment, cells were incubated with KRB buffer containing 3, 10, 17, or 24 mM glucose. Insulin released into these supernatants was measured using the human insulin ELISA kit (ALPCO, NH, USA) according to the manufacturer’s instructions. Insulin content was normalized with the protein content of corresponding cell lysates.
Immunofluorescence staining
IPCs were embedded in optimum cutting temperature (OCT) compound (Tissue-Tek, CA, USA), and sectioned for histological analysis. For staining, sections were blocked with protein block serum free solution (Dako, CA, USA) for 1 hour at room temperature, incubated with primary antibodies overnight at 4 °C, washed, then incubated with the secondary antibody for 1 hour at room temperature, and washed. For imaging, samples were mounted in fluorescent mounting medium (Dako). Representative images were taken using a confocal microscope (Carl Zeiss Inc., Oberkochen, Germany).
Statistical analyses
All values are expressed as mean ± standard error (S.E.). Statistical analysis was performed using the Student’s t-test and the Bonferroni procedure using the IBM SPSS statistics 19 software. The value of statistical significance was set at P<0.05.
Results
Morphological changes in islet-like clusters during differentiation of hADSCs using glucosamine supplemented medium
We induced differentiation of hADSCs into IPCs using a three-step protocol as described previously (protocol C) (19). To investigate the effect of glucosamine on differentiation of IPCs, we added glucosamine to our original differentiation medium at different stages of differentiation for 12 days as shown in Figure 1A. Protocol G1 included addition of glucosamine at stage 1; protocol G2 included addition of glucosamine at stage 2; and protocol G3 included addition of glucosamine at stage 3. As a control, hADSCs were differentiated in growth medium containing glucosamine only (protocol G).
Morphology of islet-like clusters differentiated using protocol C changed into a globular and smooth shape (Figure 1B). However, the islet-like clusters differentiated using protocol G showed a globular shape, although their surface was rough (Figure 1C). The cell surface was also slightly roughened in islet-like clusters differentiated using protocol G1, however, the shape of cells changed to a globular and smooth type upon differentiation using protocol G2 or G3 (Figure 1D,E,F).
Supplementation with glucosamine at a later stage of differentiation had the greatest effect on differentiation of hADSCs into IPCs
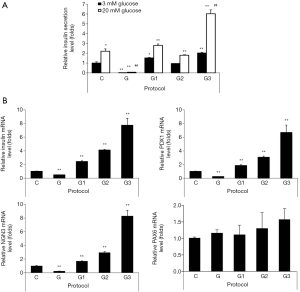
In order to determine the functionality of IPCs differentiated from hADSCs, we examined glucose-stimulated insulin secretion (GSIS) by IPCs. Basal insulin levels (upon treatment with 3 mM glucose) in IPCs differentiated using protocols G1 or G3 were significantly higher as compared with those found upon using protocol C, insulin levels were found to be the highest upon using protocol G3 (Figure 2A). The islet-like clusters obtained upon differentiation using protocol G did not produce insulin (Figure 2A). GSIS (upon treatment with 20 mM glucose) in IPCs using protocol C were increased 2.2 fold as compared with those found using 3 mM glucose (Figure 2A). The insulin releases at 20 mM glucose in differentiated IPCs by protocols G1, G2, and G3 was 1.62, 1.68 or 2.65 folds higher, respectively (Figure 2A). When we measured mRNA expression of transcriptional factors involved in regulation of the insulin gene and beta-cell differentiation, including PDX1 and NGN3 using qRT-PCR, we found that mRNA expression levels of insulin, PDX1, and NGN3 were significantly increased in IPCs differentiated using protocols G1, G2, and G3 as compared with those in IPCs differentiated using protocol C, with the highest expression seen in IPCs differentiated using protocol G3 (Figure 2B). However, there were no significant differences in PAX6 expression among groups except the marginal increase observed by protocol G3 (Figure 2B). These results indicate that glucosamine supplementation promoted the differentiation of hADSCs into IPCs.
hADSCs differentiated into IPCs using protocol G3 showed increased expression of insulin and beta-cell transcription factors
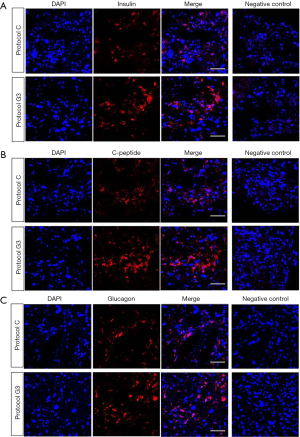
As we found that protocol G3 was the most effective for the differentiation of hADSCs into IPCs, all the experiments were then carried out using protocol G3. We examined the protein expression of insulin, glucagon, and C-peptide using immunofluorescence staining. Expression of insulin and C-peptide, known as insulin secretion markers in pancreas, was clearly increased in IPCs differentiated using protocol G3 as compared with that in IPCs differentiated using protocol C, whereas glucagon expression was not different between IPCs differentiated using different protocols (Figure 3A,B,C). We also checked the percentage of insulin-, C-peptide- and glucagon-expressing cells within the cluster. We found that approximately 44%, 52%, 66% of the DAPI-stained cells were insulin-, C-peptide- and glucagon-positive cells, respectively, in clusters by protocol C; these percentages increased to 87%, 87% and 68% in clusters by protocol G3.
When we examined the expression of beta-cell transcription factors before (day 6) and after addition of glucosamine (day 9 by Protocol G3), the mRNA expression levels of insulin, PDX1, and NGN3 were significantly increased on day 9 after differentiation of IPCs using protocol G3, as compared with those found in IPCs differentiated using protocol C (Figure 4A,B,C). These results suggest that the differentiation of hADSCs into IPCs was significantly enhanced after addition of glucosamine.

IPCs differentiated using protocol G3 increased insulin secretion in response to glucose treatment in a dose dependent manner
In order to examine the ability of insulin secretion of differentiated IPCs, they were incubated in the presence of 3, 10, 17, or 24 mM glucose. IPCs differentiated using protocol C showed 1.1-, 1.4- or 2-fold increase in insulin secretion upon treatment with 10, 17, or 24 mM glucose, respectively, as compared with that in the presence of 3 mM glucose (Figure 5A). IPCs differentiated using protocol G3 showed 1.2-, 1.5-, or 2.7-fold increase in insulin levels in the presence of 10, 17, or 24 mM glucose, respectively, as compared with that in the presence of 3 mM glucose (Figure 5A). Consistent with the results shown in Figures 2 and 3, insulin release from IPCs differentiated using protocol G3 was significantly higher in both, basal and glucose-stimulated conditions as compared with that from IPCs differentiated using protocol C (Figure 5A).
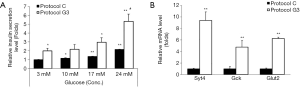
To investigate whether glucosamine-promoted insulin secretion is induced by the expression of genes related to the insulin secretion machinery, we examined the mRNA expression of Syt4, Gck, and Glut2 in the IPCs differentiated using protocol C and G3. Compared with that in IPCs differentiated using protocol C, the expressions of Syt4, Gck, and Glut2 were increased 9.3, 4.7, and 6.2 folds in IPCs differentiated using protocol G3, respectively (Figure 5B). These results indicate that insulin secretion promoted by glucosamine in the late stage of differentiation from hADSCs into IPCs.
Discussion
In this study, we found that supplementation of the IPC differentiation medium with glucosamine promotes the differentiation of hADSCs into IPCs. Addition of glucosamine at a later stage during stepwise differentiation increased the expression of beta cell-specific genes, insulin secretory genes and glucose sensor-related genes, and also increased insulin secretion.
MSCs, including bone marrow stem cells and ADSCs have been used for cell therapy for a variety of diseases, such as cardiovascular disease (20), liver disease (21) and diabetes (22). In addition, MSCs are known to be multipotent, and thus can differentiate into various cell types (such as adipocytes, osteocytes, endothelial cells, and myocytes) (23). In particular, MSCs have been found to differentiate into islet-like cells and various methods for their differentiation have been examined in order to increase their efficiency of differentiation into IPCs functionally similar to endogenous beta-cells (8,22,24). hADSCs can be conveniently extracted from tissues removed during selective cosmetic liposuction, and can also be obtained from resected adipose tissue (25). In our previous study, we established a stepwise protocol for the differentiation of hADSCs to IPCs (19). In this study, we attempted to improve the differentiation protocol by supplementation of the culture medium with glucosamine.
Glucosamine is known to be useful for the treatment of osteoarthritis, and several reports have documented the use of glucosamine for the differentiation of mouse embryonic stem cells into cartilage (18). Addition of glucosamine alone to the growth medium of hADSCs did not affect the differentiation of hADSCs to IPCs. Supplementation with glucosamine at a later stage had the greatest effect on IPC differentiation and basal insulin secretion (upon treatment with 3 mM glucose), which was about 2.65 folds as compared to that seen on IPCs differentiated using the conventional protocol (protocol C). These results indicate that glucosamine might affect the maturation stage of pancreatic beta-cells.
Pancreas is an endocrine gland that produces many important hormones that circulate in the blood, such as glucagon, insulin, somatostatin and pancreatic polypeptide which are produced from alpha-, beta-, delta-, or pancreatic polypeptide cells (pp-cells), respectively (26). PDX1 is known to be an important factor for the development of endocrine pancreas and maintenance of pancreatic beta-cell function (27) and NGN3 is an endocrine progenitor cell marker (28). Addition of glucosamine at any stage of differentiation significantly increased the expression of PDX1 and NGN3 as well as insulin mRNA, although the addition at a later stage most significantly increased expression of these genes. These results indicate that glucosamine might mostly affect the commitment to the endocrine lineage. However, the detailed mechanisms for this action of glucosamine require further investigation.
Pancreatic beta-cells secrete insulin in response to glucose. Glut2 present on the surface of beta-cells senses glucose levels; when glucose levels are elevated, glucose transferred through Glut2 is degraded by Gck to produce ATP. Increased ATP is used for insulin release by exocytosis (29-32). Therefore, Glut2 and Gck are very important for glucose sensing and glucose-responsive insulin secretion. In addition, Syt4 is known to be involved in exocytosis during secretion of insulin when insulin granules are fused to the surface of the plasma membrane (33-35). Our data show that insulin secretion is significantly increased in differentiated IPCs when glucosamine is added at a later stage. Therefore, we examined the expression of these molecules in differentiated IPCs using protocol G3 and found that their expression levels were significantly increased as compared with those seen in IPCs differentiated using the conventional protocol, namely protocol C. The increase in insulin secretion and dose-dependent glucose responsiveness of differentiated IPCs using glucosamine might also be due to the increase of the expression of molecules involved in the insulin secretion machinery.
In conclusion, we found that glucosamine supplementation promotes the differentiation of hADSCs into glucose-responsive insulin secretory cells through the increase of mRNA levels of beta-cell specific genes, including insulin, PDX1 and NGN3. In addition, glucosamine supplementation also increases mRNA expression levels of genes such as Syt4, Gck and Glut2 in differentiated IPCs. These results indicate that glucosamine might be beneficial for the differentiation of hADSCs into functional beta-cells.
Acknowledgments
Funding: This research was supported by grants from the Innovative Research Institute for Cell Therapy Project and from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant numbers: A062260, HI14C1135).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ling Lu) for the series “Stem Cell and Clinical Application” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.103). The series “Lymphedema” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rafieian-Kopaei M, Nasri H. The Ameliorative Effect of Zingiber officinale in Diabetic Nephropathy. Iran Red Crescent Med J 2014;16:e11324. [Crossref] [PubMed]
- Hendrick AM, Gibson MV, Kulshreshtha A. Diabetic Retinopathy. Prim Care 2015;42:451-64. [Crossref] [PubMed]
- Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: "double diabetes" in the Diabetes Control and Complications Trial. Diabetes Care 2007;30:707-12. [Crossref] [PubMed]
- Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69-82. [Crossref] [PubMed]
- Muniyappa R, Lee S, Chen H, et al. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008;294:E15-26. [Crossref] [PubMed]
- Vaithilingam V, Tuch BE. Islet transplantation and encapsulation: an update on recent developments. Rev Diabet Stud 2011;8:51-67. [Crossref] [PubMed]
- Serup P, Madsen OD, Mandrup-Poulsen T. Islet and stem cell transplantation for treating diabetes. BMJ 2001;322:29-32. [Crossref] [PubMed]
- Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol 2004;10:3016-20. [Crossref] [PubMed]
- Cao X, Han ZB, Zhao H, et al. Transplantation of mesenchymal stem cells recruits trophic macrophages to induce pancreatic beta cell regeneration in diabetic mice. Int J Biochem Cell Biol 2014;53:372-9. [Crossref] [PubMed]
- Ezquer FE, Ezquer ME, Parrau DB, et al. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant 2008;14:631-40. [Crossref] [PubMed]
- Lumelsky N, Blondel O, Laeng P, et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 2001;292:1389-94. [Crossref] [PubMed]
- Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26:443-52. [Crossref] [PubMed]
- Blyszczuk P, Czyz J, Kania G, et al. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci U S A 2003;100:998-1003. [Crossref] [PubMed]
- Gabr MM, Zakaria MM, Refaie AF, et al. From Human Mesenchymal Stem Cells to Insulin-Producing Cells: Comparison between Bone Marrow- and Adipose Tissue-Derived Cells. Biomed Res Int 2017;2017:3854232.
- Domouky AM, Hegab AS, Al-Shahat A, et al. Mesenchymal stem cells and differentiated insulin producing cells are new horizons for pancreatic regeneration in type I diabetes mellitus. Int J Biochem Cell Biol 2017;87:77-85. [Crossref] [PubMed]
- Derfoul A, Miyoshi AD, Freeman DE, et al. Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation. Osteoarthritis Cartilage 2007;15:646-55. [Crossref] [PubMed]
- Jeon JH, Suh HN, Kim MO, et al. Glucosamine-induced reduction of integrin beta4 and plectin complex stimulates migration and proliferation in mouse embryonic stem cells. Stem Cells Dev 2013;22:2975-89. [Crossref] [PubMed]
- Hwang NS, Varghese S, Theprungsirikul P, et al. Enhanced chondrogenic differentiation of murine embryonic stem cells in hydrogels with glucosamine. Biomaterials 2006;27:6015-23. [Crossref] [PubMed]
- Dao LT, Park EY, Hwang OK, et al. Differentiation potential and profile of nuclear receptor expression during expanded culture of human adipose tissue-derived stem cells reveals PPARgamma as an important regulator of Oct4 expression. Stem Cells Dev 2014;23:24-33. [Crossref] [PubMed]
- Fisher SA, Doree C, Mathur A, et al. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev 2016;12:CD007888. [PubMed]
- Kuo TK, Hung SP, Chuang CH, et al. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology 2008;134:2111-21, 2121.e1-3.
- Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol 2010;6:139-48. [Crossref] [PubMed]
- Bateman ME, Strong AL, McLachlan JA, et al. The Effects of Endocrine Disruptors on Adipogenesis and Osteogenesis in Mesenchymal Stem Cells: A Review. Front Endocrinol (Lausanne) 2017;7:171. [Crossref] [PubMed]
- Baertschiger RM, Bosco D, Morel P, et al. Mesenchymal stem cells derived from human exocrine pancreas express transcription factors implicated in beta-cell development. Pancreas 2008;37:75-84. [Crossref] [PubMed]
- Fraser JK, Zhu M, Wulur I, et al. Adipose-derived stem cells. Methods Mol Biol 2008;449:59-67. [PubMed]
- Ionescu-Tirgoviste C, Gagniuc PA, Gubceac E, et al. A 3D map of the islet routes throughout the healthy human pancreas. Sci Rep 2015;5:14634. [Crossref] [PubMed]
- Kaneto H, Miyatsuka T, Shiraiwa T, et al. Crucial role of PDX-1 in pancreas development, beta-cell differentiation, and induction of surrogate beta-cells. Curr Med Chem 2007;14:1745-52. [Crossref] [PubMed]
- Gomez DL, O'Driscoll M, Sheets TP, et al. Neurogenin 3 Expressing Cells in the Human Exocrine Pancreas Have the Capacity for Endocrine Cell Fate. PLoS One 2015;10:e0133862. [Crossref] [PubMed]
- Shcherbina L, Edlund A, Esguerra JL, et al. Endogenous beta-cell CART regulates insulin secretion and transcription of beta-cell genes. Mol Cell Endocrinol 2017;447:52-60. [Crossref] [PubMed]
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 2001;50:537-46. [PubMed]
- Isaac R, Vinik Y, Boura-Halfon S, et al. Prolonged Elimination of Negative Feedback Control Mechanisms Along the Insulin Signaling Pathway Impairs beta-Cell Function In Vivo. Diabetes 2017;66:1879-89. [Crossref] [PubMed]
- Nekoei SM, Azarpira N, Sadeghi L, et al. In vitro differentiation of human umbilical cord Wharton's jelly mesenchymal stromal cells to insulin producing clusters. World J Clin Cases 2015;3:640-9. [Crossref] [PubMed]
- Perelis M, Marcheva B, Ramsey KM, et al. Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 2015;350:aac4250. [Crossref] [PubMed]
- Wu B, Wei S, Petersen N, et al. Synaptotagmin-7 phosphorylation mediates GLP-1-dependent potentiation of insulin secretion from beta-cells. Proc Natl Acad Sci U S A 2015;112:9996-10001. [Crossref] [PubMed]
- Yoshihara E, Wei Z, Lin CS, et al. ERRgamma Is Required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive beta Cells. Cell Metab 2016;23:622-34. [Crossref] [PubMed]


