
Mesenchymal stem cells to treat liver diseases
Introduction
Mesenchymal stem cells (MSCs) were initially identified in the bone marrow by Friedenstein and coworkers (1), and, since then, they have been isolated from various organs, including the adipose tissue, umbilical cord and cord blood, brain, peripheral blood, synovial membranes, muscle, dermis, and liver (2-6). The International Society for Cellular Therapy (ISCT) defines MSCs as cells that can adhere to plastic; express CD73, CD90, and CD105 as cell surface antigens (≥95% positive); and differentiate into adipocytes, chondroblasts, and osteoblasts under in vitro differentiation conditions (7). MSCs are being actively studied for the regenerative treatment of incurable diseases via homing to damaged sites, differentiation into damaged target cells, or alleviation of the death of dying cells. According to ClinicalTrials.Gov, more than 1,000 clinical trials using MSCs have been registered; of these, more than 80 have targeted liver disease.
The liver has a high regenerative potential; however, long-term chronic injury, such as that due to viral hepatitis, alcohol, toxic drugs, and autoimmune attacks, lacks a complete remedy apart from liver transplantation. Since Theise et al. (8) found Y chromosome-positive hepatocytes in autopsied livers of women after therapeutic bone marrow allografts, bone marrow-derived cells, including unsorted bone marrow cells (BMCs), hematopoietic stem cells, and MSCs, have been investigated for the treatment of chronic liver diseases (9-14). In addition, the primary hepatocytes or hepatocyte-like cells derived from pluripotent stem cells are being actively explored to develop cell-based regenerative therapies for liver diseases. In this review, we focus only on MSCs that treat liver disease and discuss the potential therapeutic mechanisms, brief recent clinical advances, and future study perspectives to develop more efficient therapeutics.
Potential therapeutic mechanisms of MSCs for hepatic fibrosis
Despite reports revealing that cell therapies using BMCs, HSCs, and MSCs can improve liver function and alleviate hepatic fibrosis, their precise therapeutic mechanisms remain unclear. In this section, we summarize the potential therapeutic mechanisms underlying the effects of MSCs (Figure 1), which have been reported to have relatively diverse therapeutic roles compared to BMCs and HSCs.
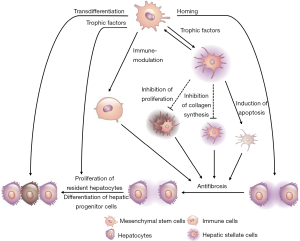
Homing of MSCs
Homing is the active migration of HSCs or lymphocytes from the BM or blood toward different organs, antigens, or cytokines via the vasculature. Recently, this term has also been applied to MSCs, considering their ability to migrate to and engraft in the injured tissues (16). Stress signaling from injured tissues triggers the migration of locally or systemically infused MSCs to the damaged site (17). Several molecules that are expressed on the MSC surface facilitate MSC rolling, adhesion, and migration into the tissue. Importantly, MSCs can be detected in the injured tissues after systemic transfusion. Green fluorescent protein (GFP)-labeled MSCs were detected in C-C motif ligand (CCL) 4-treated rat livers after infusion via peripheral or portal veins (18). Adhesion molecules (e.g., integrins, selectins, and endoglin) and chemokine receptors (CCR1, CCR7, and CCR9) are involved in MSC homing (19).
Hepatocyte-like differentiation of MSCs
MSCs possess multilineage differentiation potential for cells of all three germ layers. They can differentiate into hepatocyte-like cells both in vivo and in vitro in the presence of specific cytokines and growth factors {such as hepatocyte growth factor (HGF), oncostatin M, epidermal growth factor (EGF), insulin-like growth factor (IGF), fibroblast growth factor (FGF)-2/-4, and leukemia inhibitory factor] and chemical compounds [such as dexamethasone, insulin-transferrin-selenium, retinoic acid, nicotinamide, norepinephrine, sodium butyrate, and dimethyl sulfoxide (20)]}. Moreover, MSCs can also differentiate into the hepatocyte-like cells upon culturing with liver cells in prohepatogenic conditions (21) or in pellet cultures (22). Functionally transformed cells express hepatocyte nuclear factors (HNF)-3, GATA4, cytokeratin (CK) 19, transthyretin, alpha-fetoprotein, albumin, and CK18, which can be analyzed via flow cytometry, reverse transcription polymerase chain reaction, immunostaining, and western blotting (20).
Direct intrahepatic administration of human MSCs resulted in the differentiation of the majority of MSCs into hepatocyte-like cells in allyl alcohol-treated rat livers (23). Furthermore, although the MSC-derived hepatocyte-like cells are morphologically and functionally similar to hepatocytes, sufficient data suggesting that MSCs completely mimic hepatocytes in vivo are lacking. Moreover, studies have indicated that, in addition to their transdifferentiation into hepatocytes or hepatocyte-like cells, MSCs are able to secrete trophic factors that facilitate liver regeneration and strong immune suppression, which is important for engraftment (24).
Immunosuppressive potential of MSCs
MSCs can exhibit potent anti-inflammatory properties, such as downregulating immune cells and enhancing the secretion of immunomodulatory factors (25). They can directly inhibit the adaptive immune cells, can suppress B and T cell proliferation and function, induce apoptosis of T cells via programmed death 1, and upregulate regulatory T cell (Tregs) proliferation and functionality. Additionally, MSCs can control innate immunity by inhibiting monocyte differentiation, dendritic cell (DC) activation, and natural killer (NK) cell activation (25).
Furthermore, MSCs can secrete immunomodulatory factors, such as nitric oxide (NO), prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), human leukocyte antigen (HLA)-G, and IL-6 and -10 (25). Murine MSC-derived NO can inhibit T cell proliferation (26). PGE2 plays multifaceted roles in cell proliferation, apoptosis, tissue repair, angiogenesis, inflammation, immune surveillance, and cancer (27-29). It augments the synthesis of the anti-inflammatory cytokine IL-10 and decreases the production of the proinflammatory cytokines TNF-α, IFN-γ, and IL-12 by DCs and macrophages. Moreover, it suppresses the proliferation and differentiation of T cells, macrophages, and monocytes as well as the cytotoxic activity of NK cells and cytotoxic T lymphocytes (30-32). PGE2 directly inhibits the synthesis of IL-2, thereby promoting Th2 immune responses rather than Th1 responses, and induces differentiation and expansion of Treg cells (33). IDO and HLA-G are important for immune tolerance; they suppress the proliferation of B cells and effector T cells, maturation of DCs, and cytotoxicity of NK cells (34,35). MSCs can potentiate macrophage polarization and generation of tolerogenic DCs (26). IL-6 secreted by MSCs can inhibit T cell-mediated immunity by disrupting monocyte differentiation into DCs (36,37). In addition, IL-6 secreted by MSCs protects the lymphocytes and neutrophils against apoptosis (26,38). Thus, MSCs play a crucial role in immunosuppression, which makes them an attractive therapeutic candidate for hepatic fibrosis.
MSC therapy may trigger tissue regeneration, repair, and remodeling. MSCs exert their effects by secreting bioactive molecules that are responsible for tissue regeneration, repair, and angiogenesis. These soluble factors, known as trophic factors, are associated with not only regeneration but also reductions in inflammation, apoptosis, and fibrosis in the injured tissues (39). MSC-derived trophic factors, including growth factors [brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor, EGF, FGF-2/-4/-7/-9/-17, HGF, IGF-1, nerve growth factor, and platelet-derived growth factor (PDGF)], cytokines (IFN-γ, TNF-α, and IL-1α/β, -2, -6, -8, -10, -12, and -13), chemokines (various CCLs and C-X-C motif ligands), and antiapoptotic and angiogenic factors (VEGF), facilitate the regeneration of specific tissues (40).
Antifibrotic activities of MSCs
Fibrosis is a cardinal feature of chronic inflammation. Hepatic fibrosis results from chronic liver injury caused by alcohol, drugs, viral infection, and metabolic or inherited diseases. It is characterized by excessive ECM deposition; hepatic stellate cells are key fibrogenic cells in this process. Activated hepatic stellate cells stimulate the neighboring cells and initiate inflammatory responses. MSCs are effective in treating fibrosis due to their antifibrotic and immunosuppressive properties (41-44). Recently, MMPs, which can be inhibited by tissue inhibitor of MMP (TIMP), have been demonstrated to reduce liver fibrosis. MSCs can upregulate the expression of MMPs (43), which can degrade ECM, and downregulate TIMP expression (44); thus, MSCs regulate the balance between MMPs and TIMP to control ECM remodeling and reduce liver fibrosis. Additionally, MSCs can suppress the proliferation and activation of hepatic stellate cells via indirect mechanisms or direct cell–cell contact, inhibit collagen synthesis, and suppress overactive immune reactions; MMPs can breakdown the ECM, resulting in the apoptosis of hepatic stellate cells (42). Collectively, these mechanisms alleviate liver fibrosis.
Antioxidant activities of MSCs
Owing to their immunosuppressive, antifibrotic, and trophic properties, MSCs have also been evaluated for their antioxidant activity. Several studies have suggested that MSCs mediate strong antioxidant effects in various animal models (45-48). Typically, carbon tetrachloride (CCl4), tert-butyl hydroperoxide, paracetamol, alcohol, and thioacetamide (TAA) are used to induce oxidative stress in experimental animal models. In this process, reactive oxygen species (ROS), reactive nitrogen species, and free radicals act as mediators to initiate inflammation, hepatocellular damage, and fibrosis, although the small amount of ROS produced via the oxidation–reduction chain (cellular respiration) in the cell is crucial in cell signaling and homeostasis (49,50). MSCs can alleviate chemically induced (CCl4 and TAA) oxidative stress in vitro and in vivo (46,48). Transplantation of MSCs suppresses oxidative stress and enhances antioxidant activity by increasing the expression of superoxide dismutase, thereby reducing hepatocyte apoptosis (46,48). MSCs can surmount oxidative stress in not only hepatic fibrosis but also other diseases, such as dextran sulfate sodium-induced colitis and neurodegenerative diseases (e.g., Friedreich’s ataxia) (51). Collectively, these data highlight the efficacy of MSC infusion for the treatment of liver disease.
Clinical application of MSCs to treat liver diseases
According to ClinicalTrials.Gov, more than 80 clinical trials evaluating the treatment of liver disease using MSCs have been completed or are in progress. In Table 1, clinical studies, focusing on the patient group, source of MSC, injection route, and main improvements, are summarized (52-71). Despite differences in patient group, injection cell dose, MSC source, graft type, administration route, and study design, no significant side effects were observed in the reported clinical studies. Patient groups included acute-on-chronic liver failure (ACLF) and cirrhosis due to alcohol, HBV or HCV, primary biliary cholangitis (PBS), and autoimmune diseases-induced cirrhosis. In early studies, autologous bone marrow-derived MSCs have mainly been used; however, recently, umbilical cord, umbilical cord blood, and bone marrow-derived allogenic MSCs have also been used. Peripheral veins are mainly used for stem cell transplantation along with the portal vein and intrasplenic, intrahepatic, and hepatic arteries. Except for two other clinical outcomes, MSC transplantation typically resulted in improvement in liver functions, including AST, ALT, GGT, serum albumin, and bilirubin levels and histological score.
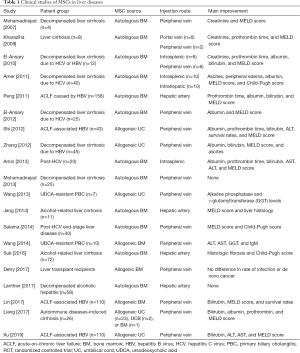
Full table
Possible risks of MSC therapy
Although MSCs have been reported to improve hepatocyte and liver function in laboratory, preclinical, and clinical trials, there are some considerations that must be noted. As described earlier, MSCs can migrate to the damaged liver and, thus, exert immunosuppressive, antifibrotic, and antioxidant effects to repair this damaged organ; however, these MSCs may exhibit fibrogenic activity. When MSCs were cocultured with the human hepatoma cell line HuH-7 in a hepatogenic differentiation medium, the MSCs expressed alpha-smooth muscle actin (α-SMA), a marker for myofibroblast differentiation. Moreover, after intrahepatic administration of MSCs into partially hepatectomized NOD/SCID mice, the MSCs expressed vimentin and α-SMA in the absence of hepatic markers (72). The transplanted MSCs exhibited very low engraftment rates in normal and acutely injured NOD/SCID mice, compared to chronically injured mice; a significant number of the MSCs injected into the site of acute liver injury exhibited a myofibroblast-like morphology (73). Collectively, these results suggest that the fibrogenic potential of MSCs could result in increased hepatic fibrosis under certain circumstances. Therefore, prior to the use of MSCs for the treatment of hepatic fibrosis, the issue of MSC-induced fibrosis needs to be evaluated in depth.
MSCs can migrate to tumors and, then, incorporate into the tumor stroma (74,75). They promote the proliferation of pre-existing tumor cells via differentiation into tumor-associated fibroblasts (TAFs) in the tumor microenvironment, inhibition of the antitumor immune response, promotion of neovascularization and tumor metastasis, and inhibition of tumor cell death (76). Transforming growth factor-β (TGF-β), commonly secreted by tumor cells, induces differentiation of MSCs into myofibroblasts, which express α-SMA, tenascin C, and fibroblast surface protein; it also increases the expression and secretion of growth-stimulating factors, such as CCL5/RANTES and stromal cell-derived factor 1 (SDF-1). Recently, researchers found that MSCs can differentiate into carcinoma-associated fibroblasts or TAFs, which express α-SMA and promote tumor growth by inducing neovascularization and expressing tumor-stimulating factors (77-81). In addition, MSCs express various antiapoptotic and prosurvival factors, including VEGF, FGF-2, PDGF, HGF, BDNF, SDF-1α, IGF-1 and -2, TGF-β, and IGF binding protein-2, through which they promote tumor growth by suppressing tumor apoptosis (40,82-86). Hypoxia, which usually occurs in tumor regions and sites of inflammation, can stimulate MSCs to produce VEGF, FGF2, HGF, IGF1, CX3CR1, and CXCR4; these factors are known to be able to protect tumor cells in tumor microenvironments (87-90).
Future prospects of MSC therapy for hepatic fibrosis
MSC priming
MSCs are known to migrate to damaged areas and express various immune cell-regulating factors, such as NO, PGE2, IDO, IL-6 and -10, and HLA-G, upon exposure to inflammatory cytokines, such as IFN-γ, TNF-α, and IL-1β (91); however, depending on the concentrations and types of inflammatory cytokines in the damaged microenvironments, MSCs may mediate myofibroblast activity (72,73). Therefore, to improve the functional activity and reduce the unwanted properties of MSCs, they can be primed with inflammatory cytokines in vitro prior to infusion. IFN-γ-primed MSCs inhibit the proliferation of activated T and NK cells by inducing IDO expression (92). In addition, HLA-A, -B, -C, and -E were elevated in IFN-γ-primed MSCs, which were less susceptible to NK cell-mediated killing and increased immunosuppression (93). Moreover, IFN-γ-primed MSCs can induce the expression of TNF-related apoptosis-inducing ligands and, thus, be used to treat cancer. Investigating the interactions of MSCs with the microenvironments of damaged areas in disease models can provide insights into the precise mechanisms underlying the therapeutic effects of MSCs, which can be applied to enhance these effects in regenerative medicine.
MSC-derived exosomes
Paracrine action is one of the key mechanisms that can be evaluated to explore the therapeutic potential of MSCs (94,95). In accordance with the effects of MSC transplantation, MSC-conditioned medium can improve liver function via paracrine factors, which comprise free soluble factors and extracellular vesicles (EVs). EVs are divided into microvesicles (0.1–1 mm in diameter) and exosomes (40–100 nm in diameter) (96,97). Exosomes originate from the inward budding of late endosomes known as multivesicular bodies; they carry various nucleic acids, lipids, and proteins. Most cells secrete EVs in response to triggers or environmental circumstances, to exchange information between the cells (98). More than 850 unique gene products and 150 miRNAs have been identified as the cargo of MSC-derived exosomes; they have been implicated, via mass spectrometry, antibody array, and microarray, in cell-to-cell communication, immune regulation, and tissue repair (99,100). Several studies have reported that MSC-derived exosomes inhibit hepatocyte epithelial-to-mesenchymal transition and collagen production (101), increase hepatocyte proliferation (102) and liver function (103), and stimulate host responses to initiate repair (104-110). Moreover, as exosomes can be more easily produced and stored than MSCs, greater quality control may be possible; exosomes can be repeatedly administered as drug; thus, they could maintain and improve their therapeutic effects more consistently over time than MSC therapy.
Genetic modification of MSCs
Despite several advantages of MSCs in treating human diseases, MSC therapy is still limited by low cell survival, engraftment, and homing efficiency to the damaged site as well as by insufficient secretion of effector molecules. To overcome these limitations, researchers have investigated genetic modifications in MSCs. Diverse prosurvival genes, such as Akt (111), heat shock protein 20 (112), SDF-1β (113), hypoxia-inducible factor-1α (114), and FGF-2 (115), have been inserted into MSCs, to prolong their survival in the target organ. Moreover, SDF-1- and CXCR4-engineered MSCs exhibited more efficient homing and engraftment in target organs, followed by enhanced regeneration of the liver, kidney, skin, and brain (116-120). To treat hepatic fibrosis, MSCs can be modified using decorin (DCN) (121), urokinase-type plasminogen activator (uPA) (122), and IL-10 (123). DCN-MSCs induce histological improvements in hepatic fibrosis and aid in the recovery of liver function in rats with TAA-induced cirrhosis via suppression of TGF-β/Smad signaling (121). MSCs expressing uPA exhibited markedly lower expression of α-SMA, TGF-β1, and collagen types I and III but increased expression of MMP-2, -3, and -9, HGF, and proliferating cell nuclear antigen; moreover, they ameliorated hepatic fibrosis (122). In addition, in liver-fibrotic rats, IL-10-MSCs improved liver histopathology and liver function but suppressed inflammation and the activation of hepatic stellate cells (123). Therefore, genetic manipulation of MSCs may greatly enhance their therapeutic functions by increasing their survival and migration to the target organs and inducing their factor expression with high therapeutic potential.
Three-dimensional (3D) culture
To increase their survival and therapeutic potential, MSCs can be cultured in 3D systems with or without biomaterial scaffolds. To date, a variety of scaffolds manufactured from natural ECM components or synthetic materials as well as decellularized organ/tissue matrices have been used to enhance the proliferation and differentiation of stem cells (124-127). Moreover, 3D spheroid MSC cultures without scaffolds have been reported to improve the differentiation efficiency of MSCs (128,129) and enhance their therapeutic potential in liver disease, peritonitis, kidney injury, and myocardial infarction (129-132). Spheroid 3D culture of MSCs increased the expression of antifibrotic factors, such as IGF-1, HGF, and IL-6; furthermore, the MSCs protected the hepatocytes injured with CCl4in vitro more effectively than 2D cultured cells. In addition, 3D spheroid-derived MSCs ameliorated hepatic fibrosis and improved liver function to a greater extent than 2D-cultured MSCs.
Conclusions
Cell-based therapies with BMCs, HSCs, hepatocytes, and MSCs are being actively used to replace liver transplantation, which is the ultimate treatment for end-stage liver disease. MSCs are being evaluated as a very suitable cell source for cell therapies that have been reported to improve the liver function. They migrate to the damaged liver tissues, differentiate into hepatocytes, reduce liver inflammatory responses and liver fibrosis, and exhibit antioxidant effects. More than 80 clinical studies on the treatment of liver disease with MSCs have been completed or are in progress; the reported clinical results suggest that MSCs are safe, have no side effects, and can improve liver function. However, despite the proven regenerative value of MSCs, their regenerative therapeutic effect is unsatisfactory. Therefore, to improve the regenerative therapeutic effects of MSCs, research on MSC-priming, MSC-derived exosomes, genetic modification, and 3D-culture methods is warranted. In addition, to improve the therapeutic efficacy of MSCs, further robust preclinical and clinical studies are necessary to standardize the optimal number of transplanted MSCs, their delivery route, and their administration frequency.
Acknowledgments
Funding: This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI17C1365), and a Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Korean government, the Ministry of Education (NRF-2017R1D1A1A02019212).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ling Lu) for the series “Stem Cell and Clinical Application” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.02.163). The series “Stem Cell and Clinical Application” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Friedenstein AJ, Deriglasova UF, Kulagina NN, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol 1974;2:83-92. [PubMed]
- Campagnoli C, Roberts IA, Kumar S, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001;98:2396-402. [Crossref] [PubMed]
- De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003;174:101-9. [Crossref] [PubMed]
- Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 2000;109:235-42. [Crossref] [PubMed]
- Jiang Y, Vaessen B, Lenvik T, et al. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol 2002;30:896-904. [Crossref] [PubMed]
- Zvaifler NJ, Marinova-Mutafchieva L, Adams G, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res 2000;2:477-88. [Crossref] [PubMed]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. [Crossref] [PubMed]
- Theise ND, Nimmakayalu M, Gardner R, et al. Liver from bone marrow in humans. Hepatology 2000;32:11-6. [Crossref] [PubMed]
- Houlihan DD, Newsome PN. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology 2008;135:438-50. [Crossref] [PubMed]
- Souza BS, Nogueira RC, de Oliveira SA, et al. Current status of stem cell therapy for liver diseases. Cell Transplant 2009;18:1261-79. [Crossref] [PubMed]
- Bird TG, Lorenzini S, Forbes SJ. Activation of stem cells in hepatic diseases. Cell Tissue Res 2008;331:283-300. [Crossref] [PubMed]
- Peggins JO, McMahon TF, Beierschmitt WP, et al. Comparison of hepatic and renal metabolism of acetaminophen in male and female miniature swine. Drug Metab Dispos 1987;15:270-3. [PubMed]
- Muraca M. Evolving concepts in cell therapy of liver disease and current clinical perspectives. Dig Liver Dis 2011;43:180-7. [Crossref] [PubMed]
- Stutchfield BM, Forbes SJ, Wigmore SJ. Prospects for stem cell transplantation in the treatment of hepatic disease. Liver Transpl 2010;16:827-36. [Crossref] [PubMed]
- Eom YW, Shim KY, Baik SK. Mesenchymal stem cell therapy for liver fibrosis. Korean J Intern Med 2015;30:580-9. [Crossref] [PubMed]
- Chapel A, Bertho JM, Bensidhoum M, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med 2003;5:1028-38. [Crossref] [PubMed]
- Sordi V, Malosio ML, Marchesi F, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 2005;106:419-27. [Crossref] [PubMed]
- Xiang GA, Zhang GQ, Fang CH, et al. Di Yi Jun Yi Da Xue Xue Bao 2005;25:994-7. [A preliminary study of the homing capacity of allograft mesenchymal stem cells to rat liver]. [PubMed]
- Brooke G, Tong H, Levesque JP, et al. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev 2008;17:929-40. [Crossref] [PubMed]
- Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest 2002;109:1291-302. [Crossref] [PubMed]
- Lange C, Bassler P, Lioznov MV, et al. Liver-specific gene expression in mesenchymal stem cells is induced by liver cells. World J Gastroenterol 2005;11:4497-504. [Crossref] [PubMed]
- Ong SY, Dai H, Leong KW. Inducing hepatic differentiation of human mesenchymal stem cells in pellet culture. Biomaterials 2006;27:4087-97. [Crossref] [PubMed]
- Jang YY, Collector MI, Baylin SB, et al. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol 2004;6:532-9. [Crossref] [PubMed]
- Xagorari A, Siotou E, Yiangou M, et al. Protective effect of mesenchymal stem cell-conditioned medium on hepatic cell apoptosis after acute liver injury. Int J Clin Exp Pathol 2013;6:831-40. [PubMed]
- De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 2012;12:574-91. [Crossref] [PubMed]
- Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 2007;109:228-34. [Crossref] [PubMed]
- Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology 2005;128:1445-61. [Crossref] [PubMed]
- Breyer RM, Bagdassarian CK, Myers SA, et al. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 2001;41:661-90. [Crossref] [PubMed]
- Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev 2007;59:207-24. [Crossref] [PubMed]
- Spaggiari GM, Abdelrazik H, Becchetti F, et al. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood 2009;113:6576-83. [Crossref] [PubMed]
- Linnemeyer PA, Pollack SB. Prostaglandin E2-induced changes in the phenotype, morphology, and lytic activity of IL-2-activated natural killer cells. J Immunol 1993;150:3747-54. [PubMed]
- Yakar I, Melamed R, Shakhar G, et al. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol 2003;10:469-79. [Crossref] [PubMed]
- Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 2003;422:897-901. [Crossref] [PubMed]
- Volarevic V, Al-Qahtani A, Arsenijevic N, et al. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity 2010;43:255-63. [Crossref] [PubMed]
- Parekkadan B, van Poll D, Suganuma K, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One 2007;2:e941. [Crossref] [PubMed]
- Djouad F, Charbonnier LM, Bouffi C, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 2007;25:2025-32. [Crossref] [PubMed]
- Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005;105:4120-6. [Crossref] [PubMed]
- Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 2009;27:1954-62. [Crossref] [PubMed]
- Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 2013;95:2196-211. [Crossref] [PubMed]
- Wang L, Wang X, Wang L, et al. Hepatic Vascular Endothelial Growth Factor Regulates Recruitment of Rat Liver Sinusoidal Endothelial Cell Progenitor Cells. Gastroenterology 2012;143:1555-63.e2. [Crossref] [PubMed]
- Higashiyama R, Inagaki Y, Hong YY, et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology 2007;45:213-22. [Crossref] [PubMed]
- Wang J, Bian C, Liao L, et al. Inhibition of hepatic stellate cells proliferation by mesenchymal stem cells and the possible mechanisms. Hepatol Res 2009;39:1219-28. [Crossref] [PubMed]
- Rabani V, Shahsavani M, Gharavi M, et al. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol Int 2010;34:601-5. [Crossref] [PubMed]
- Ali G, Mohsin S, Khan M, et al. Nitric oxide augments mesenchymal stem cell ability to repair liver fibrosis. J Transl Med 2012;10:75. [Crossref] [PubMed]
- Ayatollahi M, Hesami Z, Jamshidzadeh A, et al. Antioxidant Effects of Bone Marrow Mesenchymal Stem Cell against Carbon Tetrachloride-Induced Oxidative Damage in Rat Livers. Int J Organ Transplant Med 2014;5:166-73. [PubMed]
- Cho KA, Woo SY, Seoh JY, et al. Mesenchymal stem cells restore CCl4-induced liver injury by an antioxidative process. Cell Biol Int 2012;36:1267-74. [Crossref] [PubMed]
- Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol 2001;35:297-306. [Crossref] [PubMed]
- Quintanilha LF, Takami T, Hirose Y, et al. Canine mesenchymal stem cells show antioxidant properties against thioacetamide-induced liver injury in vitro and in vivo. Hepatol Res 2014;44:E206-17. [Crossref] [PubMed]
- Joyeux M, Rolland A, Fleurentin J, et al. tert-Butyl hydroperoxide-induced injury in isolated rat hepatocytes: a model for studying anti-hepatotoxic crude drugs. Planta Med 1990;56:171-4. [Crossref] [PubMed]
- Devasagayam TP, Tilak JC, Boloor KK, et al. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India 2004;52:794-804. [PubMed]
- Dey R, Kemp K, Gray E, et al. Human mesenchymal stem cells increase anti-oxidant defences in cells derived from patients with Friedreich's ataxia. Cerebellum 2012;11:861-71. [Crossref] [PubMed]
- Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med 2007;10:459-66. [PubMed]
- Kharaziha P, Hellstrom PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol 2009;21:1199-205. [Crossref] [PubMed]
- El-Ansary M, Mogawer S, Abdel-Aziz I, et al. Phase I Trial: Mesenchymal Stem Cells Transplantation in End Stage Liver Disease. J Am Sci 2010;6:135-44.
- Amer ME, El-Sayed SZ, El-Kheir WA, et al. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol 2011;23:936-41. [Crossref] [PubMed]
- Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology 2011;54:820-8. [Crossref] [PubMed]
- El-Ansary M, Abdel-Aziz I, Mogawer S, et al. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev Rep 2012;8:972-81. [Crossref] [PubMed]
- Shi M, Zhang Z, Xu R, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med 2012;1:725-31. [Crossref] [PubMed]
- Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol 2012;27 Suppl 2:112-20. [Crossref] [PubMed]
- Amin MA, Sabry D, Rashed LA, et al. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transplant 2013;27:607-12. [Crossref] [PubMed]
- Mohamadnejad M, Alimoghaddam K, Bagheri M, et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int 2013;33:1490-6. [PubMed]
- Wang L, Li J, Liu H, et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol 2013;28 Suppl 1:85-92. [Crossref] [PubMed]
- Jang YO, Kim YJ, Baik SK, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int 2014;34:33-41. [Crossref] [PubMed]
- Salama H, Zekri AR, Medhat E, et al. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther 2014;5:70. [Crossref] [PubMed]
- Wang L, Han Q, Chen H, et al. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev 2014;23:2482-9. [Crossref] [PubMed]
- Suk KT, Yoon JH, Kim MY, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology 2016;64:2185-97. [Crossref] [PubMed]
- Detry O, Vandermeulen M, Delbouille MH, et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: A phase I-II, open-label, clinical study. J Hepatol 2017;67:47-55. [Crossref] [PubMed]
- Lanthier N, Lin-Marq N, Rubbia-Brandt L, et al. Autologous bone marrow-derived cell transplantation in decompensated alcoholic liver disease: what is the impact on liver histology and gene expression patterns? Stem Cell Res Ther 2017;8:88. [Crossref] [PubMed]
- Lin BL, Chen JF, Qiu WH, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology 2017;66:209-19. [Crossref] [PubMed]
- Liang J, Zhang H, Zhao C, et al. Effects of allogeneic mesenchymal stem cell transplantation in the treatment of liver cirrhosis caused by autoimmune diseases. Int J Rheum Dis 2017;20:1219-26. [Crossref] [PubMed]
- Xu WX, He HL, Pan SW, et al. Combination Treatments of Plasma Exchange and Umbilical Cord-Derived Mesenchymal Stem Cell Transplantation for Patients with Hepatitis B Virus-Related Acute-on-Chronic Liver Failure: A Clinical Trial in China. Stem Cells Int 2019;2019:4130757.
- Baertschiger RM, Serre-Beinier V, Morel P, et al. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One 2009;4:e6657. [Crossref] [PubMed]
- di Bonzo LV, Ferrero I, Cravanzola C, et al. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut 2008;57:223-31. [Crossref] [PubMed]
- Kidd S, Spaeth E, Dembinski JL, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 2009;27:2614-23. [Crossref] [PubMed]
- Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 2004;96:1593-603. [Crossref] [PubMed]
- Rhee KJ, Lee JI, Eom YW. Mesenchymal Stem Cell-Mediated Effects of Tumor Support or Suppression. Int J Mol Sci 2015;16:30015-33. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007;449:557-63. [Crossref] [PubMed]
- Sappino AP, Skalli O, Jackson B, et al. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer 1988;41:707-12. [Crossref] [PubMed]
- Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005;121:335-48. [Crossref] [PubMed]
- Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410:50-6. [Crossref] [PubMed]
- Rizvi AZ, Swain JR, Davies PS, et al. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci U S A 2006;103:6321-5. [Crossref] [PubMed]
- Schichor C, Albrecht V, Korte B, et al. Mesenchymal stem cells and glioma cells form a structural as well as a functional syncytium in vitro. Exp Neurol 2012;234:208-19. [Crossref] [PubMed]
- Xu MH, Gao X, Luo D, et al. EMT and acquisition of stem cell-like properties are involved in spontaneous formation of tumorigenic hybrids between lung cancer and bone marrow-derived mesenchymal stem cells. PLoS One 2014;9:e87893. [Crossref] [PubMed]
- Li H, Feng Z, Tsang TC, et al. Fusion of HepG2 cells with mesenchymal stem cells increases cancerassociated and malignant properties: an in vivo metastasis model. Oncol Rep 2014;32:539-47. [Crossref] [PubMed]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007;445:111-5. [Crossref] [PubMed]
- Hung SC, Pochampally RR, Chen SC, et al. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 2007;25:2363-70. [Crossref] [PubMed]
- Hung SC, Pochampally RR, Hsu SC, et al. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One 2007;2:e416. [Crossref] [PubMed]
- Dias S, Shmelkov SV, Lam G, et al. VEGF(165) promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood 2002;99:2532-40. [Crossref] [PubMed]
- Crisostomo PR, Wang Y, Markel TA, et al. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. American Journal of Physiology-Cell Physiology 2008;294:C675-82. [Crossref] [PubMed]
- Sharma RR, Pollock K, Hubel A, et al. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion 2014;54:1418-37. [Crossref] [PubMed]
- Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006;24:386-98. [Crossref] [PubMed]
- Giuliani M, Bennaceur-Griscelli A, Nanbakhsh A, et al. TLR ligands stimulation protects MSC from NK killing. Stem Cells 2014;32:290-300. [Crossref] [PubMed]
- Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005;11:367-8. [Crossref] [PubMed]
- Gnecchi M, Danieli P, Malpasso G, et al. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol Biol 2016;1416:123-46. [Crossref] [PubMed]
- Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun 2015;6:8472. [Crossref] [PubMed]
- Wen D, Peng Y, Liu D, et al. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. Journal of Controlled Release 2016;238:166-75. [Crossref] [PubMed]
- Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. Taylor & Francis; 2014.
- Lai RC, Tan SS, Teh BJ, et al. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int J Proteomics 2012;2012:971907.
- Chen TS, Lai RC, Lee MM, et al. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Research 2010;38:215-24. [Crossref] [PubMed]
- Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 2013;22:845-54. [Crossref] [PubMed]
- Tan CY, Lai RC, Wong W, et al. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther 2014;5:76. [Crossref] [PubMed]
- Chen L, Xiang B, Wang X, et al. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther 2017;8:9. [Crossref] [PubMed]
- Zagoura DS, Roubelakis MG, Bitsika V, et al. Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut 2012;61:894-906. [Crossref] [PubMed]
- Kanazawa H, Fujimoto Y, Teratani T, et al. Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One 2011;6:e19195. [Crossref] [PubMed]
- Yan Y, Xu W, Qian H, et al. Mesenchymal stem cells from human umbilical cords ameliorate mouse hepatic injury in vivo. Liver Int 2009;29:356-65. [Crossref] [PubMed]
- Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 2013;10:301-12. [Crossref] [PubMed]
- Lin KC, Yip HK, Shao PL, et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol 2016;216:173-85. [Crossref] [PubMed]
- Kim DK, Nishida H, An SY, et al. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A 2016;113:170-5. [Crossref] [PubMed]
- Zhang B, Shi Y, Gong A, et al. HucMSC Exosome-Delivered 14-3-3zeta Orchestrates Self-Control of the Wnt Response via Modulation of YAP During Cutaneous Regeneration. Stem Cells 2016;34:2485-500. [Crossref] [PubMed]
- Mirotsou M, Zhang Z, Deb A, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A 2007;104:1643-8. [Crossref] [PubMed]
- Wang X, Zhao T, Huang W, et al. Hsp20‐engineered mesenchymal stem cells Are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem cells 2009;27:3021-31. [PubMed]
- Herberg S, Shi X, Johnson MH, et al. Stromal cell-derived factor-1β mediates cell survival through enhancing autophagy in bone marrow-derived mesenchymal stem cells. PLoS One 2013;8:e58207. [Crossref] [PubMed]
- Semenza GL. Hypoxia-inducible factor 1: control of oxygen homeostasis in health and disease. Pediatr Res 2001;49:614-7. [Crossref] [PubMed]
- Song H, Kwon K, Lim S, et al. Transfection of mesenchymal stem cells with the FGF-2 gene improves their survival under hypoxic conditions. Molecules & Cells (Springer Science & Business Media BV) 2005;19.
- Du Z, Wei C, Yan J, et al. Mesenchymal stem cells overexpressing C-X-C chemokine receptor type 4 improve early liver regeneration of small-for-size liver grafts. Liver Transpl 2013;19:215-25. [Crossref] [PubMed]
- Liu N, Patzak A, Zhang J. CXCR4-overexpressing bone marrow-derived mesenchymal stem cells improve repair of acute kidney injury. Am J Physiol Renal Physiol 2013;305:F1064-73. [Crossref] [PubMed]
- Yang D, Sun S, Wang Z, et al. Stromal cell-derived factor-1 receptor CXCR4-overexpressing bone marrow mesenchymal stem cells accelerate wound healing by migrating into skin injury areas. Cellular Reprogramming (Formerly" Cloning and Stem Cells") 2013;15:206-15.
- Nakamura Y, Ishikawa H, Kawai K, et al. Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell-derived factor-1. Biomaterials 2013;34:9393-400. [Crossref] [PubMed]
- Yu X, Chen D, Zhang Y, et al. Overexpression of CXCR4 in mesenchymal stem cells promotes migration, neuroprotection and angiogenesis in a rat model of stroke. J Neurol Sci 2012;316:141-9. [Crossref] [PubMed]
- Jang YO, Cho MY, Yun CO, et al. Effect of Function-Enhanced Mesenchymal Stem Cells Infected With Decorin-Expressing Adenovirus on Hepatic Fibrosis. Stem Cells Transl Med 2016;5:1247-56. [Crossref] [PubMed]
- Sun C, Li DG, Chen YW, et al. Transplantation of urokinase-type plasminogen activator gene-modified bone marrow-derived liver stem cells reduces liver fibrosis in rats. J Gene Med 2008;10:855-66. [Crossref] [PubMed]
- Lan L, Chen Y, Sun C, et al. Transplantation of bone marrow-derived hepatocyte stem cells transduced with adenovirus-mediated IL-10 gene reverses liver fibrosis in rats. Transpl Int 2008;21:581-92. [Crossref] [PubMed]
- Ji R, Zhang N, You N, et al. The differentiation of MSCs into functional hepatocyte-like cells in a liver biomatrix scaffold and their transplantation into liver-fibrotic mice. Biomaterials 2012;33:8995-9008. [Crossref] [PubMed]
- Piryaei A, Valojerdi MR, Shahsavani M, et al. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Rev Rep 2011;7:103-18. [Crossref] [PubMed]
- Tian H, Bharadwaj S, Liu Y, et al. Myogenic differentiation of human bone marrow mesenchymal stem cells on a 3D nano fibrous scaffold for bladder tissue engineering. Biomaterials 2010;31:870-7. [Crossref] [PubMed]
- Kazemnejad S, Allameh A, Soleimani M, et al. Biochemical and molecular characterization of hepatocyte‐like cells derived from human bone marrow mesenchymal stem cells on a novel three‐dimensional biocompatible nanofibrous scaffold. Journal of gastroenterology and hepatology 2009;24:278-87. [Crossref] [PubMed]
- Arufe MC, De la Fuente A, Fuentes‐Boquete I, et al. Differentiation of synovial CD‐105+ human mesenchymal stem cells into chondrocyte‐like cells through spheroid formation. Journal of cellular biochemistry 2009;108:145-55. [Crossref] [PubMed]
- Zhang X, Hu MG, Pan K, et al. 3D Spheroid Culture Enhances the Expression of Antifibrotic Factors in Human Adipose-Derived MSCs and Improves Their Therapeutic Effects on Hepatic Fibrosis. Stem Cells Int 2016;2016:4626073.
- Bartosh TJ, Ylöstalo JH, Mohammadipoor A, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A 2010;107:13724-9. [Crossref] [PubMed]
- Zhao X, Qiu X, Zhang Y, et al. Three-Dimensional Aggregates Enhance the Therapeutic Effects of Adipose Mesenchymal Stem Cells for Ischemia-Reperfusion Induced Kidney Injury in Rats. Stem Cells Int 2016;2016:9062638.
- Wang CC, Chen CH, Hwang SM, et al. Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells 2009;27:724-32. [Crossref] [PubMed]


