Outcomes with percutaneous mitral repair vs. optimal medical treatment for functional mitral regurgitation: systematic review
Mitral regurgitation (MR) is an increasingly prevalent valve disease. Severe MR is associated with progressive dilation of the left ventricle (LV) and the onset of heart failure (HF). Patients with symptoms present an annual mortality rate >5% without any intervention (1,2). The treatment of MR varies depending on the patho-physiological mechanism.
In primary or degenerative MR one of the components of the mitral apparatus (leaflets, chords or papillary muscles) is affected and valve repair or replacement is recommended when there are symptoms, ventricular dilation, pulmonary hypertension or atrial fibrillation (3,4).
In secondary or functional MR, the components of the mitral apparatus are intact; however, there is a lack of coaptation of the leaflets due to ventricular or annulus dilation. Patients with functional MR usually present LV dysfunction, and most of them undergo medical treatment. Mitral valve surgery could be considered when concomitant coronary artery bypass graft is required (3-5).
Transcatheter mitral valve repair technique has broadened the therapeutic range in the treatment of severe MR. The MitraClip system (Abbott; Menlo Park, California, USA) is a therapeutic option for patients with severe MR with high surgical risk (4,5). Treatment of patients with severe primary or degenerative MR with MitraClip has been shown to be safe and effective (6). Transcatheter mitral valve repair showed a reduction in the severity degree of MR and the improvement of functional class and quality of life (7,8).
While initial outcomes with MitraClip occurred in the field of primary or degenerative MR; most patients treated in registries had functional MR. In this group an improvement in functional class was observed in more than 75% of the cases (9-12), Therefore, transcatheter mitral valve repair along optimal medical treatment has been compared with only optimal medical therapy in two different randomized trials. In the COAPT trial (13), the MitraClip group showed a significant decrease in mortality and HF hospitalizations. In the MITRA-FR trial (14), no significant differences were observed between both groups.
The aim of this paper is (I) to review the concept, diagnosis and treatment options of the functional MR and (II) to know the clinical outcomes of the MitraClip versus medical treatment to treat functional MR.
Search strategy
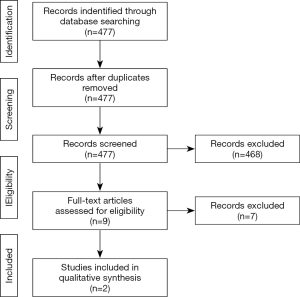
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (15) (Figure 1). The Medline through PubMed database was used to search. The present review included manuscripts published between January 2009 and September 2019. It was performed using the following search series: (“Transcatheter mitral valve repair” OR “MitraClip” OR “Percutaneous mitral repair” OR “Transcatheter mitral repair”) AND (“mitral regurgitation medical treatment” OR “functional mitral regurgitation medical treatment” OR “functional mitral regurgitation optimal medical therapy”).
Study selection
Two authors independently screened titles and abstracts of all publications (477 manuscripts), and performed the selection of studies and data extraction. In the case of disagreements, consensus meetings reached the final decision. Inclusion criteria were (I) randomized controlled trials and (II) works must compare percutaneous treatment versus optimal medical management. The exclusion criteria of the study included language other than English, duplicates, letters to editor and case reports (Figure 1).
We also reviewed the concept of functional MR, assessment of the degree, prognosis, therapy options, and finally, the most important clinical evidence of the MitraClip in functional MR.
Functional MR
Definition, mechanisms and prevalence
Functional MR could be defined as a ventricular disease where mitral valve is structurally normal, left chambers are enlarged and mitral annulus is dilated with lack of coaptation of the leaflets. The LV dysfunction and dilation lead to the displacement of the papillary muscles towards posterior and apical, modifying ventricular geometry and causing failure of coaptation of the leaflets (16). Other uncommon mechanisms of functional MR are LV dyssynchrony (17), and left atrial enlargement with annulus dilation due to atrial fibrillation (18).
Functional MR can be classified regarding the aetiology in ischaemic or non-ischaemic. The ischaemic aetiology is the most common. Non-ischaemic functional MR may be caused by different diseases: long-duration hypertension; idiopathic dilated cardiomyopathy; and myocarditis (16). Functional MR increases the preload, the stress of the LV wall and the LV workload, what contributes to a progressive HF situation within a vicious circle. Notably, the functional MR presents a dynamic nature (19).
The prevalence of moderate-to-severe functional MR varies from 6–29% in patients with diagnosis of chronic HF, increasing up to 75% in hospitalized patients due to acute HF (20).
Assessment of the severity degree of functional MR
The gold standard approach for the diagnosis of functional MR is the echocardiography. There are several echocardiographic parameters which are recommended to assess the severity degree of functional MR (4,5).
The European Society of Cardiology (ESC) guidelines define the functional MR as severe with an effective regurgitant orifice area (EROA) ≥20 mm2, and a regurgitant volume ≥30 mL (4). The American College of Cardiology/American Heart Association (ACC/AHA) guidelines define the functional MR as severe with an EROA ≥40 mm2, and a regurgitant volume ≥60 mL (5).
These differences between ESC and ACC/AHA guidelines show that the evaluation of functional MR is challenging. However, functional MR is a dynamic condition and its severity degree may change depending on the loading conditions and the phase of cardiac cycle. Thus, it is recommended to assess the severity degree of the functional MR after the optimization of medical treatment (3).
Prognostic of functional MR
Several studies have shown that functional MR present a strong negative impact on the prognosis of patients with HF in relation to the severity degree (20-23). In a meta-analysis carried out by Sannino (22), which included 53 studies and 45,900 patients with and without functional MR; functional MR was associated with an increased risk of hospitalization due to HF (RR: 2.26; 95% CI: 1.92–2.67; P<0.001); cardiac mortality (RR: 2.62; 95% CI: 1.87–3.69; P<0.001); and all-cause mortality (RR: 1.97; 95% CI: 1.71–2.27; P<0.001) (22).
Therapy options
Optimal medical treatment and cardiac resynchronization
The medical therapy for patients with functional MR is the same that the guideline-directed treatment for patients with chronic HF. The optimal medical therapy which is recommended for patients with reduced LV ejection fraction and NYHA class ≥ II includes: beta-blockers; angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; or angiotensin receptor–neprilysin inhibitor; mineralocorticoid receptor antagonists; and diuretics (24). The optimal medical treatment may promote LV reverse remodelling and improve the degree of functional MR (24).
Several studies have reported the role of cardiac resynchronization in reducing functional MR in patients with left ventricular dysfunction, wide QRS and HF symptoms at mid-term of follow-up (25,26). Thus, cardiac resynchronization is recommended by current guidelines in patients with LV dysfunction (≤35%); HF symptoms (NYHA class II–IV) despite optimal medical treatment; and a wide QRS complex on the electrocardiogram.
Cardiac resynchronization has shown to improve LV geometry and the degree of functional MR (27,28). Notably, three independent predictors of functional MR reduction after cardiac resynchronization could be highlighted: (I) an end-systolic dimension index <29 mm/m2; (II) the absence of scar at the papillary muscle insertion; and (III) anteroseptal to posterior wall radial strain dyssynchrony >200 ms (29).
Surgical treatment
Despite the increased risk, patients with severe functional MR, that undergo coronary artery bypass graft, seem to benefit from mitral valve surgery (4,5). A randomized trial, which included 301 patients, showed that patients with functional MR, who underwent coronary revascularization surgery and mitral valve repair, presented an increased complication rate. There were no differences in LV reverse remodelling and mortality rate at 2 years follow-up (30). The role of mitral valve surgery for the treatment of isolated severe functional MR remains unclear. In this context, ESC guidelines suggest mitral valve surgery in patients with relevant HF symptoms despite optimal medical therapy, LV ejection fraction over 30%, and low co-morbidities (4).
Transcatheter mitral valve intervention
Transcatheter edge-to-edge mitral valve repair
MitraClip system
The MitraClip (Abbott Vascular, Santa Clara, CA, USA) is a percutaneous device, which reduces the severity degree of MR by transcatheter approximation of the anterior and posterior mitral valve leaflets leading to a double-orifice valve similar to the Alfieri technique (6,7).
The MitraClip system consists of a steerable guide catheter and a MitraClip attached to the clip delivery system (Figure 2). The 24-Fr steerable guide catheter allows the introduction of the MitraClip delivery system, which is advanced through the guide into the left atrium via transeptal. A stabilizer keeps the system in the right position. The MitraClip consists of a cobalt-chromium clip with 2 arms covered by polyester (Figure 2). The tip of the guide catheter has a radiopaque marker. The steerable properties of the guide catheter and the MitraClip delivery system allow the precise orientation and positioning of the device. The delivery system is advanced to the point of maximum regurgitation guided by echocardiographic and fluoroscopic control (6,7). The arms can be opened and closed by a control mechanism on the MitraClip delivery system handle. On the inner of the arms are 2 grippers that help secure the leaflets. The use of the 3-dimensional transesophageal echocardiography in real-time is essential to guide the procedure, allowing to attempt the treatment of morphologically complex valves. Each leaflet is grasped between an arm and a gripper. When both leaflets are into the arms of the system, confirmed by transesophageal echocardiography, the MitraClip can be locked in the final position and released it if the result is adequate. Otherwise, the system can be re-opened and repositioned for a new attempt. The implant is performed in the cath lab with general anesthesia (6,31) (Figure 3).
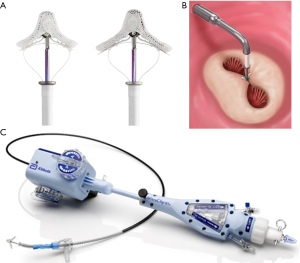
Last version of MitraClip is the XTR system (Figure 2). Compared with the first generation and the NT system, the MitraClip XTR has longer arms and grippers, which are designed to facilitate leaflet grasping in mitral valves with large coaptation gaps. In addition, the clip delivery system has been improved regarding navigation and clip positioning.
Transcatheter edge-to-edge mitral valve repair with MitraClip system has shown to be safe and effective for high-risk surgical patients with severe and symptomatic degenerative MR (32). There is scarce data regarding its use in functional MR. The ESC guidelines propose the use of the MitraClip only for symptomatic patients with severe functional MR despite optimal medical treatment (including cardiac resynchronization) and high surgical risk (4). The ACC/AHA guidelines do not report any indication for the MitraClip in patients with functional MR (5). The MitraClip system is contraindicated in patients who cannot undergo procedural anticoagulation or post procedural antiplatelet regimen; rheumatic mitral valve disease; active endocarditis; or evidence of thrombus.
PASCAL system
The PASCAL system (Edwards Lifesciences, Irvine, CA, USA) is a transcatheter mitral valve edge-to-edge repair device based on the tissue approximation with an anatomic spacer. This device consists of two wide and curved arms; two clips with capability of independent leaflet capture; and a nitinol woven spacer to optimize leaflet capture that leads to decrease the stress on the native mitral valve leaflets (33).
The CLASP Study (33), included 62 patients with MR that underwent transcatheter mitral valve repair with the PASCAL system. The successful implantation of the PASCAL device was achieved in 95% of patients. Major adverse events rate was 6.5% and all-cause mortality rate was 1.6% at 30 days follow-up. The PASCAL device showed to be feasible in decreasing the severity degree of MR; improving functional class, exercise capacity, and quality of life at 30 days.
Transcatheter direct annuloplasty mitral valve repair
Cardioband system
The Cardioband system (Edwards Lifesciences, Irvine, CA, USA) is a percutaneous mitral valve repair system, which most resembles a surgical annuloplasty ring. By interatrial approach, this device is implanted directly at the atrial side of the mitral annulus. First anchor is released in the lateral mitral commissure and additional anchors are released at short intervals until last one, which is implanted in the medial mitral commissure. Finally, the device is contract in order to remodel the mitral annulus with the aim of decreasing MR (34,35).
Messika-Zeitoun et al. (36), conducted a study including 60 patients undergoing Cardioband procedure, reporting a procedural success of 68% and a survival free of readmission for HF of 66% at 1-year follow-up.
Transcatheter indirect annuloplasty mitral valve repair
Carillon device
The Carillon Mitral Contour System (Cardiac Dimensions) consists of a distal and proximal anchor with a fixed length nitinol system that is released into the coronary sinus by a delivery system through the right external jugular vein. The distal anchor is released deep in the coronary sinus encircling the mitral annulus, and then traction is applied to constrict the coronary sinus and modify the annulus and reduce the MR (37).
The AMADEUS and TITAN trials showed safety and feasibility of Carillon device (38-40). The outcomes of the REDUCE-FMR trial at 12-month follow-up, have been presented at TCT congress 2019. The REDUCE-FMR trial randomized 120 patients, with dilated cardiomyopathy and moderate-to-severe functional MR, to Carillon implantation (87 patients) vs. sham control (33 patients). The primary efficacy endpoint of decrease in regurgitant volume was −7.1 vs. 3.3 mL (P=0.03) at 1 year follow-up. The mean change in LV end-diastolic volume was −10.4 vs. 6.5 mL (P<0.05). No differences in HF hospitalizations were found at 1-year follow-up.
ARTO system
The ARTO system (MVRx Inc., Belmont, CA, USA) consists of an interatrial septal anchor connected to a coronary sinus T-bar by a polyethylene suture, which is tensioned to decrease the anteroposterior diameter of the mitral annulus. The MAVERIC trial (phase I) (41), which included 11 patients undergoing ARTO implantation, showed safety of the device, with reduction of MR, LV size, and improvement in NYHA functional class at 30-day follow-up. Two-year follow-up showed stable safety and efficacy compared to 30-day findings.
Clinical evidence on MitraClip
The Endovascular Valve Edge-to-Edge Repair Study (EVEREST) II was the first trial of the MitraClip therapy, including 279 patients randomized to MitraClip therapy or surgical treatment (6). The MitraClip procedure showed to be safer and reported similar improvements in clinical outcomes (6). Notably, patients included in EVEREST II were low-risk surgical patients mainly affected by degenerative MR (73.4%) (6).
There are available data from several multicenter registries, mostly including patients with functional MR (7,10,12,42,43). MitraClip observational studies showed that transcatheter mitral valve repair is a safe procedure with low complication rates; effectively in reducing MR; and improves symptomatology and the quality of life (7,10,42,43). The outcomes of these registries suggest that MitraClip may improve prognosis. However, LV geometry and dysfunction, high levels of natriuretic peptides, and the impairment on NYHA class may suggest a worse prognosis in patients undergoing MitraClip.
MITRA-FR and COAPT trials
Only these two trials met the inclusion criteria of our systematic review. These trials were carried out to investigate the role of the transcatheter mitral valve repair in patients with functional MR and HF symptoms (class II-IV NYHA) despite optimal medical treatment (Figure 1) (13,14).
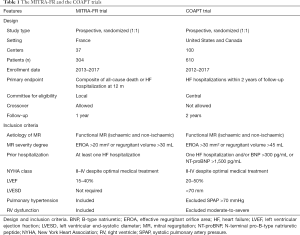
The MITRA-FR trial (Percutaneous Repair with the Mitra-Clip Device for Severe Functional/Secondary Mitral Regurgitation) (14), included 304 patients, all of them with chronic HF, left ventricular dysfunction, and severe functional MR, that were randomized to MitraClip therapy or only medical treatment. The MITRA-FR trial was major conducted by researchers in France (Table 1). Both groups show similar risk of hospitalization due to HF and risk of death at 12 months of follow-up. All all-cause mortality showed a hazard ratio of 1.11 (95% CI: 0.69–1.77); and hospitalization due to HF showed a hazard ratio of 1.13 (95% CI: 0.81–1.56). Despite the MitraClip group did not decrease LV volumes at 1-year follow-up; this group of patients showed a decrease in the degree of regurgitant volume at short-term (14).
Full table
The COAPT trial (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) (13), included 614 patients with chronic HF, left ventricular dysfunction, and moderate-to-severe or severe functional MR, that were randomized to transcatheter mitral valve repair or only medical treatment. The COAPT trial was carried out principally in the US and Canada (Table 1). The MitraClip group showed a lower risk of hospitalization due to HF, and a lower risk of death for any cause at 2 years of follow-up. The annualized rate of hospitalizations for HF showed a hazard ratio of 0.53 (95% CI: 0.40–0.70; P<0.001) at 2 years. All-cause mortality showed a hazard ratio of 0.62 (95% CI: 0.46–0.82; P<0.001) at 2 years. The MitraClip group showed a significant decreased of LV volumes at 1-year follow-up, in contrast with the outcomes of the MITRA-FR trial (13).
The outcomes at 3-year follow-up of the COAPT trial were presented at TCT congress 2019. The MitraClip showed to be safe, provided durable reduction in MR, reduced the rate of HF hospitalizations, and improved survival, quality of life and functional capacity compared to optimal medical treatment alone. In addition, patients assigned to only optimal medical treatment who crossed-over and received a MitraClip experienced fewer HF hospitalizations and deaths or HF hospitalizations within 12 months than those who did not crossover, with rates comparable to patients originally assigned to the MitraClip.
Differences between the MITRA-FR and COAPT trials
Inclusion criteria and baseline characteristics (Tables 1,2)
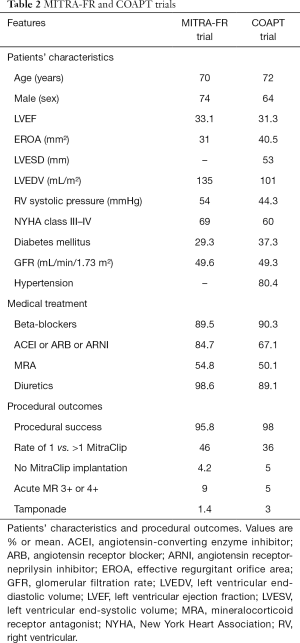
Full table
The EROA and regurgitant volume were different between both trials, lower in MITRA-FR (EROA >20 mm2) compared to COAPT (EROA >30 mm2) (13,14). In the MITRA-FR, it was required at least one hospitalization due to HF in the previous 12 months for randomization. Nevertheless, the COAPT trial did not require a recent hospitalization (13,14). However, in the COAPT trial (13), a patient could be included if BNP ≥300 pg/mL or NT-proBNT ≥1,500 pg/mL. The MITRA-FR had no restrictions on LV dimensions (14). The COAPT required a LV function between 20–50% with LV end-systolic diameter <70 mm (13). In the COAPT trial, moderate-to-severe right ventricular dysfunction and/or severe pulmonary hypertension were exclusion criteria (in contrast to MITRA-FR).
Thus, the patients included in the MITRA-FR compare to the COAPT had less severity of functional MR (EROA: 31 vs. 41 mm2), more LV dilation (135 vs. 101 mL/m2), more advanced functional class (NYHA class III/IV: 69% vs. 60%), and higher right ventricular systolic pressure (54 vs. 44 mmHg) (13,14).
Medical treatment (Table 2)
In the COAPT trial, the optimal medical treatment was centrally assessed and only patients with maximum tolerated doses could be randomized (13). In the MITRA-FR trial, patients were evaluated by the local heart team with the lack of a central assessment to optimized medical treatment. Notably, changes in medical therapy in order to up-titrate were reported at follow-up in the COAPT trial (13). In the MITRA-FR this information has not been yet reported. In the MITRA-FR compare to COAPT, there was a higher rate of renin-angiotensin-aldosterone system blockers (84.7% vs. 67.1%) (13,14). In the COAPT trial, a higher use of these drugs was reported in the MitraClip group (71.5 vs. 62.8%) (13). In addition, there was an up-titration of medical treatment during the follow-up in the MitraClip group of the COAPT trial (13).
Transcatheter mitral valve repair procedure (Table 2)
The MitraClip procedure showed to be safe in both trials with low complication rates (13,14). In both trials, the MitraClip procedure was successful (91% in the MITRA-FR; and 95% in the COAPT). Regarding the rate of 1 vs. more than 1 MitraClip implanted was 46% in the MITRAFR vs. 36% in the COAPT (13,14). The proportion of patients with residual MR (3+ or 4+) immediately after procedure was less than 10% in both trials (13,14). The differences between ESC and ACC/AHA guidelines, related to the severity degree of MR and the missing echocardiographic information at 1-year follow-up in the MITRA-FR trial, did not allow performing reliable comparisons between trials regarding residual MR (4,5,14).
Results from the MITRA-FR and COAPT trials
First, primary efficacy endpoints differ between both trials (Table 1). The MITRA-FR used the composite of death and HF hospitalization at 1-year follow-up (14). The COAPT used a single endpoint of HF hospitalization at 2 years of follow-up (13). Notably, the COAPT trial was powered for secondary endpoints: mortality; and a composite of mortality + HF admissions at 1 and 2 years (13).
In the MITRA-FR trial, the mortality rates in the MitraClip group vs. the control group at 1-year follow-up were 24.3% vs. 22.4%. In the COAPT trial, the mortality rates in the MitraClip group vs. the control group at 1-year follow-up were 18.8% vs. 23.2%. The mortality rates at 2 years follow-up in the COAPT trial were 29% in the MitraClip group vs. 46% in the control group, HR: 0.62, P<0.001). Data about mortality rates at 2 years follow-up are not yet available for the MITRA-FR trial.
Role of imaging techniques in MR
The most widely used imaging technique to measure EROA, left ventricular volume, and LV function in patients with MR is the 2-dimensional echocardiography. However, the EROA may be overestimated by the proximal isovelocity hemispheric surface area (PISA) (3), Although 2-dimensional echocardiography is the most widely used imaging technique, it may underestimate LV volume; therefore, the use of ultrasound contrast could improve the accuracy of measurement of the LV volume and the endocardial borders (44,45).
Three-dimensional echocardiography is currently recommended for the measurement of the LV volume when there is a correct visualization of the endocardial borders. The use of the 3-dimensional transesophageal echocardiography in real-time is essential to guide the transcatheter mitral valve repair procedure, allowing to attempt the treatment of morphologically complex valves (6,7).
Conclusions
Some interesting characteristics should be highlighted. In the COAPT trial, the sample size was about 2-fold larger than in the MITRA-FR; the inclusion criteria were more demanding with the requirement of an optimal medical therapy prior to randomization. However, the outcomes of the MITRA-FR and COAPT trials should be considered as complementary rather than contradictory. Before transcatheter mitral valve repair, it is necessary to assess the maximal optimization of medical treatment. The evaluation for the heart valve team, including specialists in HF, is essential in the decision-making process and the optimal management of the patient.
Transcatheter mitral valve repair with MitraClip seems to be safe and durable in patients with HF and moderate-to-severe functional MR who remained symptomatic despite maximally-tolerated optimal medical treatment. The MitraClip seems to reduce the rate of HF hospitalizations; and improve survival, quality of life and functional capacity in comparison to isolated optimal medical therapy. However, new medical treatments should be assessed and compared with invasive mitral valve procedures.
Notably, functional MR presents an active role in the progression of the cardiomyopathy. Thus, transcatheter mitral valve repair with MitraClip in selected patients, along the optimal medical therapy, may be able to break the mechanism, which leads to the end-stage disease in patients with chronic HF. In order to solve the doubts that may have generated the discrepancies between the COAPT and MITRA-FR trials, it is required better selecting the responders to functional MR correction; evaluating other percutaneous procedures (alone or in combination); as well as new medical therapies and comparing them with interventional procedures. Furthermore, large randomized trials with longer follow-up should be carried out to clarify the role of the transcatheter mitral valve repair in terms of prognosis in patients with HF.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Daniel Hernández-Vaquero) for the series “Structural Heart Disease: The Revolution” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.202). The series “Structural Heart Disease: The Revolution” was commissioned by the editorial office without any funding or sponsorship. DHV served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Translational Medicine from Aug 2019 to Jul 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Trichon BH, Felker GM, Shaw LK, et al. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol 2003;91:538-43. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395-406. [Crossref] [PubMed]
- Glower DD, Kar S, Trento A, et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II study. J Am Coll Cardiol 2014;64:172-81. [Crossref] [PubMed]
- Feldman T, Kar S, Elmariah S, et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol 2015;66:2844-54. [Crossref] [PubMed]
- Baldus S, Schillinger W, Franzen O, et al. MitraClip therapy in daily clinical practice: initial results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail 2012;14:1050-5. [Crossref] [PubMed]
- Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol 2013;62:1052-61. [Crossref] [PubMed]
- Grasso C, Capodanno D, Scandura S, et al. One- and twelve-month safety andefficacy outcomes of patients undergoing edge-to-edge percutaneous mitral valve repair (from the GRASP Registry). Am J Cardiol 2013;111:1482-7. [Crossref] [PubMed]
- Nickenig G, Estevez-Loureiro R, Franzen O, et al. Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011-2012 Pilot European Sentinel Registry. J Am Coll Cardiol 2014;64:875-84. [Crossref] [PubMed]
- Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379:2307-18. [Crossref] [PubMed]
- Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018;379:2297-306. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [Crossref] [PubMed]
- Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231-48. [Crossref] [PubMed]
- Spartera M, Galderisi M, Mele D, et al. Role of cardiac dyssynchrony and resynchronization therapy in functional mitral regurgitation. Eur Heart J Cardiovasc Imaging 2016;17:471-80. [Crossref] [PubMed]
- Delgado V, Bax JJ. Atrial functional mitral regurgitation: frommitral annulus dilatation to insufficient leaflet remodeling. Circ Cardiovasc Imaging 2017;10:e006239. [Crossref] [PubMed]
- Senni M, Adamo M, Metra M, et al. Treatment of functional mitral regurgitation in chronic heart failure: can we get a 'proof of concept' from the MITRA-FR and COAPT trials? Eur J Heart Fail 2019;21:852-61. [Crossref] [PubMed]
- Rossi A, Dini FL, Faggiano P, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011;97:1675-80. [Crossref] [PubMed]
- Kajimoto K, Sato N, Takano T, et al. Functional mitral regurgitation at discharge and outcomes in patients hospitalized for acute decompensated heart failure with a preserved or reduced ejection fraction. Eur J Heart Fail 2016;18:1051-9. [Crossref] [PubMed]
- Sannino A, Smith RL, Schiattarella GG, et al. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: a systematic review and meta-analysis. JAMA Cardiol 2017;2:1130-9. [Crossref] [PubMed]
- Goliasch G, Bartko PE, Pavo N, et al. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur Heart J 2018;39:39-46. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- St John Sutton M, Ghio S, Plappert T, et al. Cardiac resynchronization induces major structural and functional reverse remodeling in patients with New York Heart Association class I/II heart failure. Circulation 2009;120:1858-65. [Crossref] [PubMed]
- St John Sutton MG, Plappert T, Abraham WT, et al. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 2003;107:1985-90. [Crossref] [PubMed]
- van Bommel RJ, Marsan NA, Delgado V, et al. Cardiac resynchronization therapy as a therapeutic option in patients with moderate-severe functional mitral regurgitation and high operative risk. Circulation 2011;124:912-9. [Crossref] [PubMed]
- Cipriani M, Lunati M, Landolina M, et al. Prognostic implications of mitral regurgitation in patients after cardiac resynchronization therapy. Eur J Heart Fail 2016;18:1060-8. [Crossref] [PubMed]
- Onishi T, Onishi T, Marek JJ, et al. Mechanistic features associated with improvement in mitral regurgitation after cardiac resynchronization therapy and their relation to long-term patient outcome. Circ Heart Fail 2013;6:685-93. [Crossref] [PubMed]
- Michler RE, Smith PK, Parides MK, et al. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2016;374:1932-41. [Crossref] [PubMed]
- Pascual I, Arzamendi D, Carrasco-Chinchilla F, et al. Transcatheter mitral repair according to the cause of mitral regurgitation: Real-life data from the Spanish MitraClip registry. Rev Esp Cardiol (Engl Ed) 2019. [Epub ahead of print].
- Mendirichaga R, Singh V, Blumer V, et al. Transcatheter Mitral Valve Repair With MitraClip for Symptomatic Functional Mitral Valve Regurgitation. Am J Cardiol 2017;120:708-15. [Crossref] [PubMed]
- Lim DS, Kar S, Spargias K, et al. Transcatheter valve repair for patients with mitral regurgitation: 30-day results of the CLASP study. JACC Cardiovasc Interv 2019;12:1369-78. [Crossref] [PubMed]
- Maisano F, Taramasso M, Nickenig G, et al. Cardioband, a transcatheter surgical-like direct mitral valve annuloplasty system: early results of the feasibility trial. Eur Heart J 2016;37:817-25. [Crossref] [PubMed]
- Nickenig G, Hammerstingl C, Schueler R, et al. Transcatheter mitral annuloplasty in chronic functional mitral regurgitation: 6-month results with the cardioband percutaneous mitral repair system. JACC Cardiovasc Interv 2016;9:2039-47. [Crossref] [PubMed]
- Messika-Zeitoun D, Nickenig G, Latib A, et al. Transcatheter mitral valve repair for functional mitral regurgitation using the Cardioband system: 1 year outcomes. Eur Heart J 2019;40:466-72. [Crossref] [PubMed]
- Goldberg SL, Meredith I, Marwick T, et al. A randomized double-blind trial of an interventional device treatment of functional mitral regurgitation in patients with symptomatic congestive heart failure-Trial design of the REDUCE FMR study. Am Heart J 2017;188:167-74. [Crossref] [PubMed]
- Schofer J, Siminiak T, Haude M, et al. Percutaneous mitral annuloplasty for functional mitral regurgitation: Results of the CARILLON mitral annuloplasty device European Union study. Circulation 2009;120:326-33. [Crossref] [PubMed]
- Siminiak T, Wu JC, Haude M, et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN Trial. Eur J Heart Fail 2012;14:931-8. [Crossref] [PubMed]
- Lipiecki J, Siminiak T, Sievert H, et al. Coronary sinus-based percutaneous annuloplasty as treatment for functional mitral regurgitation: the TITAN II trial. Open Heart 2016;3:e000411. [Crossref] [PubMed]
- Erglis A, Narbute I, Poupineau M, et al. Treatment of secondary mitral regurgitation in chronic heart failure. J Am Coll Cardiol 2017;70:2834-5. [Crossref] [PubMed]
- Puls M, Lubos E, Boekstegers P, et al. One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: results from the German transcatheter mitral valve interventions registry. Eur Heart J 2016;37:703-12. [Crossref] [PubMed]
- Capodanno D, Adamo M, Barbanti M, et al. Predictors of clinical outcomes after edge-to-edge percutaneous mitral valve repair. Am Heart J 2015;170:187-95. [Crossref] [PubMed]
- Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation: a new conceptual framework that reconciles the results of the MITRA-FR and COAPT trials. JACC Cardiovasc Imaging 2019;12:353-62. [Crossref] [PubMed]
- Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 2012;25:3-46. [Crossref] [PubMed]