
Cystathionine gamma lyase and hydrogen sulfide: new players in orthodontic root resorption
Orthodontic tooth movement is a safe, effective, and very widely used approach to ameliorate defects in dental alignment and craniofacial architecture (1). Although orthodontics is best known for its use to enhance aesthetics, it also serves an essential role in improving functionality, particularly in repairing severe defects in dentition that arise from genetic disorders, like Cleidocranial dysostosis or cleft palate, or which occur after severe trauma. Low levels of root resorption commonly occur when teeth are subjected to orthodontic force, but only rarely is sufficient resorption triggered to be clinically relevant (2). Though rare, severe root resorption during orthodontic treatment is difficult to predict or to detect. This can result in the loss of teeth that had been healthy prior to the orthodontic procedure. Understanding the risk factors for orthodontic root resorption is therefore of considerable interest to the orthodontic community.
In a recent article, Lu, Chen and Hua from Tongji University identified cystathionine gamma lyase (CSE) activity as an aggravator of orthodontic root resorption in a mouse model (3). CSE is an enzyme that is involved in the processing of homocysteine to cysteine and alpha keto butyrate (4). Homocysteine is a non-proteinogenic, sulfur-containing amino acid formed during metabolism of the essential amino acid methionine. Levels of circulating homocysteine are determined by the balance of biosynthesis and catabolism (5). Synthesis of homocysteine via transmethylation of methionine is catalyzed by the enzymes S-adenosylmethionine synthetase, methyltransferase, and S-adenosylhomocysteine hydrolase in three sequential steps. Catabolism of homocysteine can occur by two pathways, remethylation to methionine and trans-sulfuration to cysteine. The enzyme methyltetrahydrofolate reductase (MTHFR) is involved in remethylation. During transulferation homocysteine is first processed to cystathione, by cystathione-β-synthase (CAB) and then is converted to cysteine by CSE. Mutations in both MTHFR and CBS are associated with elevated circulating homocysteine levels, which are linked to cardiovascular disease (6), clots in veins, complications in pregnancy, and various other conditions including, autism (7), cognitive impairment or dementia (8), Down’s syndrome, osteoporosis (9), movement disorder, migraines, multiple sclerosis, and polycystic ovary syndrome. Mutations in CSE by contrast are generally benign, but have been associated with intellectual disability (10).
In addition to playing a role in the very important process of catabolism of homocysteine, CSE also produces hydrogen sulfide (H2S) as a byproduct of the enzymatic reaction it facilitates. H2S has recently emerged as a signaling molecule functioning in processes that include neuromodulation in the brain and smooth muscle relaxation (11). It can also modulate inflammation, insulin-release and angiogenesis (12). Recent studies provide evidence that H2S also has a role in controlling osteoclast activity. For example, H2S was reported to trigger osteoclast production and receptor activator of nuclear factor kappa B-ligand (RANKL) expression in rats (13), and H2S produced by CSE was shown to promote orthodontic tooth movement (14,15). The mechanism by which H2S exerts its effects on osteoclasts and bone cells are not well characterized, but evidence suggests that it stimulates sirtuin 1 (16) and inhibits cAMP phosphodiesterase activity (17), both plausible regulators of osteoclasts and osteoblasts (Figure 1). It was therefore reasonable to hypothesize that CSE, and H2S produced by CSE, might have a role in orthodontic induced root resorption, a process that includes both stimulating uncoupled resorption and inflammation.
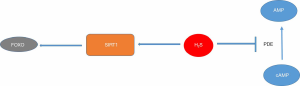
In their recent article, Lu and colleagues first showed evidence by microCT of teeth that root resorption increased with age in mice to a maximum age of 52 weeks, but that CSE knockouts had lower levels of root resorption. Root resorption also increased over a period of three weeks in wild type mice compared with no force controls, or mice in which CSE was knocked out. This data was measured first by microCT, then results were confirmed by analysis of hematoxylin and eosin stained sections. Tissues contained less RANKL and osteoprotegerin mRNA in CSE knockout mice, and the RANKL/osteoprotegerin ratio was higher in wild type mice compared with CSE knockouts. Consistent with these data, CSE mice had fewer root-associated odontoclasts. Taken together, this suggests that mice with no CSE, and presumably lower H2S levels, display fewer odontoclasts and less root resorption.
Because there are conditions in humans that can result in increases or decreases in CSE activity, there is potential immediate clinical relevance to these data. In humans, severe deficiency in CSE, leading to cystathioninuria, is most typically described as benign, but evidence for neurological defects in childhood has been presented (18). Excess of H2S from CSE activity can be the result of hyperhomocysteinemia (19). This can result from kidney disease, lack of B vitamins in the diet, hypothyroidism, alcoholism, and certain types of medications (19). Might the rare patients who have clinically significant orthodontic-induced root resorption exhibit excess CSE activity leading to root resorption? Although there is no data that directly addresses this question, indirect evidence suggests that this is unlikely. Chronic binge drinkers, where H2S would be expected to be higher (20), display reduced orthodontic tooth movement compared with controls (21).
Can strategies to increase CSE activity or local H2S be used to increase rates of tooth movement, or could local inhibition of CSE activity and reduction in H2S be used to curtail root resorption? In Lu et al., the rate of tooth movement in the CSE knockout was not reported. Based on previous research by the same authors it would presumably be slower than wild type. How much relative to root resorption is a crucial question. It is possible that root resorption is more responsive to CSE activity than bone resorption. In that case a small increase in CSE activity may have little effect on tooth movement rate, but may greatly enhance root resorption. Likewise, reductions in CSE activity may not greatly influence tooth movement, but may be associated with large decreases in root resorption. It is also possible that root resorption is less sensitive to CSE activity changes than tooth movement, in which case it can be imagined that local addition of H2S might significantly enhance tooth movement, while largely sparing the teeth from additional root resorption. Future studies carefully examining the effects of changes in CSE activity, and local H2S levels, on both tooth movement rates and root resorption will be crucial.
Although the interpretation presented in the present study is that CSE triggers root resorption through H2S, this was not directly demonstrated. The enzymatic pathways associated with the metabolism of methionine and catabolism of homocysteine are complex, and some elements, homocysteine for example, have direct effects on bone and other connective tissues that could influence root resorption. If H2S production is key, then better understanding of how it exerts effects on cells would increase our understanding of root resorption and orthodontic tooth movement. Currently three distinct mechanisms of H2S signaling have been identified: (I) reduction and/or direct binding of metalloprotein heme centers; (II) serving as a potent antioxidant through reactive oxygen species/reactive nitrogen species scavenging [probably the mechanism of induction of Sirt1 (22)]; or (III) persulfidation, the post-translational modification of proteins by addition of a thiol (-SH) group onto reactive cysteine residues (23,24). Further understanding of the role of CSE and H2S in root resorption will depend on identifying the mechanisms that support CSE’s ability to stimulate bone and root resorption.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.169). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wise GE, King GJ. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res 2008;87:414-34. [Crossref] [PubMed]
- Rody WJ Jr, Holliday LS, McHugh KP, et al. Mass spectrometry analysis of gingival crevicular fluid in the presence of external root resorption. Am J Orthod Dentofacial Orthop 2014;145:787-98. [Crossref] [PubMed]
- Lu C, Chen L, Hua Y. Cystathionine gamma lyase aggravates orthodontic root resorption in mice. Ann Transl Med 2019;7:787. [Crossref] [PubMed]
- Zhao K, Li H, Li S, et al. Regulation of cystathionine gamma-lyase/H2S system and its pathological implication. Front Biosci (Landmark Ed) 2014;19:1355-69. [Crossref] [PubMed]
- Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr 2004;24:539-77. [Crossref] [PubMed]
- Wen YD, Wang H, Zhu YZ. The Drug Developments of Hydrogen Sulfide on Cardiovascular Disease. Oxid Med Cell Longev 2018;2018:4010395.
- Bełtowski J. Hydrogen sulfide in pharmacology and medicine--An update. Pharmacol Rep 2015;67:647-58. [Crossref] [PubMed]
- Panthi S, Chung HJ, Jung J, et al. Physiological Importance of Hydrogen Sulfide: Emerging Potent Neuroprotector and Neuromodulator. Oxid Med Cell Longev 2016;2016:9049782.
- Petramala L, Acca M, Francucci CM, et al. Hyperhomocysteinemia: a biochemical link between bone and cardiovascular system diseases? J Endocrinol Invest 2009;32:10-4. [PubMed]
- Kraus JP, Hasek J, Kozich V, et al. Cystathionine gamma-lyase: Clinical, metabolic, genetic, and structural studies. Mol Genet Metab 2009;97:250-9. [Crossref] [PubMed]
- Cao X, Ding L, Xie ZZ, et al. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid Redox Signal 2019;31:1-38. [Crossref] [PubMed]
- Huang CW, Moore PK. H2S Synthesizing Enzymes: Biochemistry and Molecular Aspects. Handb Exp Pharmacol 2015;230:3-25. [Crossref] [PubMed]
- Irie K, Ekuni D, Yamamoto T, Morita M, et al. A single application of hydrogen sulphide induces a transient osteoclast differentiation with RANKL expression in the rat model. Arch Oral Biol 2009;54:723-9. [Crossref] [PubMed]
- Pu H, Hua Y. Hydrogen sulfide regulates bone remodeling and promotes orthodontic tooth movement. Mol Med Rep 2017;16:9415-22. [Crossref] [PubMed]
- Liu F, Wen F, He D, et al. Force-Induced H2S by PDLSCs Modifies Osteoclastic Activity during Tooth Movement. J Dent Res 2017;96:694-702. [Crossref] [PubMed]
- Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004;303:2011-5. [Crossref] [PubMed]
- Bucci M, Papapetropoulos A, Vellecco V, et al. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol 2010;30:1998-2004. [Crossref] [PubMed]
- Espinós C, García-Cazorla A, Martínez-Rubio D, et al. Ancient origin of the CTH alelle carrying the c.200C>T (p.T67I) variant in patients with cystathioninuria. Clin Genet 2010;78:554-9. [Crossref] [PubMed]
- Zaric BL, Obradovic M, Bajic V, et al. Homocysteine and Hyperhomocysteinaemia. Curr Med Chem 2019;26:2948-61. [Crossref] [PubMed]
- Sarna LK, Siow YL. O K. The CBS/CSE system: a potential therapeutic target in NAFLD? Can J Physiol Pharmacol 2015;93:1-11. [Crossref] [PubMed]
- de Araujo CM, Johann AC, Camargo ES, et al. The effects of binge-pattern alcohol consumption on orthodontic tooth movement. Dental Press J Orthod 2014;19:93-8. [Crossref] [PubMed]
- Liu AJ, Li B, Yang M, et al. Sirtuin 1 mediates hydrogen sulfide-induced cytoprotection effects in neonatal mouse cardiomyocytes. Chin Med J 2017;130:2346-53. [PubMed]
- Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 2011;51:169-87. [Crossref] [PubMed]
- Filipovic MR, Zivanovic J, Alvarez B, et al. Chemical Biology of H2S Signaling through Persulfidation. Chem Rev 2018;118:1253-337. [Crossref] [PubMed]


