
Clinical application of stem cell in patients with end-stage liver disease: progress and challenges
Introduction
End-stage liver disease (ESLD), decompensated liver cirrhosis or liver failure, is the final stage of liver disease and is the major cause of mortality worldwide. Patients with ESLD should be referred to liver transplantation. However, serious problems, including shortage of donors, surgical complications, organ rejection and high cost, have restricted the use of liver transplantation (1). Other treatment strategies have been explored to mitigate the clinical challenge.
Stem cell therapy, an emerging field, has brought substantial benefits to patients with various diseases and may also be a potential alternative for patients with ESLD. Considerable preclinical research and clinical trials have been performed. This article discusses the potential of stem cell in liver diseases. In particular, we focus on the clinical progress and challenges of multipotent stem cells in the field of ESLD.
Hepatic stem cells
In response to mild acute liver injury and partial hepatectomy, the mature liver cells begin to proliferate to reconstitute the liver mass, whereas in the setting of severe or chronic injury in which the proliferative capacity of hepatocytes and cholangiocytes is blocked, hepatic stem cells may contribute to liver regeneration (2).
Adult hepatic stem cells, mainly referred to hepatic progenitor cells (HPCs) in humans and oval cells in rodents, maintain quiescent in normal liver homeostatic condition. They are able to differentiate into hepatocyte and cholangiocyte lineages with stimulus from stem cell niche. Stem cell niche includes not only the site where stem cells are located but also the surrounding cells and the composition of microenvironment, all of which help to maintain the characteristics of stem cells by preserving their quiescent state in normal condition and regulating their proliferation and differentiation capacity after activation (3). The well-known niche for hepatic stem cells is the Canal of Hering. Hepatic stem cells can be activated with signaling factors and cytokines released by damaged hepatocytes, inflammatory cells and adjacent liver cells including Kupffer cells, hepatic stellate cells and liver sinusoidal endothelial cells (2,4).
However, the benefit of HPCs to chronic liver injury is controversial. Other than hepatocytes and cholangiocytes, HPCs may also differentiate into myofibroblasts and promote the development of fibrosis and even cirrhosis during chronic liver injury (5,6). The precise mechanism underlying hepatic differentiation of HPCs is still unclear, thus more efforts are needed to govern HPCs committed differentiation to hepatocytes and cholangiocytes both in vivo and vitro.
Pluripotent stem cells
Embryonic stem cells (ESCs) are pluripotent cells isolated from the inner mass of an embryo. They can differentiate into any cell type of three embryonic germ layers (7). Although human ESC-derived hepatocyte-like-cells (HLCs) have been reported to rescue liver function and promote liver repair in mice (8,9), the clinical application of ESCs and their differentiated progeny is still limited owing to the safe issue (tumorigenesis) and ethical concerns. Induced pluripotent stem cells (iPSCs) have pluripotent properties like ESCs. However, iPSCs may develop gene mutation during differentiation, which may even lead to teratoma formation (10).
Both HPCs and iPSC are considered as candidates for hepatocytes and hepatocyte-like cells generation. However, long-term culture of cells with traditional two dimensional (2D) system in vitro may lead to the loss of stem cells characteristics and chromosome alterations in higher passages (11). Moreover, cells grown in 2D culture system cannot recapitulate in vivo cell polarization and cellular interaction. Recently, these gaps are partly filled by 3D culture technology, or term organoid culture techniques. Organoids retain a tissue structure and function similar to the original tissue in vivo under physiological and pathological state, paving a way for the study of tissue development, physiology and disease (11). Organoids can be generated from both tissue-resident stem/progenitor cells and pluripotent stem cells (12). Liver organoids allow long-term expansion of liver stem cells with the preservation of their stable chromosome and structural level over months, and these cells can differentiate into functional hepatocytes in vitro and upon transplantation in vivo (13), which has the advantage over iPSCs who have an unpredictable degree of differentiation and a higher risk for tumorigenesis. Up to now, most liver organoids studies are in preclinical stage.
Multipotent stem cells
Multipotent stem cells, also known as adult stem cells, are characterized by a relatively limited differentiation potential and are able to generate specialized cell types of adult tissue (14). The two major populations of adult stem cells are hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs). They may be mobilized and migrate to the injured liver, which form the basis of regenerative medicine for the treatment of specific diseases.
Hematopoietic stem cells
Human bone marrow (BM) and peripheral blood are easily available sources for HSCs isolation. HSCs are able to self-renew and differentiate to replenish short-lived mature blood cell types (15). HSCs also have the potential of plasticity, meaning that HSCs can generate non-hematopoietic cell types when exposed to appropriate stimuli, for example hepatocytes (16). Surface marker cluster of differentiation (CD) 34 is often used to identify HSCs in human beings and CD133 may represent a more stem cell-enriched subpopulation of the CD34+cells (7).
BM is the classical stem cell niche for HSC. A body of evidence has proved the importance of CXCR4/stromal-derived factor-1 (SDF-1) axis in the mobilization of HSCs from BM and in the homing of HSCs to damaged liver (17,18). Granulocyte colony-stimulating factor (G-CSF) has been proved to antagonize CXCR4 and SDF-1 interaction and mobilize HSC from BM to peripheral circulation (19,20). It is thought that the secretion of SDF increases during tissue injury, and these CXCR4 positive stem cells may response to the increased SDF-1gradient by chemotaxis thereby migrating to damaged liver (21). Except for SDF-1, hepatocyte growth factor (HGF) is also upregulated in liver injury (22). HGF can facilitate the mobilization of stem cell via HGF/c-MET axis (23).
The administration of G-CSF would significantly increase peripheral CD34+ cell counts and improve the outcome of patients with ESLD. Salama et al. (24) separated CD34+ and CD133+ stem cells in patients with ESLD after G-CSF administration for five consecutive days. These patients were subsequently infused with CD34+ and CD133+ through portal vein, and their liver functions were greatly improved, suggesting that these autologous stem cells infusion may serve as a possible therapeutic protocol. Gordon et al. (25) obtained CD34+ cells from peripheral blood after G-CSF mobilization and observed the improvement of serum bilirubin and albumin in patients with liver failure after these CD34+ cells infusion, and no adverse effect related to the procedure were observed. The mechanism of G-CSF in protecting against liver injury may be multiple, including but not limiting to its potential of mobilizing and homing HSCs (26). G-CSF may also increase the level of HGF and induce the proliferation of HPCs (27).
Although the promising studies have been reported (Table 1), a multicenter, open-label, randomized, controlled phase 2 trial performed by Newsome and colleagues found that G-CSF or autologous HSC infusions did not improve the disease severity, fibrosis, or quality of life in patients with compensated cirrhosis, as no evidence of differences in the change of model for end-stage liver disease (MELD) scores over time between the treatment and control groups was observed (28). Moreover, serious adverse events, such as ascites and encephalopathy, were more frequent in patients receiving stem cell transplantation. The reason why this study observed the absence of any effect of HSCs on the fibrosis and liver function might be that the dose and viability of transfused HSCs (0.2×106/kg) in this report was lower than that in other studies (31). Besides, patients enrolled in Newsome study are heterogeneous at baseline. Some patients with ascites, variceal bleeding and/or encephalopathy are classified to compensated cirrhosis. The different background of the enrolled patients may affect the results. It may be hasty to doubt the effect of HSCs in ESLD. Thus, rigorous randomized and controlled clinical trials are required to confirm the efficacy of stem cell therapy in patients with severe ESLD.

Full table
In adults, HSCs predominantly reside in marrow cavity of long bones. MSCs are another major stem cell type in BM. Some investigators infused a mixed cell population derived from BM into the liver and also found the promising results. Bai et al. (29) demonstrated that the infusion of autologous BM mononuclear cells (MNCs) in patients with decompensated cirrhosis was safe and effective, significantly improving liver function and short-term quality of life. Terai et al. (30) treated 9 cirrhotic patients with autologous MNCs through peripheral vein. Significant improvements in serum albumin, total protein and Child-Pugh scores were observed after MNCs infusion. AFP and proliferating cell nuclear antigen (PCNA) expressions were markedly elevated in liver biopsy tissue. However, it was unclear which subpopulation in the MNCs imparts the maximum benefits owing to the infused unsorted BM-derived stem cell. Herein, most clinical trials observed short-term adverse effects after stem cell transplantation. Nevertheless, concerns regarding the medium- to long-term adverse effects after stem cell therapy including progressive liver fibrosis and hepatocellular carcinoma should also be noted, as it has reported that BM- derived cells may contribute to the development of liver fibrosis and hepatocellular carcinoma (7). Thus, more clinical trials are needed to monitor the adverse effects for a long enough time to confirm the safety of stem cell therapy.
The mechanism of HSCs in alleviating liver damage is yet not fully understood. BM-derived HSCs of host origin can migrate to the liver and differentiate into hepatocytes and cholangiocytes (32). However, some publications demonstrated that the new appearance of hepatocytes in animal liver was caused by the fusion of HSCs with resident hepatocytes rather by the trans-differentiation of HSCs to hepatocytes (33-35). Some authors proposed that epithelial cells in the lung, skin, and liver could develop from BM-derived cells through a mechanism other than cell fusion (36). Other explanations for HSCs in the improvement of liver function are the induction of angiogenesis by releasing vascular endothelial growth factor (VEGF) and the suppression of apoptosis by upregulating the Bcl-2 (2 B-cell leukemia/lymphoma 2) gene (37,38). In addition, HSCs may stimulate the oval cells in the liver and facilitate liver regeneration (39).
MSCs
MSCs are defined as a heterogeneous subset of cells characterized with potency of self-renewing, giving rise to various cell lineages, homing to damaged sites, modulating the host immune system and paracrine actions, which make them numerically the most favored cell type under clinical trials at present (40). MSCs can be defined based on the propensity to adhere to the plastic surface of culture vessels, the differentiation potential and the expression of some special surface biomarkers, positive for CD90, CD29, CD44 and CD105 and negative for CD45, CD34, CD117 and human leukocyte antigen (HLA)-DR (41).
MSCs trials are happening with high activity in the field of liver diseases. Accumulating evidences have validated therapeutic effects of MSCs in experimental animal models of liver injury (42,43), paving the way to the execution of clinical trials. Clinical trials of human MSCs are evolving rapidly with ambitious goals of improving the outcome of patients with ESLD.
MSCs are typically isolated from BM and the clinical application of these undifferentiated cells has attracted a lot of attentions (Table 2). Peng et al. (44) recruited 527 patients with liver failure caused by hepatitis B, among whom 53 received a single transplantation of autologous BM MSCs through hepatic artery. In transplantation group, short-term outcomes were favorable, with the significant improvement of ALB and TBIL levels, MELD scores and prothrombin time (PT) at 2–3 weeks after transplantation, whereas long-term benefits were limited. Lin et al. (45) transplanted allogeneic BM-derived MSCs to patients with hepatitis B virus (HBV)-related acute-on-chronic liver failure (ACLF), and found that serum total bilirubin levels and MELD scores were markedly improved in patients with MSCs infusion. No severe infusion-related side effects were reported. In the study of Kharaziha et al. (46), MSCs injection through either peripheral or the portal vein decreased the MELD scores, serum creatinine and bilirubin levels in patients with liver cirrhosis. And no adverse effects were reported. El-Ansary et al. (47) and Salama et al. (48) also proved the significant improvement in liver function by autologous BM-MSCs in liver cirrhosis induced by hepatitis C.
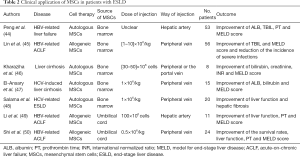
Full table
For clinical use, the isolation of MSC from BM is an invasive and painful procedure. Besides, the proportion of MSCs in BM is very low (0.001–0.01%), and their number and differentiation potential may decline with increasing age (41,51). Other adult tissues, such as umbilical cord blood (UC) and adipose tissue (AD), are alternative options for MSCs isolation. These MSCs isolated from different tissue share some common features, morphological and immunophenotypic properties.
The promising results of UC- MSCs have been observed in experimental animal models of liver cirrhosis and liver failure (52,53). For clinical trial, Li et al. (49) reported that MELD scores, PT and liver function were markedly improved in patients with HBV-related ACLF after UC-MSCs transplantation, and the inspiring results were also reported in another study in which patients suffering ACLF treated with UC-MSCs (50). Although AD-MSCs transplantation improved serum biochemical parameters and slowed disease progression in experimental model of ACLF (54), no too many studies have been conducted in clinical trials.
Although MSCs have the capacity of differentiating into hepatocytes in acute and chronic liver injury, the mechanism of MSCs in liver injury might be primarily based on their immunomodulatory properties and paracrine action (55-57). With the potential of homing to the damaged sites and the pleiotropic properties of anti-apoptosis, anti-fibrosis, angiogenesis, anti-inflammation and growth factor production, MSCs contribute to the improvement of ESLD (58). In addition, MSCs might communicate with immune cells to regulate the inflammatory process.
Challenges to be addressed
Although multipotent stem cells, particularly MSCs transplantation, might become a potentially efficacious tool to treat ESLD and large numbers of preclinical and clinical studies have been performed, there are still some vital challenges to be resolved before stem cells widely used in clinic.
Which are more safe, convenient and cost-effective, autologous or allogenic stem cells in the field of liver disease?
Autologous stem cells are generally advantaged in terms of ethical consideration and risks of sensitization and cell rejection. Autologous HSCs are more common than allogenic HSCs in the field of liver disease, as allogenic HSCs may cause inflammatory neuropathy and graft versus-host disease (59). It is a little complicated when comes to MSCs. There is an argument on the pros and cons of autologous and allogeneic MSCs transplantation. Autologous MSCs are safe and cause no transplant rejection. However, autologous MSCs derived from aged donors show increased number of senescent cells and low capacity of proliferation and differentiation (60). System disease may also affect the characteristic of autologous MSCs. Allogenic MSCs isolated from young and healthy donors are more effective and powerful than those from older and systemic sick individuals. Moreover, allogenic MSCs can be expanded and cryopreserved in vitro, which make them available off the shelf in emergency settings where stem cells are required immediately, whereas the extraction and large-scale expansion of autologous cells are expensive and time consuming (61).
However, the safety implication of allogenic MSCs in an immunocompetent host needs reexamined. Previously, MSCs are usually considered to be immunoprivileged because of the absence or low expression of major histocompatibility complex (MHC) class II and costimulatory molecules (62), and allogenic MSCs may escape the recipient’s immune surveillance. Nonetheless, several studies have documented that the transplantation of allogeneic MSCs could elicit specific cellular and humoral immune responses, which may reduce the survival of allogenic MSCs in vivo (63,64). It is likely that compared with autologous MSCs, more allogenic MSCs are needed to ensure the comparable therapeutic effects. Therefore, allogenic MSCs are not fully immunoprivileged and their long-term benefits await more clinical validation.
Which is the optimal route for stem cells transplantation?
The common routes for stem cell transplantation include peripheral vein, portal vein and hepatic artery. Peripheral vein is the most common and may be also the most safe and accessible route for transplantation (47,48,65,66). Nevertheless, infused stem cells may be trapped in the lung following peripheral intravenous injection (67-69). In the study of Eggenhofer et al. (69), these pulmonary trapped stem cell were short-term survival and disappeared from the lung, and the MSCs observed in the liver were MSC debris or phagocytosed MSC rather than living MSC. In summary, intravenously infused MSCs lead to a reduction in the number of viable stem cells homing to the target organ, which may inhibit stem cell harvest and decrease the treatment efficacy of stem cells. Although there were no significant differences in the therapeutic effects among the three transplanted routes in animal models and in some patients (46,70,71), the literatures always consisted of small cohort group not adequately characterized and might exist bias. Hence, investigators have explored and tried other routes for cell transplantation.
Theoretically, direct administration of stem cells into the vessels supplying the liver (the portal vein and hepatic artery) may be more effective. Although published reports have proved the safety and efficacy of stem cells injected through portal vein or hepatic artery (46,71-73), the invasive approaches may carry substantial risks: hemorrhea and thrombosis. The potential of portal hypertensive may increase if stem cells are delivered directly to the liver. Other adverse side effects, such as radiocontrast nephropathy and hepatorenal syndrome, although rare, were also observed in decompensated cirrhosis after infusion of HSCs through hepatic artery (74). What described above has raised the significant concerns about the methodology used to deliver the stem cells.
What is the optimal dose of stem cells for single injection? And what is the schedule of injection?
The impact associated with the aforesaid pulmonary trapping may be mitigated if sufficient cells are infused (68). The dose of cell infusion affects the treatment effect and larger number of MSCs administration may induce more beneficial effects. However, as shown in Tables 1 and 2, there was obvious heterogeneity among studies regarding the dose of stem cell transplantation, which greatly limits the comparison of treatment effects across studies.
In addition, the therapeutic benefit of stem cells transplantation is transient. MSCs administration improved liver functions and decreased MELD scores in 3 patients on month six post-transplantation. Among these 3 patients, the MELD score of 1 patient came back to pre-transplantation status on month 12 post-transplantation (75). Repeated cell infusions seemed to induce more lasting clinical efficacy and improvement in liver function than single infusion (65). It is likely that the duration of therapeutic effects is limited after stem cell single injection. Therefore, the frequency of cell transplants to guarantee the sustained therapeutic effects in patients is still an open question and a matter of intensive investigation. Further studies may explore the standardized cell dose per injection and the life span of the injected cells, determining the frequency of stem cell infusion to guarantee the long-term therapeutic benefits in patients.
Except for these challenges above, other technical issues, for example the cryopreserved methods in vitro, are being explored. Stem cell should be cryopreserved without compromising their phenotypic characteristics, their proliferation and differentiation potential. During cryopreservation, the cooling rate, whether too fast or too slow, may cause cell injury. Cryoprotectants which are used to avoid minimize the freezing damage, can cause cell injury as well. Different storage period and temperature also have a great effect on the function of cryopreserved stem cells (41). At present, strategies are being explored to refine the cryopreservation procedure and to minimize the cryoprotectants toxicity and freezing-induced damage.
Conclusions
ESLD is a severe clinical disease with high morbidity and mortality. Stem cell therapies are promising agents and may act as a novel alternative for patients still waiting for liver transplantation. But stem cell transplantation is still in the early stage of development. And there still some challenges need to be addressed. Future clinical trials should be planned with standardized protocols to define the nature of stem cell, the transplantation procedure and even the evaluation system regarding efficacy. Optimism has been high for clinical application of stem cell therapy in the future.
Acknowledgments
Funding: This research is partly supported by the Foundation of Science and Technology Department of Sichuan Province in China (2019YFS0209 and 2019YFS0028).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ling Lu) for the series “Stem Cell and Clinical Application” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.153). The series “Stem Cell and Clinical Application” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Terai S, Sakaida I. Current status of autologous bone marrow cell infusion therapy for liver cirrhosis patients. Hepatol Res 2008;38 Suppl 1:S72-75. [Crossref] [PubMed]
- Rehman K, Iqbal MJ, Zahra N, Akash MS. Liver stem cells: From preface to advancements. Curr Stem Cell Res Ther 2014;9:10-21. [Crossref] [PubMed]
- Kordes C, Haussinger D. Hepatic stem cell niches. J Clin Invest 2013;123:1874-80. [Crossref] [PubMed]
- Van Haele M, Roskams T. Hepatic progenitor cells: An update. Gastroenterol Clin North Am 2017;46:409-20. [Crossref] [PubMed]
- Pan XR, Jing YY, Liu WT, et al. Lipopolysaccharide induces the differentiation of hepatic progenitor cells into myofibroblasts via activation of the hedgehog signaling pathway. Cell Cycle 2017;16:1357-65. [Crossref] [PubMed]
- Sekiya S, Miura S, Matsuda-Ito K, et al. Myofibroblasts derived from hepatic progenitor cells create the tumor microenvironment. Stem Cell Reports 2016;7:1130-9. [Crossref] [PubMed]
- Houlihan DD, Newsome PN. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology 2008;135:438-50. [Crossref] [PubMed]
- Tolosa L, Caron J, Hannoun Z, et al. Transplantation of HESC-derived hepatocytes protects mice from liver injury. Stem Cell Res Ther 2015;6:246. [Crossref] [PubMed]
- Woo DH, Kim SK, Lim HJ, et al. Direct and indirect contribution of human embryonic stem cell-derived hepatocyte-like cells to liver repair in mice. Gastroenterology 2012;142:602-11. [Crossref] [PubMed]
- Peterson SE, Loring JF. Genomic instability in pluripotent stem cells: Implications for clinical applications. J Biol Chem 2014;289:4578-84. [Crossref] [PubMed]
- Nantasanti S, de Bruin A, Rothuizen J, et al. Concise review: Organoids are a powerful tool for the study of liver disease and personalized treatment design in humans and animals. Stem Cells Transl Med 2016;5:325-30. [Crossref] [PubMed]
- Prior N, Inacio P, Huch M. Liver organoids: From basic research to therapeutic applications. Gut 2019;68:2228-37. [Crossref] [PubMed]
- Huch M, Gehart H, van Boxtel R, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015;160:299-312. [Crossref] [PubMed]
- Tolosa L, Pareja E, Gomez-Lechon MJ. Clinical application of pluripotent stem cells: An alternative cell-based therapy for treating liver diseases? Transplantation 2016;100:2548-57. [Crossref] [PubMed]
- Esrefoglu M. Role of stem cells in repair of liver injury: Experimental and clinical benefit of transferred stem cells on liver failure. World J Gastroenterol 2013;19:6757-73. [Crossref] [PubMed]
- Mosaad YM. Hematopoietic stem cells: An overview. Transfus Apher Sci 2014;51:68-82. [Crossref] [PubMed]
- Dalakas E, Newsome PN, Harrison DJ, et al. Hematopoietic stem cell trafficking in liver injury. Faseb J 2005;19:1225-31. [Crossref] [PubMed]
- Ahmadi AR, Chicco M, Wesson RN, et al. Stem cell mobilization is lifesaving in a large animal preclinical model of acute liver failure. Ann Surg 2018;268:620-31. [Crossref] [PubMed]
- Gaia S, Smedile A, Omede P, et al. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol 2006;45:13-9. [Crossref] [PubMed]
- Duan XZ, Liu FF, Tong JJ, et al. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis b virus-associated acute-on-chronic liver failure. World J Gastroenterol 2013;19:1104-10. [Crossref] [PubMed]
- Kucia M, Ratajczak J, Reca R, et al. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol Dis 2004;32:52-7. [Crossref] [PubMed]
- Kollet O, Shivtiel S, Chen YQ, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human cd34+ stem cell recruitment to the liver. J Clin Invest 2003;112:160-9. [Crossref] [PubMed]
- Lehwald N, Duhme C, Wildner M, et al. Hgf and sdf-1-mediated mobilization of cd133+ BMSC for hepatic regeneration following extensive liver resection. Liver Int 2014;34:89-101. [Crossref] [PubMed]
- Salama H, Zekri AR, Zern M, et al. Autologous hematopoietic stem cell transplantation in 48 patients with end-stage chronic liver diseases. Cell Transplant 2010;19:1475-86. [Crossref] [PubMed]
- Gordon MY, Levicar N, Pai M, et al. Characterization and clinical application of human cd34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells 2006;24:1822-30. [Crossref] [PubMed]
- Zhang L, Kang W, Lei Y, et al. Granulocyte colony-stimulating factor treatment ameliorates liver injury and improves survival in rats with d-galactosamine-induced acute liver failure. Toxicol Lett 2011;204:92-9. [Crossref] [PubMed]
- Spahr L, Lambert JF, Rubbia-Brandt L, et al. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: A randomized trial. Hepatology 2008;48:221-9. [Crossref] [PubMed]
- Newsome PN, Fox R, King AL, et al. Granulocyte colony-stimulating factor and autologous cd133-positive stem-cell therapy in liver cirrhosis (realistic): An open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol Hepatol 2018;3:25-36. [Crossref] [PubMed]
- Bai YQ, Yang YX, Yang YG, et al. Outcomes of autologous bone marrow mononuclear cell transplantation in decompensated liver cirrhosis. World J Gastroenterol 2014;20:8660-6. [Crossref] [PubMed]
- Terai S, Ishikawa T, Omori K, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells 2006;24:2292-8. [Crossref] [PubMed]
- Verma N, Singh A, Singh V. Haematopoietic stem cells in cirrhosis. Lancet Gastroenterol Hepatol 2018;3:298. [Crossref] [PubMed]
- Theise ND, Nimmakayalu M, Gardner R, et al. Liver from bone marrow in humans. Hepatology 2000;32:11-6. [Crossref] [PubMed]
- Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 2003;422:897-901. [Crossref] [PubMed]
- Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature 2003;422:901-4. [Crossref] [PubMed]
- Fujino H, Hiramatsu H, Tsuchiya A, et al. Human cord blood cd34+ cells develop into hepatocytes in the livers of nod/scid/gamma(c)null mice through cell fusion. Faseb J 2007;21:3499-510. [Crossref] [PubMed]
- Harris RG, Herzog EL, Bruscia EM, et al. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science 2004;305:90-3. [Crossref] [PubMed]
- Tang J, Xie Q, Pan G, et al. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg 2006;30:353-61. [Crossref] [PubMed]
- Chen Z, Chua CC, Ho YS, et al. Overexpression of bcl-2 attenuates apoptosis and protects against myocardial i/r injury in transgenic mice. Am J Physiol Heart Circ Physiol 2001;280:H2313-20. [Crossref] [PubMed]
- Pai M, Zacharoulis D, Milicevic MN, et al. Autologous infusion of expanded mobilized adult bone marrow-derived cd34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol 2008;103:1952-8. [Crossref] [PubMed]
- Tao YC, Wang ML, Chen EQ, et al. Stem cells transplantation in the treatment of patients with liver failure. Curr Stem Cell Res Ther 2018;13:193-201. [Crossref] [PubMed]
- Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, et al. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 2015;71:181-97. [Crossref] [PubMed]
- Wang D, Zhang H, Liang J, et al. Effect of allogeneic bone marrow-derived mesenchymal stem cells transplantation in a polyi:C-induced primary biliary cirrhosis mouse model. Clin Exp Med 2011;11:25-32. [Crossref] [PubMed]
- Zhang Y, Cai W, Huang Q, et al. Mesenchymal stem cells alleviate bacteria-induced liver injury in mice by inducing regulatory dendritic cells. Hepatology 2014;59:671-82. [Crossref] [PubMed]
- Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis b: Short-term and long-term outcomes. Hepatology 2011;54:820-8. [Crossref] [PubMed]
- Lin BL, Chen JF, Qiu WH, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis b virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology 2017;66:209-19. [Crossref] [PubMed]
- Kharaziha P, Hellstrom PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: A phase i-ii clinical trial. Eur J Gastroenterol Hepatol 2009;21:1199-205. [Crossref] [PubMed]
- El-Ansary M, Abdel-Aziz I, Mogawer S, et al. Phase ii trial: Undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev Rep 2012;8:972-81. [Crossref] [PubMed]
- Salama H, Zekri AR, Medhat E, et al. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther 2014;5:70. [Crossref] [PubMed]
- Li YH, Xu Y, Wu HM, et al. Umbilical cord-derived mesenchymal stem cell transplantation in hepatitis b virus related acute-on-chronic liver failure treated with plasma exchange and entecavir: A 24-month prospective study. Stem Cell Rev Rep 2016;12:645-53. [Crossref] [PubMed]
- Shi M, Zhang Z, Xu R, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med 2012;1:725-31. [Crossref] [PubMed]
- Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294-301. [Crossref] [PubMed]
- Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol 2012;27 Suppl 2:112-20. [Crossref] [PubMed]
- Guo G, Zhuang X, Xu Q, et al. Peripheral infusion of human umbilical cord mesenchymal stem cells rescues acute liver failure lethality in monkeys. Stem Cell Res Ther 2019;10:84. [Crossref] [PubMed]
- Gilsanz C, Aller MA, Fuentes-Julian S, et al. Adipose-derived mesenchymal stem cells slow disease progression of acute-on-chronic liver failure. Biomed Pharmacother 2017;91:776-87. [Crossref] [PubMed]
- Gazdic M, Arsenijevic A, Markovic BS, et al. Mesenchymal stem cell-dependent modulation of liver diseases. Int J Biol Sci 2017;13:1109-17. [Crossref] [PubMed]
- Xagorari A, Siotou E, Yiangou M, et al. Protective effect of mesenchymal stem cell-conditioned medium on hepatic cell apoptosis after acute liver injury. Int J Clin Exp Pathol 2013;6:831-40. [PubMed]
- Zhu X, He B, Zhou X, et al. Effects of transplanted bone-marrow-derived mesenchymal stem cells in animal models of acute hepatitis. Cell Tissue Res 2013;351:477-86. [Crossref] [PubMed]
- Trounson A, McDonald C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 2015;17:11-22. [Crossref] [PubMed]
- Doi Y, Sugahara H, Yamamoto K, et al. Immune-mediated peripheral neuropathy occurring simultaneously with recurrent graft-versus-host disease after allogenic hematopoietic stem cell transplantation. Leuk Res 2012;36:e63-5. [Crossref] [PubMed]
- Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev 2006;5:91-116. [Crossref] [PubMed]
- Mamidi MK, Dutta S, Bhonde R, et al. Allogeneic and autologous mode of stem cell transplantation in regenerative medicine: Which way to go? Med Hypotheses 2014;83:787-91. [Crossref] [PubMed]
- Ding DC, Chang YH, Shyu WC, et al. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplant 2015;24:339-47. [Crossref] [PubMed]
- Schu S, Nosov M, O'Flynn L, et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med 2012;16:2094-103. [Crossref] [PubMed]
- Griffin MD, Ryan AE, Alagesan S, et al. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: What have we learned so far? Immunol Cell Biol 2013;91:40-51. [Crossref] [PubMed]
- Zekri AR, Salama H, Medhat E, et al. The impact of repeated autologous infusion of haematopoietic stem cells in patients with liver insufficiency. Stem Cell Res Ther 2015;6:118. [Crossref] [PubMed]
- Wang Q, Qian S, Li J, et al. Combined transplantation of autologous hematopoietic stem cells and allogenic mesenchymal stem cells increases t regulatory cells in systemic lupus erythematosus with refractory lupus nephritis and leukopenia. Lupus 2015;24:1221-6. [Crossref] [PubMed]
- Wang N, Shao Y, Mei Y, et al. Novel mechanism for mesenchymal stem cells in attenuating peritoneal adhesion: Accumulating in the lung and secreting tumor necrosis factor alpha-stimulating gene-6. Stem Cell Res Ther 2012;3:51. [Crossref] [PubMed]
- Watanabe T, Kajiume T, Takaue Y, et al. Decrease in circulating hematopoietic progenitor cells by trapping in the pulmonary circulation. Cytotherapy 2001;3:461-6. [Crossref] [PubMed]
- Eggenhofer E, Benseler V, Kroemer A, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol 2012;3:297. [Crossref] [PubMed]
- Sun L, Fan X, Zhang L, et al. Bone mesenchymal stem cell transplantation via four routes for the treatment of acute liver failure in rats. Int J Mol Med 2014;34:987-96. [Crossref] [PubMed]
- Truong NH, Nguyen NH, Le TV, et al. Comparison of the treatment efficiency of bone marrow-derived mesenchymal stem cell transplantation via tail and portal veins in ccl4-induced mouse liver fibrosis. Stem Cells Int 2016;2016:5720413.
- Deng Q, Cai T, Zhang S, et al. Autologous peripheral blood stem cell transplantation improves portal hemodynamics in patients with hepatitis b virus-related decompensated cirrhosis. Hepat Mon 2015;15:e32498. [Crossref] [PubMed]
- Sharma M, Rao PN, Sasikala M, et al. Autologous mobilized peripheral blood cd34(+) cell infusion in non-viral decompensated liver cirrhosis. World J Gastroenterol 2015;21:7264-71. [Crossref] [PubMed]
- Mohamadnejad M, Namiri M, Bagheri M, et al. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol 2007;13:3359-63. [Crossref] [PubMed]
- Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med 2007;10:459-66. [PubMed]


