
Which is better for articular cartilage regeneration, cultured stem cells or concentrated stromal cells?
Introduction
Articular cartilage has poor regeneration ability after damage. Consequently, research on cartilage regeneration for treatment of early or moderate stage OA (osteoarthritis) patients has been regarded as a ‘never ending story’ (1,2). There are two major factors dictating this poor regeneration: First, cartilage regeneration is limited because the half-life of cartilage tissue is much longer than that of other tissues, resulting in secondary damage or degeneration during regeneration and remodeling. Second, cellular migration is limited by the cartilage tissue structure, and the chondrocytes in the damaged lesion cannot participate fully in regeneration. Thus, cartilage regeneration is restricted by the limited number of participating surrounding tissue cells (3).
ACI (autologous chondrocyte implantation) was developed for treatment of cartilage defects by utilizing chondrocytes (4). However, harvest of normal cartilage tissue from the knee joint and implantation of the cultivated cells requires two surgical processes, which increases time and cost burdens for both surgeon and patient (5).
Arthritis treatment studies using stem cells from various sites such as bone marrow, adipose tissue, umbilical cord, menstrual blood, and amniotic fluid have been conducted (6-8). Use of ADSVF (adipose derived stromal vascular fraction) and BMAC (bone marrow aspirate concentrate) for cartilage repair have been studied and clinically applied (9-12).
BMAC has been in clinical application since the early 2000s and recently, long-term results have been reported (13-15). Bone marrow-derived stem cells have shown equivalent effects to ACI in short and ten-year long-term data, supporting the use of various sources for cartilage regeneration (16,17).
ADSVF production involves the harvest of adipose tissue and requires centrifugation following enzyme treatment to yield a substantial number of stromal cells. Although treatments using ADSVF have shown favorable outcomes, the relative efficacy of cultivated adipose-derived stem cell (ADSC) versus ADSVF for cartilage regeneration is unclear.
ADSC for cartilage regeneration
Homogenous stem cells can be used for cartilage regeneration following cultivation of stem cells from adipose tissue. However, the relationship between the number of stem cells and clinical results remains unclear.
Jo et al. studied the safety and efficacy of intra-articular injection of ASDC on 18 OA patients of Kellgren-Lawrence grade 3 or 4, dividing them into low-dose (1.0×107 cells, n=3), mid-dose (5.0×107, n=3), and high-dose (1.0×108, n=12) groups (18). There were no treatment-related adverse events, and the high-dose group showed the most favorable results concerning pain and function. Furthermore, the high-dose group showed a decrease in cartilage defects and qualitative improvement after six months in serial MRI evaluations and second-look arthroscopy. A two-year follow-up study reported high efficacy and safety in the high-dose group compared to the low and mid-dose groups, supporting use of the high-dose ADSC injection (19).
However, research by Pers et al. presented the opposite results. In their study, 18 OA patients of Kellgren-Lawrence grade 3 or 4 were divided into three equal groups of low-dose (2×106 cells), mid-dose (10×106), and high-dose (50×106). After six months, the low-dose group showed the most significant improvement in pain and function, and there were no adverse effects in any of the three groups. Pers et al. suggested that the reason for this outcome was the immunomodulatory function of paracrine effects that followed ADSC injection to the group with the highest baseline pain (20).
The two studies mentioned above consisted of only 18 patients each, and because the two studies showed opposite results, the optimum dosage of ASDC is still uncertain. Further and larger studies on the efficacy and safety of dosage of injected stem cells are required.
ADSVF for cartilage regeneration
ADSC-based treatment has shown favorable outcomes, but the stem cell culture and expansion require substantial time, cost, and the inconvenience of two stage treatment. ADSVF-based treatment has been drawing attention as an alternative, and many studies are in progress (21). Centrifugation or the micro-fragmentation of the adipose tissue is used for ADSVF production. ADSVF that has been obtained through the aforementioned process comprises heterogenous cell populations, including mesenchymal progenitor/stem cells, preadipocytes, endothelial cells, pericytes, T-cells, and M2 macrophages (17,22).
A double-blinded RCT (randomized controlled trial) study on ADSVF treatment was recently held on 16 bilateral knee OA patients (23). One knee underwent intra-articular injection of 4 mL of ADSVF, the other knee was injected with 4 mL of HA (hyaluronic acid), and the results were compared. At a 12-month follow-up, the ADSVF injection group showed significant improvement in VAS (visual analogue scale), WOMAC (Western Ontario and McMaster Universities Arthritis Index), and ROM (range of motion), whereas the HA group did not show such improvement. Additionally, the ADSVF group showed significant improvement in radiologic review of WORMS (Whole-Organ Magnetic Resonance Imaging Score) and MOCART (MR observations of cartilage repair tissue) measurement compared with the HA group (23). Although the study supported use of the ADSVF treatment, the research also consisted of only 16 patients and lacks arthroscopic measurements. Therefore, more ADSVF studies are required because previous research involved a small number of patients, and insufficient follow-up studies.
The relationship between stromal cells and stem cells
The initial stage of adipose tissue-derived stem cell culture involves cytolyzing the adipose tissue and plating the derived cells on culture flasks. Then following the stem cell culture protocol, the culture-derived cells are utilized as the adipose tissue-derived stem cells. Various differentiation stages of stromal cells are used in the culture. Under a specific culture condition, stromal cells are transformed into cells with stem cell characteristics. Thus, cells derived from adipose tissue show a quantitative relationship as described in Figure 1.
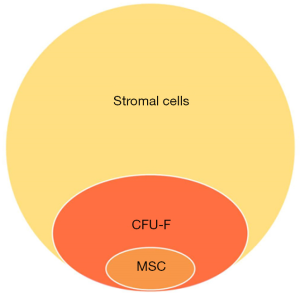
An increased number of stem cells participating in cartilage regeneration can result in a better outcome. However, not all stem cells applied in surgery or treatment directly regenerate into cartilage, and most contribute to cartilage regeneration by their paracrine effect. Therefore, we can estimate an efficacy limit at a certain stem cell number. Meanwhile, there are a variety of stages of differentiated cells concentrated in the ADSVF, and more effective cartilage regeneration can be achieved by the growth factors, cytokines, etc. secreted from each cell. Therefore, basic research on the differences between ADSVF and ADSC is necessary.
Comparison of ADSVF and ADSC
A study by Yokota et al. directly compared ADSC and ADSVF treatment methods. In the study, 59 knees of 49 patients underwent intra-articular injection of 12.75×106 cells of ADSC, and 69 knees of 38 patients underwent intra-articular injection with 5 mL prepared ADSVF and the two groups were compared. VAS and Knee injury and Osteoarthritis Outcome Score (KOOS) were assessed at baseline and at 1, 3, and 6 months, and the Outcome Measures in Rheumatology-Osteoarthritis Research Society International (OMERACT-OARSI) criteria were used to determine a positive response. Neither group showed major adverse effects, although the ADSVF group showed more minor adverse effects. The ADSC group showed an earlier recovery than the ADSVF group in the KOOS symptom, but the two groups showed no difference in response after six months. There was also no significant difference in OMERACT-OARSI Responder Rate. The only difference was that the ADSC group showed greater improvement in VAS score than the ADSVF group (24).
The study by Yokota et al. is important because it is the first attempt to compare directly the ADSC and ADSVF treatment methods. However, the study lacks relevance in selecting the patient group because it is a retrospective study. Also, even though it is the first attempt to compare the two treatments, it lacks any comparison of radiologic and arthroscopic data. It is also difficult to conclude which treatment is superior because the number of patients was small, and the result showed no significant difference between the two groups; although the ADSC group showed a better response in the study, indicators other than VAS score showed similar results. Thus, if the VAS score alone is considered the clinical differentiator, we question whether it is appropriate to use the ADSC method, which requires significant time and cost compared to ADSVF.
Conclusions
There have been various developments and studies on treatments for articular cartilage regeneration. Past studies were focused primarily on cartilage regeneration, whereas current studies are focused on various methods and objectives including comparison of surgical and non-surgical cartilage regeneration treatment, safety, cost-effectiveness, and methods to reduce discomfort of patients and increase recovery (25,26). It is inconclusive, based on the available evidence, whether the stem cell or stromal cell concentration method is more effective for cartilage regeneration. If the two methods show similar clinical outcomes, we expect that the one-step, stromal cell concentration-based cartilage regeneration method will be employed more actively in the future.
Acknowledgments
The authors wish to acknowledge the support of The Catholic University of Korea Uijeongbu St. Mary's Hospital Clinical Research Laboratory Foundation made in the program year of 2019.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim SH, Ha CW, Park YB, et al. Intra-articular injection of mesenchymal stem cells for clinical outcomes and cartilage repair in osteoarthritis of the knee: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg 2019;139:971-80. [Crossref] [PubMed]
- Borakati A, Mafi R, Mafi P, et al. A Systematic Review And Meta-Analysis of Clinical Trials of Mesenchymal Stem Cell Therapy for Cartilage Repair. Curr Stem Cell Res Ther 2018;13:215-25. [Crossref] [PubMed]
- Lo Monaco M, Merckx G, Ratajczak J, et al. Stem Cells for Cartilage Repair: Preclinical Studies and Insights in Translational Animal Models and Outcome Measures. Stem Cells Int 2018;2018:9079538.
- Shetty AA, Kim SJ, Shetty V, et al. Autologous bone-marrow mesenchymal cell induced chondrogenesis: single-stage arthroscopic cartilage repair. Tissue Eng Regen Med 2017;14:479-80. [Crossref] [PubMed]
- Shetty AA, Kim SJ, Bilagi P, et al. Autologous collagen-induced chondrogenesis: single-stage arthroscopic cartilage repair technique. Orthopedics 2013;36:e648-52. [Crossref] [PubMed]
- Huri PY, Hamsici S, Ergene E, et al. Infrapatellar Fat Pad-Derived Stem Cell-Based Regenerative Strategies in Orthopedic Surgery. Knee Surg Relat Res 2018;30:179-86. [Crossref] [PubMed]
- Shin YS, Yoon JR, Kim HS, et al. Intra-Articular Injection of Bone Marrow-Derived Mesenchymal Stem Cells Leading to Better Clinical Outcomes without Difference in MRI Outcomes from Baseline in Patients with Knee Osteoarthritis. Knee Surg Relat Res 2018;30:206-14. [Crossref] [PubMed]
- Jin YZ, Lee JH. Mesenchymal Stem Cell Therapy for Bone Regeneration. Clin Orthop Surg 2018;10:271-8. [Crossref] [PubMed]
- Jang Y, Koh YG, Choi YJ, et al. Characterization of adipose tissue-derived stromal vascular fraction for clinical application to cartilage regeneration. In Vitro Cell Dev Biol Anim 2015;51:142-50. [Crossref] [PubMed]
- Khanmohammadi M, Golshahi H, Saffarian Z, et al. Repair of Osteochondral Defects in Rabbit Knee Using Menstrual Blood Stem Cells Encapsulated in Fibrin Glue: A Good Stem Cell Candidate for the Treatment of Osteochondral Defects. Tissue Eng Regen Med 2019;16:311-24. [Crossref] [PubMed]
- Murata D, Akieda S, Misumi K, et al. Osteochondral Regeneration with a Scaffold-Free Three-Dimensional Construct of Adipose Tissue-Derived Mesenchymal Stromal Cells in Pigs. Tissue Eng Regen Med 2018;15:101-13. [Crossref] [PubMed]
- Vines JB, Aliprantis AO, Gomoll AH, et al. Cryopreserved amniotic suspension for the treatment of knee osteoarthritis. J Knee Surg 2016;29:443-50. [Crossref] [PubMed]
- Shapiro SA, Kazmerchak SE, Heckman MG, et al. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med 2017;45:82-90. [Crossref] [PubMed]
- Kim JD, Lee GW, Jung GH, et al. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol 2014;24:1505-11. [Crossref] [PubMed]
- Centeno C, Pitts J, Al-Sayegh H, et al. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int 2014;2014:370621.
- Teo AQA, Wong KL, Shen L, et al. Equivalent 10-Year Outcomes After Implantation of Autologous Bone Marrow-Derived Mesenchymal Stem Cells Versus Autologous Chondrocyte Implantation for Chondral Defects of the Knee. Am J Sports Med 2019;47:2881-7. [Crossref] [PubMed]
- Han S, Sun HM, Hwang KC, et al. Adipose-Derived Stromal Vascular Fraction Cells: Update on Clinical Utility and Efficacy. Crit Rev Eukaryot Gene Expr 2015;25:145-52. [Crossref] [PubMed]
- Jo CH, Lee YG, Shin WH, et al. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial. Stem Cells 2014;32:1254-66. [Crossref] [PubMed]
- Jo CH, Chai JW, Jeong EC, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med 2017;45:2774-83. [Crossref] [PubMed]
- Pers YM, Rackwitz L, Ferreira R, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a Phase I dose-escalation trial. Stem Cells Transl Med 2016;5:847-56. [Crossref] [PubMed]
- Kim EJ, Seo SG, Shin HS, et al. Platelet-Derived Growth Factor Receptor-Positive Pericytic Cells of White Adipose Tissue from Critical Limb Ischemia Patients Display Mesenchymal Stem Cell-Like Properties. Clin Orthop Surg 2017;9:239-48. [Crossref] [PubMed]
- Bianchi F, Maioli M, Leonardi E, et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 2013;22:2063-77. [Crossref] [PubMed]
- Hong Z, Chen J, Zhang S, et al. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. Int Orthop 2019;43:1123-34. [Crossref] [PubMed]
- Yokota N, Hattori M, Ohtsuru T, et al. Comparative Clinical Outcomes After Intra-articular Injection With Adipose-Derived Cultured Stem Cells or Noncultured Stromal Vascular Fraction for the Treatment of Knee Osteoarthritis. Am J Sports Med 2019;47:2577-83. [Crossref] [PubMed]
- Yokota N, Yamakawa M, Shirata T, et al. Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regen Ther 2017;6:108-12. [Crossref] [PubMed]
- Peeters CM, Leijs MJ, Reijman M, et al. Safety of intraarticular cell-therapy with culture-expanded stem cells in humans: a systematic literature review. Osteoarthritis Cartilage 2013;21:1465-73. [Crossref] [PubMed]


