
Fusion imaging for transcatheter mitral and tricuspid interventions
Introduction
There is an increasing number of options for transcatheter mitral and tricuspid valve intervention, particularly for those with functional regurgitation (1-5). These include both valve repair and replacement devices. Paravalvular leaks are also generally managed by transcatheter closure if feasible. Since direct exposure and visualization of the cardiac structures is not possible like in traditional open cardiac surgery, these procedures are performed under real-time fluoroscopic and echocardiographic guidance (6).
Echocardiography provides live, continuous, high frame rate visualization of soft tissues in 2- or 3-dimensions without any radiation exposure, but some device components are difficult to visualize. These include thin components or wires and certain metallic structures that can cause shadowing and blooming artifact. The transesophageal echocardiography (TEE) probe’s maximum sector volume size is also limited and non-cardiac anatomical landmarks are usually not seen. Therefore, fluoroscopy is also required to monitor the equipment involved in the procedure and the device position in a wider field of view. The limitations of fluoroscopy are the opposite compared to echocardiography, including inability to visualize soft tissue, radiation exposure and having only 2-dimensional views.
Most structural intervention hybrid operating rooms or cardiac catheterization laboratories are set up to display information from fluoroscopy and echocardiography separately. Pre-procedure imaging such as computed tomography or magnetic resonance imaging scans are also not usually viewed on the same system. Procedural cardiologists or surgeons and interventional imagers must, therefore, mentally combine the complementary data from all imaging modalities during catheter manipulation. Adding to the complexity of this task is the fact that the modalities view the heart from different perspectives and display them in different orientations.
New systems now exist to better combine complementary imaging modalities during structural heart interventions. Commonly used fusion, or hybrid, imaging systems include the Philips EchoNavigator and Siemens Syngo TrueFusion. Generally, they require compatible same brand echocardiography and fluoroscopy equipment in addition to specialized hardware and software that is needed to process and create hybrid images. Computed tomography and magnetic resonance imaging datasets can also be used in the fusion imaging systems to allow importation of anatomic targets and features.
Static fusion imaging: “Roadmapping”
Static fusion imaging typically refers to the use 3-dimensional data sets acquired prior to the planned procedure that are then combined with intraprocedural fluoroscopy. It most commonly the fusion of computed tomography and fluoroscopy. This is done using software algorithms using anatomic landmarks to register the computed tomography dataset position and scale with fluoroscopy, such as by using a pigtail catheter in the aortic root. This registration can then be manually fine tuned if needed. Once the computed tomography datasets are registered, structures from computed tomography can be overlaid on a real-time 2-dimensional fluoroscopy screen to assist with procedural guidance by showing anatomical structures usually not seen by fluoroscopy alone (7).
Magnetic resonance imaging-fluoroscopy fusion imaging is also possible. The dataset is similarly registered to fluoroscopy using software algorithms in combination with anatomic landmarks and manual fine tuning (8). Compared to computed tomography, specific potential benefits of magnetic resonance-fluoroscopy fusion include a reduction in ionizing radiation exposure for the patient and the ability to incorporate dynamic cardiac and respiratory motion in the preprocedural scan, which may improve the accuracy of procedural imaging alignment.
Current available systems are Syngo DynaCT (Siemens Healthcare, Erlangen, Germany) and HeartNavigator (Philips Healthcare, Andover, MA, USA).
Although there is potential for static fusion imaging to assist with guidance of structural heart procedures, there are important concerns regarding the accuracy of this technology. For example, misalignment with fluoroscopy due to registration error and changes in patient positioning during the procedure must be avoided. Periodic motion, like respiratory or cardiac movements, can be corrected by increasing C-arm spin rates or specialized ECG-gating algorithms. However, nonperiodic and unpredictable anatomic motion introduced by intracardiac catheter manipulation are difficult to predict with software algorithms and remain a challenge with no clear solution at this time. Lastly, changes in the cardiac structure dimensions between preprocedural imaging and the time of a procedure, which may be seen with changes in loading conditions, are not accounted for with current software versions.
Dynamic fusion imaging
Dynamic fusion refers to the combination of real-time imaging data, as opposed to a previously collected dataset. Two main characteristics are required for live image guidance of percutaneous structural heart disease interventions: detailed characterization of soft tissue anatomy and the ability to localize devices with high precision and accuracy. As previously mentioned, fluoroscopy and echocardiography suffer from different technical limitations and thus offer unique advantages during a transcatheter procedure. Fusion of echocardiographic and fluoroscopic imaging mixes these attributes and may improve the interpretation of device orientation relative to surrounding cardiac structures as well as facilitate faster and safer catheter manipulation (9).
Co-registration is first performed when starting a procedure with dynamic fusion imaging. This involves spatially and temporally aligning the transesophageal (TEE) transducer within the fluoroscopy field by following software package specific steps. Real-time tracking of the TEE probe in fluoroscopic space is then based on a software algorithm that compares the live fluoroscopic appearance with a 3-dimensional model of the TEE transducer head to predict the TEE probe position and rotation relative to the C-arm. This software-determined TEE probe position is usually also displayed on screen to allow for direct visual confirmation that there are no registration errors. The accuracy of image registration is improved by using multiple sequential fluoroscopic images of the TEE probe at different C-arm angles (9-11). Depending on the fusion platform, registration may need to be repeated if the patient table height is changed.
Currently available fusion imaging software packages, like EchoNavigator (Philips Healthcare, Best, The Netherlands) and TrueFusion (Siemens Healthcare, Erlangen, Germany), includes automated registration and display of live 2-dimensional, 3-dimensional and colour Doppler transesophageal echocardiographic images whenever fluoroscopy is in use.
After this is done, the fusion imaging software can customize which parts of the data inputs are displayed by using various overlay options. The overlay image is also adjusted immediately if the fluoroscopic projection angle is changed. Screen layout of the hybrid images and raw source data is customizable and the system can be controlled by an operator using a wireless input device or by another individual in the control room. Newer versions of fusion platforms now allow the interventional imager to control overlay options and place custom anatomic markers directly on the echocardiography system, increasing the convenience, speed and functionality during cases. Additional control options of these commercial systems include image cropping (to remove soft tissue details not relevant for the procedure), overlay translucency adjustment (to prevent fluoroscopy from being obscured) and placement of persistent anatomic reference markers in fluoroscopic space (to mark key cardiac structures even when not in view of the echocardiographic imaging volume, such as the left upper pulmonary vein or fossa ovalis).
Early experience using both static and dynamic fusion imaging systems for transcatheter mitral and tricuspid interventions has been described.
Transcatheter native mitral valve intervention
Different options for transcatheter mitral valve intervention, particularly for those with functional mitral regurgitation, include edge-to-edge repair, annuloplasty and valve replacement. The role of echocardiography is critical during these procedures due to the need for an optimized transseptal puncture, steering of large systems in the left atrium, and precise therapeutic soft tissue targets.
Edge-to-edge repair
Transcatheter edge-to-edge repair is the most commonly performed transcatheter atrioventricular valve intervention to date. This technique replicates the surgical Alfieri stitch repair, which opposes the anterior and posterior leaflets to create a double orifice mitral valve to improve coaptation and reduce regurgitation (12). The MitraClip device (Abbot Vascular, Santa Clara, CA, USA) has been implanted in more than 100,000 patients worldwide since its introduction to the market and is available in two sizes (NTR and XTR) in the current third generation version and four sizes (additional NTW and XTW) in the upcoming fourth generation.
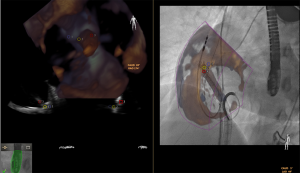
Specific advantages of a fusion imaging system for this procedure include assistance during transseptal puncture (13) (Figure 1), steering of the device in the left atrium to the valve, monitoring the guide sheath relative to the interatrial septum and allowing device trajectory and position to be assessed by fluoroscopy (Figure 2). It is possible that these advantages may lead to shorter procedure times with reduced radiation exposure as well as improved procedural and clinical outcomes.
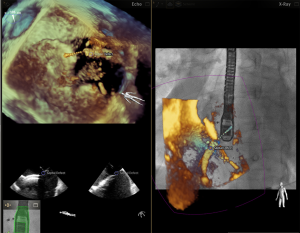
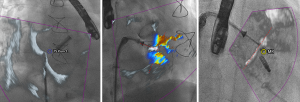
Afzal and colleagues published a study in 2017 in which they performed a pre-post analysis of transseptal puncture using fusion imaging for either MitraClip or left atrial appendage closure at two centres in Germany (14). Of the 88 patients included in the analysis, 32 received a MitraClip device. They found that the time required for a successful transseptal puncture was reduced from a mean of 23.2±9.6 to 18.5±5.6 minutes after the introduction of fusion imaging into their clinic. The success rate was equal in both groups and no complications were seen. Although patients were matched based on procedure type and the implant team, limitations of this study include the variability in patient anatomy and associated difficulty of transseptal puncture, increased experience of the operators in the post-fusion timeframe, and lack of statistical power for safety endpoints.
Sundermann and colleagues published a small study in 2014 comparing radiation dose, fluoroscopy time and procedure time for Mitraclip in a pre-post analysis of 42 patients treated in Zurich, Switzerland (15). They found that after the installation of a fusion imaging system, there was no change to any of their outcomes. No difference in safety outcomes was reported. Limitations of this study include the small sample size limiting the ability to detect differences, especially in the context of patient and procedure complexity heterogeneity. Additionally, increase experience of the operators over time was also not accounted for.
Aside from these two small observational studies, the remaining literature of fusion imaging for MitraClip implantation is limited to case reports (9,16,17).
The PASCAL system (Edwards Lifesciences, Irvine, CA, USA) is an investigational device that also allows for a transcatheter edge-to-edge repair of the atrioventricular valves. It differs from the MitraClip in that independent arm grasping is possible, it is larger in size, it has a central spacer component and the material, flexibility and grasping forces are different (18). There is currently no data on the use of fusion imaging specifically for PASCAL implantation. The potential advantages of using fusion imaging during this procedure would be expected to be the same as those for the MitraClip device.
Annuloplasty and chordal replacement
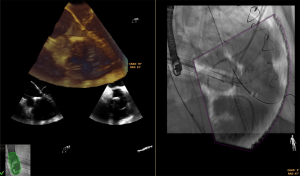
Mitral annuloplasty has been a longstanding component of a surgical mitral valve repair, and data has suggested increased repair durability when it is performed in addition to a leaflet-based repair. There is currently one transcatheter annuloplasty device with CE mark approval for commercial use, which is the Cardioband device (Edwards Lifesciences, Irvine, CA, USA) (19,20). This direct annuloplasty device is implanted through a transseptal puncture. A steerable guide catheter is then placed on the mitral annulus at the lateral trigone a few millimeters external to the mitral leaflet edge. From this initial position, a flexible dacron band with a central cinching wire is deployed by inserting sequential anchors through it into the ventricular myocardium from the left atrium. This is done along the circumference of the mitral annulus until the medial trigone is reached. At the end of the procedure, the band is slowly cinched under live echocardiographic control to titrate the final band size to the best effect on mitral regurgitation. Fusion imaging during this procedure has been reported previously by our group (Figure 3) (21,22).
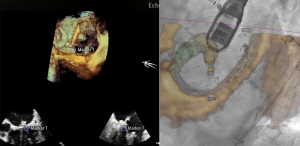
Transapical off-pump chordal replacement can be performed with the NeoChord (NeoChord Inc, St. Louis Park, Minnesota, USA) device to replicate chordal replacement surgery for degenerative mitral regurgitation. The advantage of this device is that the procedure is less invasive compared to conventional cardiac surgery, including the ability to avoid cardiopulmonary bypass (23).
The only report on the use of fusion imaging for Cardioband and NeoChord implantation was published in 2017 by von Bardeleben and colleagues (24). A combined, single-stage procedure including NeoChord and Cardioband implantation for degenerative mitral regurgitation in a high risk 63-year-old male. Fusion imaging was useful in this case because it allowed for the computed tomography-derived ideal transseptal puncture site and anchor locations to be marked and visualized on fluoroscopy.
Valve replacement
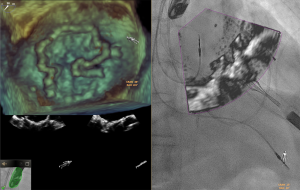
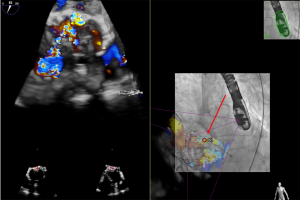
Several investigational transcatheter mitral valve replacement devices currently exist. The valves with the greatest clinical experience worldwide are the Medtronic Intrepid and Abbott Tendyne (4,5,25). Extensive imaging for planning and procedure guidance is needed for these complex procedures. There is currently no data on the use and benefits of fusion imaging for transcatheter mitral valve replacement, but some institutions have adopted more routine use during these specific procedures (Figure 4).
Transcatheter native tricuspid valve intervention
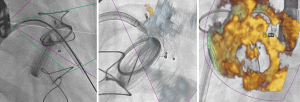
The field of transcatheter tricuspid valve intervention, including repair and replacement, is in its infancy but beginning to grow. Edge-to-edge transcatheter valve repair with the MitraClip is the most common procedure in the international multi-centre TriValve registry (1,26). The tricuspid Cardioband device is now commercially available in Europe. Other devices under investigation include the PASCAL edge-to-edge device, TriCinch (4Tech, Galway, Ireland) annuloplasty device, Trialign (Mitralign, Tewksbury, Massachusetts, USA) annuloplasty device, FORMA (Edwards Lifesciences, Irvine, California, USA) coaptation device NaviGate valved stent replacement (NaviGate Cardiac Structures, Lake Forest, California, USA) (2,27,28). Outside of individual case experience using fusion imaging during transcatheter tricuspid valve repair or replacement, there is no data evaluating procedural outcomes (Figures 5,6).
Transcatheter prosthetic valve intervention
Paravalvular leak around a prosthetic valve is a difficult to treat clinical entity that can be complicated by heart failure and significant hemolysis. This is particularly true for those in the mitral or aortic positions. While surgical replacement is an option, it is associated with a high risk of adverse outcomes (29). Therefore, transcatheter closure is often the preferred method of intervention if it is technically feasible. Mitral paravalvular leaks are best visualized by live 3D echocardiography, which provides the location and size of the leak(s) in an easy to understand perspective. Unfortunately, visualization of the catheters and devices in the ventricle is limited due to shadowing from the prosthesis. Therefore, fluoroscopy is still critical in these cases.
There is currently only minimal data on the use of fusion imaging for aortic and mitral paravalvular leak closure (21,30). Since the anatomic target is often quite small and the specific information needed from both echocardiography and fluoroscopy during catheter manipulation is limited, this potential application of fusion imaging can help localize a leak on fluoroscopy and may be particularly useful (Figures 7,8).
Conclusions
Fusion imaging allowing the simultaneous display and interpretation of multiple imaging modalities at once continues to evolve from a technologic standpoint. While these advancements increase the accuracy of final hybrid images and available overlay options, there need to be more studies evaluating whether this technology has an impact on procedural and clinical outcomes. The heterogeneity in patient anatomy, procedural complexity, device-specific procedural steps, operator skill and experience, and proprietary imaging systems are challenges in research on fusion imaging. At this time, there is insufficient data to suggest that it should be widely implemented in transcatheter procedure rooms. Rather, it is currently operator dependent on whether the use of fusion imaging adds to a specific procedure, and therefore, a matter of case-by-case operator preference.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Daniel Hernández-Vaquero) for the series “Structural Heart Disease: The Revolution” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The series “Structural Heart Disease: The Revolution” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Taramasso M, Alessandrini H, Latib A, et al. Outcomes After Current Transcatheter Tricuspid Valve Intervention. JACC Cardiovasc Interv 2019;12:155-65. [Crossref] [PubMed]
- Ho EC, Fam NP. Transcatheter therapies for tricuspid regurgitation: coaptation augmentation devices. Minerva Cardioangiol 2018;66:718-28. [Crossref] [PubMed]
- Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med 2018;379:2307-18. [Crossref] [PubMed]
- Bapat V, Rajagopal V, Meduri C, et al. Early Experience With New Transcatheter Mitral Valve Replacement. J Am Coll Cardiol 2018;71:12-21. [Crossref] [PubMed]
- Muller DWM, Farivar RS, Jansz P, et al. Transcatheter Mitral Valve Replacement for Patients With Symptomatic Mitral Regurgitation. J Am Coll Cardiol 2017;69:381-91. [Crossref] [PubMed]
- Zamorano JL, Badano LP, Bruce C, et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur Heart J 2011;32:2189-214. [Crossref] [PubMed]
- Krishnaswamy A, Tuzcu EM, Kapadia SR. Integration of MDCT and fluoroscopy using C-arm computed tomography to guide structural cardiac interventions in the cardiac catheterization laboratory. Catheter Cardiovasc Interv 2015;85:139-47. [Crossref] [PubMed]
- Faranesh AZ, Kellman P, Ratnayaka K, Lederman RJ. Integration of cardiac and respiratory motion into MRI roadmaps fused with x-ray. Med Phys 2013;40:032302. [Crossref] [PubMed]
- Faletra FF, Pedrazzini G, Pasotti E, Murzilli R, Leo LA, Moccetti T. Echocardiography–X-Ray Image Fusion. JACC Cardiovasc Imaging 2016;9:1114-7. [Crossref] [PubMed]
- Wiley BM, Eleid MF, Thaden JJ. Fusion Imaging for Procedural Guidance. Rev Esp Cardiol (Engl Ed) 2018;71:373-81. [Crossref] [PubMed]
- Clegg SD, Chen SJ, Nijhof N, et al. Integrated 3D Echo-X Ray to Optimize Image Guidance for Structural Heart Intervention. JACC Cardiovasc Imaging 2015;8:371-4. [Crossref] [PubMed]
- Alfieri O, Maisano F, De Bonis M, et al. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg 2001;122:674-81. [Crossref] [PubMed]
- Pozzoli A, Taramasso M, Russo M, et al. Echo-navigation to guide challenging transseptal puncture during transfemoral repair of mitral and tricuspid valve. J Cardiovasc Med (Hagerstown) 2018;19:73-4. [Crossref] [PubMed]
- Afzal S, Veulemans V, Balzer J, et al. Safety and efficacy of transseptal puncture guided by real-time fusion of echocardiography and fluoroscopy. Neth Heart J 2017;25:131-6. [Crossref] [PubMed]
- Sündermann SH, Biaggi P, Grünenfelder J, et al. Safety and feasibility of novel technology fusing echocardiography and fluoroscopy images during MitraClip interventions. EuroIntervention 2014;9:1210-6. [Crossref] [PubMed]
- Thaden JJ, Sanon S, Geske JB, et al. Echocardiographic and Fluoroscopic Fusion Imaging for Procedural Guidance: An Overview and Early Clinical Experience. J Am Soc Echocardiogr 2016;29:503-12. [Crossref] [PubMed]
- Jone PN, Haak A, Petri N, et al. Echocardiography-Fluoroscopy Fusion Imaging for Guidance of Congenital and Structural Heart Disease Interventions. JACC Cardiovasc Imaging 2019;12:1279-82. [Crossref] [PubMed]
- Praz F, Spargias K, Chrissoheris M, et al. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: a multicentre, prospective, observational, first-in-man study. Lancet 2017;390:773-80. [Crossref] [PubMed]
- Maisano F, Taramasso M, Nickenig G, et al. Cardioband, a transcatheter surgical-like direct mitral valve annuloplasty system: early results of the feasibility trial. Eur Heart J 2016;37:817-25. [Crossref] [PubMed]
- Nickenig G, Hammerstingl C, Schueler R, et al. Transcatheter Mitral Annuloplasty in Chronic Functional Mitral Regurgitation. JACC Cardiovasc Interv 2016;9:2039-47. [Crossref] [PubMed]
- Faletra FF, Pozzoli A, Agricola E, et al. Echocardiographic-fluoroscopic fusion imaging for transcatheter mitral valve repair guidance. Eur Heart J Cardiovasc Imaging 2018;19:715-26. [Crossref] [PubMed]
- Pozzoli A, Zuber M, Taramasso M, et al. 3D echo-fluoro fusion imaging to guide Cardioband transcatheter mitral annuloplasty. Eur Heart J Cardiovasc Imaging 2018;19:827. [Crossref] [PubMed]
- Colli A, Adams D, Fiocco A, et al. Transapical NeoChord mitral valve repair. Ann Cardiothorac Surg 2018;7:812-20. [Crossref] [PubMed]
- von Bardeleben RS, Colli A, Schulz E, et al. First in human transcatheter COMBO mitral valve repair with direct ring annuloplasty and neochord leaflet implantation to treat degenerative mitral regurgitation: Feasibility of the simultaneous toolbox concept guided by 3D echo and computed tomography fu. Eur Heart J 2018;39:1314-5. [Crossref] [PubMed]
- Beller JP, Rogers JH, Thourani VH, et al. Early clinical results with the Tendyne transcatheter mitral valve replacement system. Ann Cardiothorac Surg 2018;7:776-9. [Crossref] [PubMed]
- Taramasso M, Hahn RT, Alessandrini H, et al. The International Multicenter TriValve Registry. JACC Cardiovasc Interv 2017;10:1982-90. [Crossref] [PubMed]
- Ho EC, Ong G, Fam NP. Transcatheter tricuspid valve intervention. Curr Opin Cardiol 2019;34:164-72. [Crossref] [PubMed]
- Fam NP, Braun D, von Bardeleben RS, et al. Compassionate Use of the PASCAL Transcatheter Valve Repair System for Severe Tricuspid Regurgitation. JACC Cardiovasc Interv 2019;12:2488-95. [Crossref] [PubMed]
- Alkhouli M, Rihal CS, Zack CJ, et al. Transcatheter and Surgical Management of Mitral Paravalvular Leak: Long-Term Outcomes. JACC Cardiovasc Interv 2017;10:1946-56. [Crossref] [PubMed]
- Pozzoli A, Taramasso M, Zuber M, et al. Management of Aortic Prosthetic Leaks. In: Surgical Management of Aortic Pathology. Vienna: Springer Vienna, 2019:719-30.


