
The association of white matter hyperintensities with stroke outcomes and antiplatelet therapy in minor stroke patients
Introduction
White matter hyperintensities (WMHs) or leukoaraiosis, as one of the main magnetic resonance imaging (MRI) expressions of cerebral small vessel disease (CSVD) (1), are detected in 64–86% of stroke patients (2,3). WMHs may have important clinical implications as predictors of poor functional outcome, increased mortality, and long-term recurrence after ischemic stroke (2,4,5). However, the clinical significance of WMHs on stroke outcomes was inconclusive (6).
Data also suggest that stroke patients with WMHs may respond differently to treatment and secondary prevention (7-9). However, the therapeutic effect of stroke preventive therapies in patients with WMHs have yet to be reported from a randomized controlled trial. However, the interaction of WMHs severities with antiplatelet therapies for stroke outcomes has rarely been reported.
Moreover, the distribution of WMH seems to be an even more critical factor. Periventricular white matter hyperintensities (PVWMHs) and subcortical deep white matter hyperintensities (SDWMHs) are supposed to have different pathophysiological significance, thus distribution of WMHs may be a critical factor for clinical consequences after stroke (10-13). Investigations on WMHs will help to stratify risks in stroke patients.
Accordingly, we sought to characterize WMHs in a population of CSVD by examining minor stroke patients within the Clopidogrel in High-risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial and assess the relationship between WMHs and outcomes of stroke, as well as response to antiplatelet treatments.
Methods
Study design and participants.
We derived data from the CHANCE trial. Details about the rationale, design, and results of the trial have been published elsewhere (14,15). In brief, CHANCE was a randomized, double-blind, placebo-controlled clinical trial conducted in China between October 2009 and July 2012 to compare the efficacy and safety of combination therapy with Clopidogrel and Aspirin (Clopidogrel: loading dose of 300 mg followed by 75 mg daily for 90 days; Aspirin: loading dose of 75–300 mg followed by 75 mg daily for 21 days) vs. aspirin alone (loading dose of 75–300 mg followed by 75 mg daily for 90 days) in patients with minor stroke or high-risk TIA (transient ischemic attack). The inclusion criteria were as followed: age 40 years or older and diagnosis of an acute minor ischemic stroke (NIHSS ≤3) or high-risk TIA (ABCD2 ≥4) (16) within 24 hours after symptom onset. A total of 5,170 patients with minor stroke or TIA were included in the trial.
Standard protocol approvals, registrations, and patient consents
The CHANCE trial is listed on clinicaltrials.gov (NCT00979589). The protocol and data collection of the trial were approved by the ethics committee of Beijing Tiantan Hospital and all participating centers. All participants or their representatives provided written informed consent before inclusion into the study.
Data collection
The data were collected through face-to-face interview by trained and certified neurologists who were blinded to patients’ treatment allocation and clinical information. Patient demographic information, vascular risk factors, symptoms of the qualifying event, pretreatment modified Rankin Scale score, stroke severity, treatment allocation, and time from index event to randomization were collected. Vascular risk factors included history of stroke or TIA, myocardial infarction, angina, known atrial fibrillation, hypertension, diabetes, hyperlipidemia, and smoking.
Participants of the imaging subgroup
Patients who were required to undergo magnetic resonance (MR) examinations (3.0 or 1.5 T) at baseline with the following sequences were included in the imaging subgroup: T1 weighted imaging, T2 weighted imaging, fluid-attenuated inversion recovery (FLAIR), diffusion weighted imaging (DWI), and 3D time-of-flight MR angiography (MRA). Those without any of the aforementioned sequences of MR examinations at baseline were excluded from the imaging subgroup. As reported in our previous article (17), baseline characteristics of patients in the trial with and without the MR sequences were similar.
Image analysis and interpretation
All images were centrally read by 2 readers (L Zong and C Zhang), who were blinded to patient information. Neuroimage markers of CSVD including WMHs, lacunes, cerebral microbleeds (CMBs), and perivascular spaces (PVSs) were evaluated.
FLAIR sequences were used to evaluate the degree and distribution of WMHs. WMHs were scored using Fazekas scale (18). PVWMHs were defined as lesions contiguous with the margins of the lateral ventricles and extending up to and including 10 mm from the lateral ventricle into the white matter. SDWMHs were defined as lesions that were separated from the margins of the lateral ventricles, regardless of their distance to the margins of the lateral ventricles. PVWMHs and SDWMHs were evaluated separately and totaled as Fazekas scores. The severity of WMHs was rated by Fazekas scores (mild 0–2; moderate 3–4; severe 5–6). PVWMHs and SDWMHs were both divided into low [0–1] and high [2–3] groups.
The inter-rater agreement for the rating of WMHs was assessed on a random sample of 100 subjects at 8-week intervals. The intrareader reliability analysis showed a good reliability with κ values of 0.73 and 0.79 for PVWMHs and SDWMHs respectively.
Other neuroimaging markers of CSVD were assessed as following standards (19). Lacunes were defined as focal lesions 3 to 15 mm in size, with the same signal characteristics as cerebrospinal fluid on all MRI sequences, and surrounded by a hyperintense rim on FLAIR images, mainly situated in basal ganglia or white matter. Lacunes were initially assessed on T1WI. CMBs were defined as a round or ovoid area of homogeneous signal loss on SWI, 2 to 10 mm in diameter with blooming effect. Multiple CMBs were defined as ≥5 CMBs. PVSs were defined as small (<3 mm) punctate (if perpendicular to the plane of scan) or linear (if longitudinal to the plane of scan) hyperintensities on T2WI in the basal ganglia or centrum semiovale. Burden of PVSs was then stratified into 4 groups: 0, 1–10, 11–20, >20. Severe PVSs were defined as >10 PVSs in the basal ganglia, or >20 in the centrum semiovale.
Outcomes
Stroke outcomes included initial stroke severity measured by the NIHSS at admission, functional stroke outcome measured by 3-month mRS, and stroke recurrence (ischemic stroke and hemorrhagic stroke). The initial stroke severity was categorized as low (NIHSS 0–1) and high (NIHSS 2–3) levels. Patients’ functional outcomes were dichotomized into good and poor categories by applying the mRS score cutoff 1 (no significant disability)/2 (slight disability).
Statistical analysis
We presented categorical variables as percentages and continuous variables as mean with SD or median with interquartile range. The continuous variables between the 3 groups were compared by one-way analysis of variance or Kruskal-Wallis test. Categorical variables were compared by the χ2 test. We assessed the associations between WMHs (including Fazekas scale score ≥2 for the PVWMHs and SWMHs) and outcomes (NIHSS, mRS, and stroke recurrence) using multivariable Logistic regression models. Adjusted factors for NIHSS included age, gender, medical history, smoking, multiple CMBs, lacunes, and severe PVSs. Adjusted factors for mRS and stroke recurrence included age, gender, medical history, baseline NIHSS, smoking, antiplatelet therapy within 90 days, multiple CMBs, lacunes, and severe PVSs. In addition, we assessed whether the treatment effect differed in certain prespecified subgroups by testing the treatment-by-subgroup interaction effect with the use of Cox models. The level of significance was P<0.05 (2-sided). All analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Study participants and characteristics
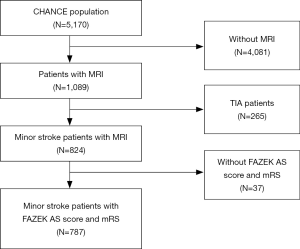
Among the 5,170 patients enrolled in the CHANCE trial, 1,089 patients at 45 sites underwent MR examinations at baseline. After excluding TIA patients and those without Fazekas scores and mRS at 3 months, 787 patients undergoing baseline MR examinations with all the required sequences were included in this subgroup analysis (Figure 1).
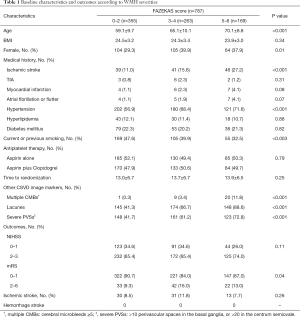
Among the 787 patients, there were 355 (45.1%), 263 (33.4%), and 169 (21.5%) patients identified as mild level [0–2], moderate level [3–4], and severe level [5–6] based on Fazekas scores respectively. Patients with higher Fazekas scores were older, more likely to be female (P=0.01), and had more vascular risk factors, such as ischemic stroke history (P<0.001), hypertension (P<0.001), and smoking (P<0.003) (Table 1). Patients with severe WMHs were more likely to have multiple CMBs (P<0.001), lacunes (P<0.001), severe PVSs (P<0.001), and worse functional outcomes (P=0.04). There was no statistic difference in NIHSS scores and stroke recurrence among 3 groups. Recurrent ischemic stroke occurred in 90 (8.7%) patients, and none developed hemorrhage stroke (Table 1).
Full table
Association of WMHs with stroke outcomes
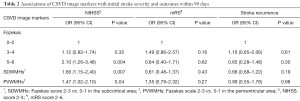
Table 2 shows associations of the severity and location of WMHs with outcomes. After adjustment for age, gender, final diagnosis, medical history, antiplatelet therapy within 90 days, and other CSVD markers (multiple CMBs, lacunes, and severe PVSs), severity of WMHs (OR 2.10; 95% CI, 1.26–3.48; P=0.004), SDWMHs (OR 1.66; 95% CI, 1.15–2.40; P=0.007) and PVWMHs (OR 1.47; 95% CI, 1.02–2.10; P=0.04) were associated with higher NIHSS scores. After adjustment, the severity and location of WMHs were not related to higher mRS scores and stroke recurrence.
Full table
Effect of randomized interventions
The risk of stroke recurrence and functional outcome did not differ for dual antiplatelet therapy versus aspirin alone amongst patients with different WMH features. There were no significant interactions in any of the subgroups (P>0.10 for all comparisons) (Table 3). Since none of patients developed hemorrhage stroke, there was no suggestion that the dual antiplatelet therapy increased intracranial hemorrhage in patients with severe WMHs.

Full table
Discussion
Our study reveals that high WMHs burden, especially SDWMHs burden, substantially impacts initial stroke severity in minor stroke; WMHs might not be a predictor of mRS and stroke recurrence in 3 months.
Our results agree with findings from the studies showing that the WMHs were highly predictive for NIHSS on admission (20,21). The probable reason is that loss of white matter tract organization resulted in reduced functional network integrity. It is difficult to compensate for deficits caused by acute ischemic stroke. This hypothesis will require confirmation in additional studies. One intriguing finding is that SDWMHs and PVWMHs both predict severe NIHSS, while SDWMHs might be more relevant. The two subtypes of WMHs have different but converging trajectories, possibly because of overlapping but not identical mechanisms and pathogenesis involved (22). Neuropathological differences are reported that PVWMHs are more likely due to diminished cerebral vasomotor reactivity and subsequent hypoperfusion, while SDWMHs are related to microangiopathy (22). In addition, the functional significance of the two subtypes is possible to be different. In a study involving stroke patients, SDWMHs had a stronger association with cortical perfusion, although SDWMHs counted for only 1/3 of the total WMHs volume, with PVWMHs counting for 2/3 (23,24). That supports our finding, that the two subtypes are differently associated with initial stroke severity in CSVD.
Some studies suggested that patients with WMHs have a higher risk of intracranial hemorrhage and disability after stroke (25,26). Particularly, periventricular WMH volume was associated with accelerated functional decline (27). However, we demonstrated that WMHs has no impact on short-term functional outcome. In addition, several studies indicate that PVWMHs were significantly associated with poor functional outcome at 3 months (12,13,28). However, mRS does not seem to corelate with the location of WMHs in our study. This might be due to the fact that we exclusively included patients with minor stroke who have relatively good functional outcomes. Because of the limited sample size, the result is not conclusive. Further studies are needed to fully understand the association and mechanism.
Whether WMHs are risk factors of intracranial hemorrhage is controversial in prior studies (6,25). We did not observe a statistically significant treatment interaction between WMHs and dual vs. mono antiplatelet therapy. Therefore, the presence of WMHs might not impact on clinical decision making regarding the second preventive treatment.
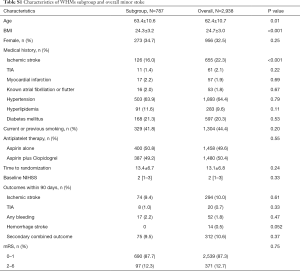
Our study had several limitations. First, potential selection bias might have existed since this subgroup analysis included only approximately 20% of patients from 45 of 114 participating sites providing MRIs. However, baseline characteristics of patients in the trial with and without the MR sequences were similar (Table S1). Second, the CHANCE trial enrolled only Chinese patients. Hence the external generalizability of the findings of this subgroup analysis needs further validation in Western populations. Third, MRI-based WMHs grading using the Fazekas scale is well established, frequently used in clinical research, and has been shown to correlate well with the WMHs volume. However, the volume of WMHs might be a more precise way to define the severity of CSVD (29). Forth, some patients were scanned with 1.5 T MRI and some other with 3.0 T MRI. This might cause inconsistency in the evaluation of image markers of CSVD. Fifth, in CHANCE study, minor stroke was defined as an acute ischemic stroke with NIHSS ≤3. However, the standardized surrogate information of the stroke severity might include infarct size on DWI at presentation. Sixth, in our study, stroke subtypes were not determined based on the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria. There are suggestions that stroke outcomes may be affected by WMHs differentially depending on stroke subtypes (29).
Full table
Conclusions
In summary, in patients with minor stroke, both SDWMHs and PVWMHs were predictors for initial stroke severity. WMHs might not be associated with the effect of secondary stroke preventative therapies.
Acknowledgements
The authors thank all the participants who gave their time to the study.
Funding: This study was supported by grants from the Ministry of Science and Technology of the People’s Republic of China (2016YFC0901001, 2016YFC0901002, 2017YFC1310901), grants from Beijing Municipal Commission of Health and Family Planning (No. 2016-1-2041, SML20150502), National Natural Science Foundation of China (81801139), and Beijing Municipal Science & Technology Commission, code: D151100002015003.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol and data collection of the trial were approved by the ethics committee of Beijing Tiantan Hospital and all participating centers. All participants or their representatives provided written informed consent before inclusion into the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S. What Are White Matter Hyperintensities Made Of? Relevance to Vascular Cognitive Impairment. J Am Heart Assoc 2015;4:001140. [Crossref] [PubMed]
- Fu JH, Lu CZ, Hong Z, et al. Extent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischaemic stroke. J Neurol Neurosurg Psychiatry 2005;76:793-6. [Crossref] [PubMed]
- Li L, Simoni M, Kuker W, et al. Population-based case-control study of white matter changes on brain imaging in transient ischemic attack and ischemic stroke. Stroke 2013;44:3063-70. [Crossref] [PubMed]
- Arsava EM, Rahman R, Rosand J, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology 2009;72:1403-10. [Crossref] [PubMed]
- Hasan TF, Barrett KM, Brott TG, et al. Severity of White Matter Hyperintensities and Effects on All-Cause Mortality in the Mayo Clinic Florida Familial Cerebrovascular Diseases Registry. Mayo Clin Proc 2019;94:408-16. [Crossref] [PubMed]
- Palumbo V, Boulanger JM, Hill MD, et al. Leukoaraiosis and intracerebral hemorrhage after thrombolysis in acute stroke. Neurology 2007;68:1020-4. [Crossref] [PubMed]
- Atchaneeyasakul K, Leslie-Mazwi T, Donahue K, et al. White Matter Hyperintensity Volume and Outcome of Mechanical Thrombectomy With Stentriever in Acute Ischemic Stroke. Stroke 2017;48:2892-4. [Crossref] [PubMed]
- Curtze S, Melkas S, Sibolt G, et al. Cerebral computed tomography-graded white matter lesions are associated with worse outcome after thrombolysis in patients with stroke. Stroke 2015;46:1554-60. [Crossref] [PubMed]
- Han SW, Song TJ, Bushnell CD, et al. Cilostazol decreases cerebral arterial pulsatility in patients with mild white matter hyperintensities: subgroup analysis from the Effect of Cilostazol in Acute Lacunar Infarction Based on Pulsatility Index of Transcranial Doppler (ECLIPse) study. Cerebrovasc Dis 2014;38:197-203. [Crossref] [PubMed]
- de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 2000;47:145-51. [Crossref] [PubMed]
- O'Brien J, Desmond P, Ames D, et al. A magnetic resonance imaging study of white matter lesions in depression and Alzheimer's disease. Br J Psychiatry 1996;168:477-85. [Crossref] [PubMed]
- Kang HJ, Stewart R, Park MS, et al. White matter hyperintensities and functional outcomes at 2 weeks and 1 year after stroke. Cerebrovasc Dis 2013;35:138-45. [Crossref] [PubMed]
- Liou LM, Chen CF, Guo YC, et al. Cerebral white matter hyperintensities predict functional stroke outcome. Cerebrovasc Dis 2010;29:22-7. [Crossref] [PubMed]
- Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11-9. [Crossref] [PubMed]
- Wang Y, Johnston SC. Rationale and design of a randomized, double-blind trial comparing the effects of a 3-month clopidogrel-aspirin regimen versus aspirin alone for the treatment of high-risk patients with acute nondisabling cerebrovascular event. Am Heart J 2010;160:380-6.e1. [Crossref] [PubMed]
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283-92. [Crossref] [PubMed]
- Liu L, Wong KS, Leng X, et al. Dual antiplatelet therapy in stroke and ICAS: Subgroup analysis of CHANCE. Neurology 2015;85:1154-62. [Crossref] [PubMed]
- van Straaten EC, Fazekas F, Rostrup E, et al. Impact of white matter hyperintensities scoring method on correlations with clinical data: the LADIS study. Stroke 2006;37:836-40. [Crossref] [PubMed]
- Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822-38. [Crossref] [PubMed]
- Helenius J, Henninger N. Leukoaraiosis Burden Significantly Modulates the Association Between Infarct Volume and National Institutes of Health Stroke Scale in Ischemic Stroke. Stroke 2015;46:1857-63. [Crossref] [PubMed]
- Helenius J, Goddeau RP Jr, Moonis M, et al. Impact of Leukoaraiosis Burden on Hemispheric Lateralization of the National Institutes of Health Stroke Scale Deficit in Acute Ischemic Stroke. Stroke 2016;47:24-30. [Crossref] [PubMed]
- Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993;43:1683-9. [Crossref] [PubMed]
- Wen W, Sachdev P, Shnier R, et al. Effect of white matter hyperintensities on cortical cerebral blood volume using perfusion MRI. Neuroimage 2004;21:1350-6. [Crossref] [PubMed]
- Sachdev P, Wen W. Should we distinguish between periventricular and deep white matter hyperintensities? Stroke 2005;36:2342-3; author reply 3-4. [Crossref] [PubMed]
- Kongbunkiat K, Wilson D, Kasemsap N, et al. Leukoaraiosis, intracerebral hemorrhage, and functional outcome after acute stroke thrombolysis. Neurology 2017;88:638-45. [Crossref] [PubMed]
- Inzitari D, Pracucci G, Poggesi A, et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ 2009;339:b2477. [Crossref] [PubMed]
- Dhamoon MS, Cheung YK, Bagci A, et al. Periventricular White Matter Hyperintensities and Functional Decline. J Am Geriatr Soc 2018;66:113-9. [Crossref] [PubMed]
- Kissela B, Lindsell CJ, Kleindorfer D, et al. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race. Stroke 2009;40:530-6. [Crossref] [PubMed]
- Ryu WS, Woo SH, Schellingerhout D, et al. Stroke outcomes are worse with larger leukoaraiosis volumes. Brain 2017;140:158-70. [Crossref] [PubMed]


