
Dihydroartemisinin: from malaria to the treatment of relapsing head and neck cancers
Head and neck squamous cell carcinoma (HNSCC) represents the 7th most common neoplasia worldwide. Eight hundred and ninety thousand new cases and 450,000 deaths were recorded in 2018 (1). Alcohol abuse, tobacco, human papillomavirus (HPV) infection increase the risk for HNSCC development. Despite the new diagnostic and therapeutic advances, HNSCC relapsed in more than 60% of patients (2). Currently, the management of HNSCC tumor relapses should take in consideration several aspects of the patients, such as previous treatment and clinical status. The EXTREME trial established that the combination of cetuximab, an anti-epidermal growth factor receptor (EGFR) monoclonal antibody, with standard treatment, fluorouracil plus platinum, significantly increases overall survival, progression free survival and overall response (3). Indeed, 90% of head and neck squamous-cell carcinoma are characterized by an high expression of EGFR (4), only few patients find a benefit from the new therapeutic regimen compared to standard treatment (36% vs. 20%) (3). The inherent and acquired molecular resistance mechanisms to anti EGFR treatment have been widely investigated in non-small cell lung cancer (NSCLC), in which mutations in the EGFR tyrosine kinase (TK) domain have been found to impair EGFR TK inhibitor treatment sensitivity, the same mechanism does not occur in HNSCC (5). Moreover, the KRAS mutational status determines anti EGFR treatment resistance in colon rectal cancer, but this event has been rarely found in HNSCC (6). Several studies have profiled the gene mutational and expression landscapes of different HNSCC tumors casuistries to understand the molecular mechanisms of drug resistance and provide new treatment strategies for HNSCC relapsing patients management. TP53 is the most frequently mutated gene in HPV negative HNSCC patients, but it is not directly targetable. Intriguingly, alpelisib treatment, a direct phosphatidylinositol 3-kinase alpha (PI3Kα) inhibitor, has been discovered to impinge on mutant gain-of-function p53-Myc dependent gene signature, which expression level correlated with alpesilib-response in patient-derived xenografts (PDX) and cell lines of HNSCC (7). Conversely to TP53, EGFR overexpression and PIK3CA amplification were diagnosed both in HPV-negative and in HPV-positive patients (8). The aberrant activation of PI3K/Akt/mTOR pathway has been shown to be a resistance mechanism to EGFR inhibitors. HNSCC patients harboring PI3KCA mutation, indeed, relapse suddenly after few weeks of cetuximab treatment response. However, anti PI3KCA monotherapy failed due to new emerging mechanisms of resistance (9,10).
Based on clinical and experimental results, monotherapy appears inadequate for treatment of HNSCC relapsed patients, whose inherent and acquired drug resistant mechanisms should be bypassed through the use of a drug combination therapy. Accordingly, Chaib et al., investigated the efficacy of the association of osimertinib, a third generation of EGFR TK inhibitor, with dihydroartemisinin (DHA), an artemisinin semi-synthetic derivative prescribed for malaria treatment that has, also, showed anticancer activity (11). Notably, they assessed viability of two different HNSCC cell lines, Cal-27 and FaDu, treated either with the two compounds alone or in association. They also compared the effect of the osimertinib/DHA combination with the association of osimertinib with three different target drugs, currently in clinical trial for HNSCC management, thus to target key pathways involved in the acquired resistance to the EGFR inhibitor drugs. In particular they combined osimertinib with either R428, an inhibitor of the receptor tyrosine kinase (RTXs) AXL, or BML258, a sphingosine kinase 1 (SPHK1) inhibitor. Transactivation of EGFR by either over expression of AXL or sphingosine kinase 1 (SPHK1) aberrant activity has been associated to cetuximab-resistance in HNSCC (Figure 1). In addition, they co-treated cells with osimertinib and TPX-0005, an inhibitor of the Src/janus-like kinase 2 (JAK2)/focal adhesion kinase (FAK) pathways. Aberrant activation of EGFR downstream pathways such as Src and JAK leads to the activation of YES associated protein 1 (YAP1), FAK and the signal transducer and activator of transcription 3 (STAT3); thereby resulting in the overexpression of several genes involved in HNSCC tumorigenesis and drug resistance mechanisms (Figure 1). All the tested drugs increased osimertinib citotoxicity in vitro, however, combination with DHA showed very low combination index value compared to the other treatments. Effectively, the combination of osimertinib with DHA significantly affected Cal-27 and FaDu xenografts tumor volume size compared to single treatment with osimertinib or DHA. Remarkably, while the single treatment with osimertinib promoted expression of several proteins involved in anti EGFR-resistance like YAP1, FAK, STAT3 and Src; the combined treatment with DHA significantly reduced the expression of these targets. In addition, after seven days of combined treatment, Cal-27 and FaDu cells showed a reduction in AXL, transmembrane protein CUB domain-containing protein 1 (CDCP1) and MET RTK mRNA levels compared to the same cells treated with osimertinib alone. Previous evidences demonstrated that DHA treatment impinges concurrently on several pathways. In particular, it induces reactive oxygen species (ROS) release, cell cycle arrest through downregulation of the cyclin-depedent kinase 4 (CDK4), apoptosis, inhibition of angiogenesis by affecting vascular endothelial growth factor (VEGF) production, ferroptosis by inhibiting GPx4 and Ras expression (12). DHA also increases cisplatin (CDDP) citotoxicity in HNSCC cell lines impairing JAK2/STAT3 signaling (13) (Figure 1). Accordingly, as Chaib et al., reported in their work, DHA treatment alone induced a strong reduction of the downstream effectors of the JAK2/FAK/Src pathways more than the combined treatment with osimertinib, which activities impinge mainly on AXL and phosphor-Akt axis.
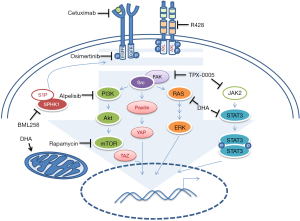
Although this study highlighted an interesting potential role of DHA in inhibiting key targets involved in the anti EGFR therapy resistance, the experiments have been performed on previously untreated HNSCC cell lines. The use of an in vitro generated anti EGFR-resistant HNSCC cell model might help to better understand the mechanisms by which DHA impinges on resistance processes, as well as to provide evidences of the effectiveness of osimertinib in a HNSCC relapsing model. Osimertinib, indeed, is a mutant-specific, but not wild-type, inhibitor of EGFR-TKI, designed to overcome EGFRT790M resistance mutation in lung cancer. It exhibited reduced pharmacologic activity and EGFR phosphorylation inhibition activity toward EGFR wild type cancer cell lines compared to EGFR mutated-ones (14). As mentioned above, 90% of HNSCC patients present EGFR-overexpression, but only 1% of them harbor EGFR gene mutation (8). Moreover, neither Cal-27 nor FaDu cell lines carry any EGFR mutation. In particular, Cal-27 cells harbor three EGFR gene copies without any activating mutation (15), while FaDu cells have a moderate EGFR expression due to the absence of further EGFR copy numbers (16). Currently, osimertinib is prescribed as first-line treatment for EGFRT790M metastatic NSCLC, while there are no clinical trials testing the efficacy of this compound in HNSCC. Additional biological and molecular studies are mandatory to provide evidences of the effectiveness of osimertinib in HNSCC.
Overcoming inherent and acquired resistance mechanisms is a pivotal issue for the management of HNSCC relapsing patients. Clinical trials testing the efficacy of multi agent combination treatment provided contradictory results so far. In this regard, a multi target agent, as artemisinin and its derivatives (ARTs), represents a potential option for HNSCC relapsing patients treatment. Treatment with DHA, indeed, significantly increases the cytotoxic effects of chemotherapy conventional treatment, such as epirubicin in chemo resistant breast cancer cells (17), gemcitabine in pancreatic cancer cells and hepatoma in vitro and in vivo models (18,19), cyclophosphamide and cisplatin in NSCLC in vivo models (20), temozolomide in glioma cells (21). Moreover, the published results of clinical trials that enrolled cancer patients treated with standard chemo therapy plus ARTs had proved safe and effective (12). However, there are not clinical studies that investigate the effectiveness of ARTs in HNSCC patients so far. In this regard, the repurposing of ‘old’ drugs, as DHA, is an attractive opportunity for management of HNSCC relapsing patients, due to low risk profile, low studies costs and a fast timeline development.
Acknowledgments
Funding: GB acknowledges the support of AIRC IG 2017 - ID. 20613, Regione Lazio and MAECI Italy/USA bilateral grant program.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.104). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Argiris A, Karamouzis MV, Raben D, et al. Head and neck cancer. Lancet 2008;371:1695-709. [Crossref] [PubMed]
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116-27. [Crossref] [PubMed]
- Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011;11:9-22. [Crossref] [PubMed]
- Cohen EE, Kane MA, List MA, et al. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res 2005;11:8418-24. [Crossref] [PubMed]
- Sheu JJ, Hua CH, Wan L, et al. Functional genomic analysis identified epidermal growth factor receptor activation as the most common genetic event in oral squamous cell carcinoma. Cancer Res 2009;69:2568-76. [Crossref] [PubMed]
- Ganci F, Pulito C, Valsoni S, et al. PI3K inhibitors curtail MYC-dependent mutant p53 gain-of-function in head and neck squamous cell carcinoma. Clin Cancer Res 2020;clincanres.2485.2019. [Epub ahead of print].
- Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck 2016;38 Suppl 1:E1625-38. [Crossref] [PubMed]
- Juric D, Castel P, Griffith M, et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kalpha inhibitor. Nature 2015;518:240-4. [Crossref] [PubMed]
- Lui VW, Hedberg ML, Li H, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov 2013;3:761-9. [Crossref] [PubMed]
- Wong YK, Xu C, Kalesh KA, et al. Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action. Med Res Rev 2017;37:1492-517. [Crossref] [PubMed]
- Slezakova S, Ruda-Kucerova J. Anticancer Activity of Artemisinin and its Derivatives. Anticancer Res 2017;37:5995-6003. [PubMed]
- Jia L, Song Q, Zhou C, et al. Dihydroartemisinin as a Putative STAT3 Inhibitor, Suppresses the Growth of Head and Neck Squamous Cell Carcinoma by Targeting Jak2/STAT3 Signaling. PLoS One 2016;11:e0147157. [Crossref] [PubMed]
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. [Crossref] [PubMed]
- Licitra L, Perrone F, Tamborini E, et al. Role of EGFR family receptors in proliferation of squamous carcinoma cells induced by wound healing fluids of head and neck cancer patients. Ann Oncol 2011;22:1886-93. [Crossref] [PubMed]
- Kjær I, Lindsted T, Frohlich C, et al. Cetuximab Resistance in Squamous Carcinomas of the Upper Aerodigestive Tract Is Driven by Receptor Tyrosine Kinase Plasticity: Potential for mAb Mixtures. Mol Cancer Ther 2016;15:1614-26. [Crossref] [PubMed]
- Chen K, Shou LM, Lin F, et al. Artesunate induces G2/M cell cycle arrest through autophagy induction in breast cancer cells. Anticancer Drugs 2014;25:652-62. [PubMed]
- Hou J, Wang D, Zhang R, et al. Experimental therapy of hepatoma with artemisinin and its derivatives: in vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res 2008;14:5519-30. [Crossref] [PubMed]
- Wang SJ, Gao Y, Chen H, et al. Dihydroartemisinin inactivates NF-kappaB and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro and in vivo. Cancer Lett 2010;293:99-108. [Crossref] [PubMed]
- Zhou HJ, Zhang JL, Li A, et al. Dihydroartemisinin improves the efficiency of chemotherapeutics in lung carcinomas in vivo and inhibits murine Lewis lung carcinoma cell line growth in vitro. Cancer Chemother Pharmacol 2010;66:21-9. [Crossref] [PubMed]
- Huang XJ, Li CT, Zhang WP, et al. Dihydroartemisinin potentiates the cytotoxic effect of temozolomide in rat C6 glioma cells. Pharmacology 2008;82:1-9. [Crossref] [PubMed]


