
20/20 in 2020: seeking clarity on the management of stage III melanoma in a rapidly changing treatment environment
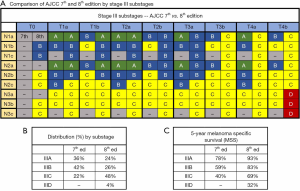
Clinical management of cutaneous melanoma, especially resectable stage III disease, has rapidly evolved over the past decade, and critical to developing an accurate treatment plan has been disease staging. The American Joint Committee on Cancer (AJCC) 7th edition melanoma staging guideline was published in 2009 (1), and more recently in January 2018, the updated 8th edition was released (2). Most importantly, the newer 8th edition makes specific modifications in staging with regards to tumor (T) and nodal (N) definitions and led to the division of stage III disease into 4, rather than 3 substages, with the majority of stage III patients now falling under stage IIIC (48%) vs. the 7th edition which classified a majority of these patients as Stage IIIB (42%) (Figure 1A,B). The underlying intent of these staging modifications is to enhance ability to estimate patient prognosis, primarily melanoma specific survival (MSS), by readily identifying lower-risk IIIA and higher-risk IIID patients (Figure 1C).
During the almost 10-year tenure of the 7th edition, two critical changes in the management of stage III disease occurred. Firstly in 2017, the Multicenter Selective Lymphadenectomy Trial-II (MSLT-II) trial was published (3). This trial with 1,934 patients determined that while immediate completion lymph node dissection (CLND) following a positive sentinel lymph node (SLN) biopsy does improve locoregional disease control, it does not result in meaningful improvements in 3-year disease free survival. Thus, a shift in clinical paradigm was born, and patients were no longer required to undergo CLND after local excision. Instead, patients could be followed by frequent high-performance ultrasound following SLN biopsy.
Secondly, the late 2000s and early 2010s brought about the rise of systemic targeted therapies (anti-BRAF/MEK) and immunotherapies (anti-PD-1 and anti-CTLA-4 antibodies) for patients with advanced melanoma. In fact three treatment regimens have been recently approved for use in the post-surgical adjuvant setting and have become the primary therapies used in this setting, namely: nivolumab (anti-PD-1 antibody for resected stage III and stage IV disease); dabrafenib plus trametinib (BRAF/MEK inhibitors for resected stage III tumors that harbor the BRAF V600E/K mutation); and pembrolizumab (anti-PD-1 antibody for resected stage III melanoma) (4-6). An important point to recognize is that all of the trials that led to approval of these agents were based on 7th edition staging, focused on the primary endpoint of recurrence free survival (RFS), and due to their temporal overlap with MSLT-II, required all patients to undergo CLND following local excision and SLN biopsy prior to receiving adjuvant intervention.
Data from one of these trials, EORTC125/Keynote-054, was recently re-evaluated retrospectively in the context of the AJCC 8th edition. In this paper, Eggermont et al. (7) sought to determine the effect of the new staging system on results of this trial by retrospectively restaging all patients by the 8th edition criteria. In particular, the authors examined how the effect of adjuvant pembrolizumab on RFS in substages changed and assessed if the new staging system conferred any predictive benefit in the trial population.
As anticipated, there were changes in the distribution of patients in substages. Per their findings, patient distribution by the AJCC 7th edition substage criteria was 15.0% in IIIA, 46.3% in IIIB, and 38.7% in IIIC, whereas after re-classification according to the 8th edition it was 8% in IIIA, 34.7% in IIIB, 49.7% in IIIC, 3.7% in IIID, and 3.8% unknown.
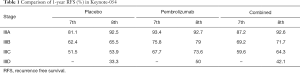
A 1-year RFS benefit from adjuvant pembrolizumab was seen in all 8th edition substages, but the absolute benefit in IIIA disease was lower in patients staged according to the 8th edition (92.5% vs. 92.7%) than in those staged according to 7th edition (81.1% vs. 93.4%), which suggests a lower-risk population for which therapy may be less beneficial (Table 1).
Full table
No statistically significant difference in hazard ratios [IIIA (0.76), IIIB (0.59), IIIC (0.48), and IIID (0.69)] was seen amongst the substage, demonstrating the lack of predictive benefit of the 8th edition in this setting.
Looking at combined RFS for both arms, patients staged by the 8th edition had improved outcomes in the IIIA, IIIB, and IIIC substages as compared to patients staged according to 7th edition criteria, with the new IIID substage identifying a smaller population of patients with poorer RFS outcomes, as would be anticipated (Table 1).
Overall, the results of this retrospective analysis by Eggermont et al. demonstrate that the benefit of adjuvant pembrolizumab on one-year RFS is seen in all 8th edition substages. The absolute benefit of adjuvant pembrolizumab for stage IIIA patients by the 8th edition was minimal compared to the 7th edition analysis, while there was simultaneously an approximate halving of the IIIA population [8% (8th) vs. 15% (7th)]. The effect of this on RFS confirms that the patients moved to other substages were patients with higher risk disease. The data also delineate another prognostic extreme in stage IIID (4% of patients in this trial), for which the RFS benefit of adjuvant anti-PD-1 therapy was notable (50% vs. 33.3%). Interestingly, similar prognostic and predictive results were found during retrospective re-staging of the clinical trial assessing adjuvant treatment of stage III melanoma by the targeted agents dabrafenib and trametinib (8).
Going forward, however, surgical practice is again changing in a way that will impact the staging system and our ability to use data from it. Just as requirement of a sentinel node biopsy improved the accuracy of the 8th edition, the decreased use of CLND will reduce the accuracy of staging in patients with positive sentinel nodes. The 8th edition recognizes the challenge of nodal staging without CLND by creating a new coding designation of pN1a(sn). This designation will include patients who truly have only one microscopically positive node (N1a) but also include patients who would have had additional positive nodes had they undergone CLND (N2a and N3a). As such, overall representation of the IIIA population in trials done without a requirement for CLND will likely increase from the 8% seen in this trial staged by 8th edition criteria toward the 15% seen in patients staged by 7th edition criteria, driven predominantly by greater inclusion of patients with worse prognosis. A similar shift will likely occur in clinical practice. This will complicate application of the data from this trial, and other trials requiring CLND, to patients who have not undergone this procedure. In clinical practice, patients with pN1a(sn) disease likely will not have the same prognosis as patients who are pN1a after CLND, and they should be informed of this when being counseled about adjuvant therapy.
It is important to improve our ability to define prognosis on the basis of characteristics of the primary and the sentinel node without additional data from CLND. One such effort is based on a retrospective review of patients treated on EORTC protocols who underwent CLND. The CLND resulted in upstaging 19% of these patients as defined by N status but only 5–6% were moved to different substages. A combination of ulceration and tumor burden was able to define 3 risk categories based solely on information derived from the tumor and the SLN biopsy (9).
Further analysis of the Keynote-054 data would provide additional insight into how patients shift out of and into the various substages when comparing the 7th and 8th editions. While it is likely that most substage migration is unidirectional and results in upstaging, this granular information may further inform how much of the migration noted was due to additional positive nodes found on CLND versus influences of tumor characteristics. More generally, the authors did observe migration out of the lower risk IIIA [15% (7th) vs. 8% (8th)] and IIIB (46.3% vs. 34.7%) substages into higher risk IIIC (38.7% vs. 39.7%) and the newly founded IIID (3.7%).
The representativeness of the population enrolled in this trial compared with the more general population can be estimated by comparison of 8th edition-based patient distributions in the Keynote-054 dataset (n=1,019) versus the larger AJCC database (n=4,582) used to establish the staging criteria. There were a lower total percentage of IIIA [8% (Keynote-054) vs. 22% (AJCC)] and IIIC (39.7% vs. 48%) patients in the trial population, and an increased representation of IIIB patients (34.7% vs. 25.5%), and a similar ratio of IIID (3.7% vs. 4.5%) patients. The reduced size of the IIIA cohort in Keynote-054 relative to the general population may reflect provider hesitation to offer 1 year of adjuvant therapy to lower risk patients during the period of trial accrual. This also indicates that the problem of defining which stage III patients do not require adjuvant therapy may apply to more patients than would be thought from the patients in this trial.
As CLND is abandoned in clinical practice, and likely in trial design as well, the question of which clinical outcome measure is most meaningful for patients and for research should be re-examined. RFS is rapidly assessable in a 12–24-month timeline and can assess both locoregional and distant metastatic recurrence (DMFS: distant metastasis-free survival). Overall survival (OS) or MSS, while acknowledged to be the most meaningful measure for patients, now takes much longer to read out due to the availability of effective systemic therapies and is affected by those therapies in addition to the trial treatment. In Keynote-054, the reported total rates for locoregional (LR) recurrence by 8th edition stage at 1.25-year median follow-up were 4.9% (IIIA), 10.2% (IIIB), 15.2% (IIIC), and 23.7% (IIID) and in total, 132 of 1,019 patients (13.0%) experienced local recurrence, even after undergoing CLND. In comparison, in MSLT-II the rate of locoregional/nodal recurrence following CLND at 3-year was 8% as compared to a much higher 23% in patients not undergoing CLND. In Keynote-054, the rates for distant metastasis with or without LR recurrence in all patients, however, are even higher (IIIA: 3.7%, IIIB: 20.3%, IIIC: 23.1%, IIID: 39.5%, unknown: 23.1%), suggesting that even with all patients undergoing CLND the propensity for disease spread to some extent lessens the benefits of local control. Locoregional recurrences in carefully followed patients can usually be controlled by surgery, thus they are not threatening in and of themselves but rather as harbingers of distant spread. Perhaps, as CLND for positive SLN becomes a thing of the past, DMFS will be found to have a better correlation with OS and might replace RFS as the primary endpoint.
It is likely that many future improvements in disease staging will come from the bench rather than the bedside. One approach has been to investigate the prognostic ability of a 31 gene expression profile of the tumor cells themselves. The 28 genes of interest were chosen from a literature review of genes that had been found to be important in melanoma growth and metastasis in in vitro studies (10). This test has been evaluated for ability to stratify patients into prognostic groups, and early work has been done to integrate this study with the AJCC staging system (11). It is also being explored as a method to predict sentinel node positivity. The challenges with incorporating this test into clinical medicine have been thoughtfully reviewed (12), and likely much of this will apply to other approaches as well. This review also discusses other gene expression profiles being studied.
A second approach is to study primary melanomas for T-cell fraction and repertoire of T-cell clonality measured by high-throughput sequencing of the T-cell receptor beta chain, focusing on the immune response rather than the tumor itself. This approach has been reported in 199 stage T2–T4 melanomas; it was second only to tumor thickness as a prognostic factor, and appeared to add value to tumor thickness more than any histopathologic variable tested (13).
Yet another strategy is transcriptomic analysis of tumor infiltrating lymphocytes: work in this area has yielded a 53 gene melanoma immune profile with prognostic value in early stage melanoma (14). Addition of a calculation of the ratio between cytotoxic T lymphocytes and macrophages added to the value of this test.
Most current molecular approaches focus on prognosis, but to be of value they must either significantly complement, if not altogether supplant, the AJCC staging system. As Keynote-054 suggests, lack of predictive power is a limitation of the AJCC staging system, and lab-based approaches that render predictive value for efficacy and toxicity of any given therapy would undoubtedly add value to the management of patients and the design of clinical trials. One notable example are mutations in BRAF signaling for which targeted therapy exists, but such mutations only occur in up to 50% of melanomas (15,16).
Taken together, the last ten years mark a time of rapid evolution in the management of advanced melanoma. Our current staging system clearly has improved prognostic capabilities, but abandonment of CLND will necessitate changes in future. Results from high quality clinical trials and basic research, albeit sometimes challenging to apply, have changed our understanding of the disease biology and are offering therapies for patients that at one point had none. We can all look forward to advances in staging methods that will both improve prognostic ability and also lead to predictive capabilities as more and more therapeutic targets and pathways emerge.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.02.69). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199-206. [Crossref] [PubMed]
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:472-92.
- Faries MB, Thompson JF, Cochran AJ, et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med 2017;376:2211-22. [Crossref] [PubMed]
- Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med 2018;378:1789-801. [Crossref] [PubMed]
- Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N Engl J Med 2017;377:1813-23. [Crossref] [PubMed]
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med 2017;377:1824-35. [Crossref] [PubMed]
- Eggermont AMM, Blank CU, Mandala M, et al. Prognostic and predictive value of AJCC-8 staging in the phase III EORTC1325/KEYNOTE-054 trial of pembrolizumab vs placebo in resected high-risk stage III melanoma. Eur J Cancer 2019;116:148-57. [Crossref] [PubMed]
- Hauschild A, Dummer R, Schadendorf D, et al. Longer Follow-Up Confirms Relapse-Free Survival Benefit With Adjuvant Dabrafenib Plus Trametinib in Patients With Resected BRAF V600-Mutant Stage III Melanoma. J Clin Oncol 2018;36:3441-9. [Crossref] [PubMed]
- Verver D, van Klaveren D, van Akkooi ACJ, et al. Risk stratification of sentinel node-positive melanoma patients defines surgical management and adjuvant therapy treatment considerations. Eur J Cancer 2018;96:25-33. [Crossref] [PubMed]
- Gerami P, Cook RW, Wilkinson J, et al. Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma. Clin Cancer Res 2015;21:175-83. [Crossref] [PubMed]
- Zager JS, Gastman BR, Leachman S, et al. Performance of a prognostic 31-gene expression profile in an independent cohort of 523 cutaneous melanoma patients. BMC Cancer 2018;18:130. [Crossref] [PubMed]
- Grossman D, Kim CC, Hartman RI, et al. Prognostic gene expression profiling in melanoma: necessary steps to incorporate into clinical practice. Melanoma Manag 2019;6:MMT32. [Crossref] [PubMed]
- Pruessmann W, Rytlewski J, Wilmott J, et al. Molecular analysis of primary melanoma T cells identifies patients at risk for metastatic recurrence. Nat Cancer 2020. doi: 10.1038/s43018-019-0019-5. [Crossref]
- Gartrell-Corrado RD, Chen AX, Rizk EM, et al. Linking transcriptomic and imaging data defines features of a favorable tumor immune microenvironment and identifies a combination biomarker for primary melanoma. Cancer Res 2020;80:1078-87. [Crossref] [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [Crossref] [PubMed]
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol 2012;30:2375-83. [Crossref] [PubMed]


