
Mesenchymal stem cell therapy for liver fibrosis/cirrhosis
Introduction
Liver disease account for 3.5% of all deaths worldwide every year (1). Fibrosis is the most common events of all chronic and iterative liver injury (2). Chronic damages such as viral hepatitis and alcohol impaired the hepatocytes as well as endothelial barrier, and induce the inflammatory cell infiltration, which leading to the extensive production of collagen and extracellular matrix (ECM) by activated hepatic stellate cells (HSCs) (3). At the end stage, liver fibrosis progress to cirrhosis, for which there is no effective therapy but liver transplantation only. However, shortage of donors, the high costs, and the long-term requirement for immunosuppressants to prevent rejection remain the major limitations in liver transplantation (4). Therefore, alternative therapeutic strategies to reverse fibrogenesis are urgently needed.
Cell-based therapy using mesenchymal stem cells (MSCs) have been confirmed to have beneficial effects on liver fibrosis in several basic and clinical studies (5). MSCs are multipotent stromal cells that can be easily isolated from various tissue sources, expanded in vitro and are of inherently low immunogenicity (6). The therapeutic advantages of MSCs include self-renewal, engraftment ability, immunomodulation, multilineage differentiation, and trophic factor secretion, which promote the repairment and regeneration of damaged tissue (3). Since firstly isolated from rodent bone marrow (BM) in 1976 (7), accumulating studies from in vitro and in vivo have revealed the potency of MSCs in liver disease (Figure 1). Several animal studies already showed that MSCs can safely ameliorate liver fibrosis/cirrhosis and improve liver function (8-11). Thus, pre-clinical and clinical trials are underway to determine the therapeutic potential and safety of MSC-based therapy in liver disease.
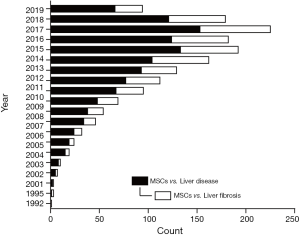
In this review, we will focus on recent clinical applications using MSCs and summarize the immunomodulatory mechanisms of MSCs in the treatment of liver fibrosis/cirrhosis.
Sources and advantages of MSCs
MSCs are nearly immunoprivileged because they lack expression of class II major histocompatibility (MHC) antigens and have low levels of class I MHC molecules. In addition, MSCs do not express co-stimulatory molecules required for immune recognition such as CD80, CD86 and CD40, which forms the basis of their allogeneic application (12). Therefore, both autologous and allogeneic MSCs has been employed in clinical applications. Recent studies reported that more than 2,000 patients have received autologous or allogeneic MSCs for treatments of different diseases in the worldwide (13).
Allogeneic BM has been the main source for MSC-based experimental studies and clinical trials, such as the treatment of graft-versus-host disease (GvHD), osteogenesis imperfecta, and liver disease (14). As the invasive nature of the isolation procedure and the limited frequency of MSCs (0.001% to 0.01% of total mononuclear cells) in the BM, alternative allogeneic sources would be required (15). Although originally isolated from BM, MSCs can also be isolated from other adult tissue sources including adipose tissue, dental pulp, and muscle and from rather “fetal origin” such as umbilical cord, amniotic tissue, and placenta (16). In addition, embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) has emerged as novel sources of MSCs (17). MSCs from different sources are found to exhibit similar phenotypes as defined by ISCT minimum criteria (18), yet there were some differences in the proliferation capacity, differentiation potential, levels of paracrine factors, and immunomodulatory properties (19-21). It has been found that adipose tissue contains MSCs at the highest frequency (0.02% to 0.06%) (22) and MSCs from umbilical cord have highest proliferation capacity, compared with the BM-MSCs (14). Therefore, adipose tissue and umbilical cord have been regarded as additional sources of MSCs for experimental and clinical needs. Adipose-derived MSCs (AD-MSCs) have shown great potential in liver regeneration for treating acute or chronic liver injury with respect to their abundance source and minimally invasive isolation procedures (23). Umbilical cord MSCs (UC-MSCs) have higher hepatic differentiation potential, thus may be advantageous over BM-MSCs for the treatment of end-stage liver disease (24). Concerning these differences, the properties and qualities of MSC from different sources should be carefully evaluated for therapeutic use. More advanced protocols comply with current good manufacturing practice (cGMP) requirements are needed to improve the ex vivo expansion and differentiation potential of MSCs. The safety and efficacy of human MSCs for patients with liver disease should be further evaluated.
It has been suggested that the therapeutic benefits of MSCs may prefer paracrine soluble factors [e.g., prostaglandin E2 (PGE2), hepatocyte growth factor (HGF), indoleamine 2,3-dioxygenase (IDO) and vascular endothelial growth factor (VEGF), etc.] to alter the microenvironment rather than differentiation alone (25). Thus, MSC conditioned medium (MSC-CM) may serve as an alternative approach versus direct transplantation of MSCs in the therapy of liver disease. MSC-CM has been found to significantly inhibit apoptosis and promote proliferation of hepatocytes in different murine models of acute liver injury (26,27). MSC-CM could also increase the secretion of anti-inflammatory IL-10 and improve survival rate of rats with D-galactosamine-induced liver failure (28). However, Lotfinia et al. reported that MSC-CM can ameliorate the histopathological and biochemical parameters of livers but fail to provide a significant survival benefit in mice with thioacetamide (TAA)-caused liver failure (29). A recent study compares the therapeutic effects of MSC-CM or MSCs in TAA-induced fulminant hepatic failure (FHF) and CCl4-induced chronic liver fibrosis in mice (30). MSC treatment effectively improves the liver function and survival rate in both FHF and liver fibrosis mice. With respect to MSC-CM, it dramatically improves liver fibrosis, but partially ameliorates FHF with no significant survival benefit (30). These data suggested that protective effects of MSC-based therapy of acute liver failure require their successful engraftment in the injured liver where they directly or indirectly modulate the tissue microenvironment that continually keep liver regeneration and recovery.
Notably, exosome is an important component of paracrine factors secreted by MSCs. Thus, MSC-CM can also play the therapeutic role in liver injury via exosomes (31). Similar to exosomes in general, MSC-derived exosomes (MSC-Exs) carry abundance of cargos, including nucleic acids, proteins and lipids, resulting in the alteration of various activities in target cells through different signaling pathways. MSC-Exs could migrate to sites of injury and been taken up by AML12 hepatocytes (32). After transplantation, menstrual blood-derived MSC-Exs markedly improve liver function by inhibiting apoptosis of hepatocytes, and enhanced survival rates of FHF mice (32). Jiang et al. reported that human umbilical cord MSC-Exs presented more distinct antioxidant and hepatoprotective effects compared to bifendate (DDB) treatment (33). AD-MSC-Exs significantly ameliorate acute liver failure and suppress inflammasome activation in macrophages (34). Although MSC-Exs have great therapeutic potential, the short of reproducible and efficient production methods remains a major limitation. Therefore, it needs to develop improved approaches for the isolation, characterization, purification and storage of exosomes that suitable for clinical application. In sum, the therapeutic potential of MSC-CM and MSC-Exs presents exciting novel cell-free therapy for intervention in liver diseases.
Clinical trials using MSCs in treatment of liver fibrosis/cirrhosis
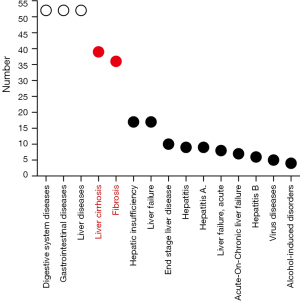
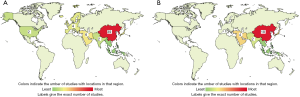
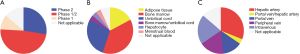
According to ClinicalTrials.gov (https://clinicaltrials.gov/), there are 59 registered clinical trials using MSCs have been conducted in the treatment of liver disease, most of which focus on liver cirrhosis or fibrosis (29 trials) (Figure 2). In particular, China alone conducts almost 50% of the clinical trials registered (Figure 3), as the research on stem cell and regenerative medicine is set as a key task in the twelfth five-year plan and the national key research and development program of China. In the trials focus on liver cirrhosis/fibrosis, most of them are in mixture of phase I/II studies (48.28%), phase II (27.59%), and phase I (10.34%), whereas no trials are in phase III (Figure 4A). More than 50% of MSCs are autologous. Bone marrow, umbilical cord and adipose tissue are the three major sources of MSCs (Figure 4B). In addition, over 48% of studies are treated via hepatic artery or portal vein (Figure 4C), reflecting the fact that the characteristics of hepatic hemodynamics should be considered to improve the efficiency of stem cells in the treatment of liver diseases. The hepatic artery seems to be the better delivery route and shows better homing efficacy (20–30%) compared with portal vein (5%) (35).
In general, treatment using MSCs appear to be safe and well-tolerated, and could improve liver function in patients with liver fibrosis/cirrhosis. The completed clinical trials are summarized in Table 1. Results obtained in a two case pilot study demonstrated that autologous BM-MSC transplantation twice via portal vein combined with one year of Pioglitazone therapy is safe and transiently improve Model for End-Stage Liver Disease (MELD) score in one patient with compensated cirrhosis (36). Sakai et al., in a phase I study, showed that serum albumin concentrations were improved in two cirrhosis patients who received autologous AD-MSCs infusion through intrahepatic arterial. No serious side-effects were observed (37). In a phase I–II clinical trial, eight patients with liver cirrhosis received injection of autologous BM-MSCs stimulated to hepatic lineage via peripheral or portal vein. All patients had reduced volumes of ascites, improved liver function [e.g., MELD score, prothrombin complex from international normalized ratio (INR), and serum creatinine] and no adverse effects were observed (38). A phase II study reported by Suk et al. showed that collagen proportionate area as well as Child-Pugh score was significantly improved in patients with alcoholic cirrhosis after intrahepatic arterial injection of autologous BM-MSCs. Interestingly, the twice injection in comparison with single injection of BM-MSCs did not have improved effects on the fibrosis condition (39). UC-MSC transplantation was tested in a phase I-II trial of patients with hepatitis B cirrhosis. Intravenous infusion of UC-MSCs significantly reduced the volume of ascites, improved liver function (e.g., serum albumin, serum bilirubin) and decrease the MELD Na score with no significant adverse effects and complications (40). Although these studies indicate the clinical safety and therapeutic potential of MSC transplantation for patients with liver fibrosis/cirrhosis, future larger-scale studies are needed to determine the appropriate cell source, delivery route, dosage, therapeutic window, and the anti-fibrosis mechanisms of MSCs.
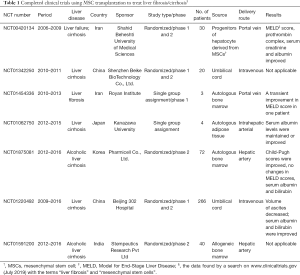
Full table
Therapeutic mechanisms of MSCs on hepatic immune microenvironment
The liver is an important immunological organ with many types of immune cells such as macrophages, Kupffer cells (KCs), neutrophils, dendritic cells (DCs), natural killer (NK) cells, T cells and B cells. These resident immune cells and non-hematopoietic cell populations in the liver combine to create a unique growth factor/cytokine microenvironment, and play essential roles in determining the balance between inflammation and tolerance in the healthy liver (41). The immune-mediated inflammatory effects play a dual role in the pathogenesis of fibrosis/cirrhosis such that, fibrosis/cirrhosis itself also leads to immune system dysregulation. The immune system mediates chronic damage (e.g., alcohol and virus infection, etc.) induced hepatocyte injury, driving fibrogenesis by HSC activation. In turn, liver fibrosis and subsequent cirrhosis lead to impairment of the immune system with dysregulated immune cell function and inability to protect the patients from bacterial infection (42). The ultimate consequence of the intrahepatic immune response depends on the function of innate immune cells (e.g., macrophages and NK cells), as well as on the balance between anti- and pro-inflammatory adaptive immune cells (e.g., T and B cells) (43).
Although early therapeutic interest in MSCs revolved around their capacity of hepatic differentiation, accumulating evidences indicated that therapeutic mechanisms of MSCs in liver disease might be primarily dependent on their wide array of immunomodulatory effects (4). MSCs are now considered to attenuate acute or chronic liver inflammation and consequent hepatocyte injury by regulating the immune cell function creating a hepato-protective environment through the paracrine effects and/or via cell-cell interaction (44). MSCs have the ability to regulate both the innate and adaptive immune response. Here, we will summarize the immunomodulatory effects of MSCs in liver fibrosis/cirrhosis (Figure 5).
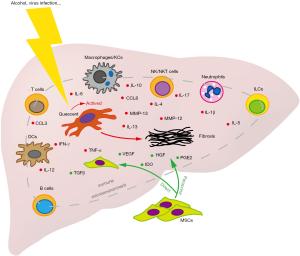
Effects of MSCs on innate immune response
Macrophages and KCs
Macrophages exert profound effects on pathogenesis of chronic liver injury, including inflammation and fibrosis (45,46). KCs are organ-resident macrophages arisen from self-renewing yolk sack-derived local macrophages, which constitute 15% of the total cell population in liver and compose about 80% of all body’s macrophages in the health state (43). KCs play a major role in maintaining liver immune tolerance by secreting the anti-inflammatory cytokine IL-10 during homeostasis (47). During liver injury, resident KCs can activate HSCs via production of pro-inflammatory cytokines [e.g., tumor necrosis factor (TNF)-α and MCP-1] as well as profibrotic cytokines TGF-β and platelet-derived growth factor (PDGF) (48). However, KCs can also secret matrix metalloproteinases (MMP-12 and MMP-13) that enhance ECM degradation, which favor the resolution of fibrosis (49,50). Thus, a given cell could be both pathogenic and beneficial. The double-edged sword of infiltrated macrophage activity in liver fibrosis progression and recovery has also been illustrated in genetic ablation mice model. Using selective macrophage depleted mice (CD11b-DTR), Duffield et al. found that ablation of infiltrated macrophages during fibrosis progression ameliorates fibrosis; while ablation of these macrophages at the recovery phase exacerbates fibrosis (46). These data suggest that functionally distinct subpopulations of macrophages may play opposing roles during the progression of liver fibrosis.
In most occasions, there are two forms of macrophages: the activated M1 macrophage and the alternatively activated M2 macrophage. Activation of M1 inflammatory macrophages by Th1 lymphokines (e.g., IFN-γ), pathogen components, or degraded matrix, leads to release pro-inflammatory cytokines such as TNF-α, interleukin (IL)-1β, and IL-6, which induces liver damage and ultimately the development of fibrosis (16, 17). The M2 anti-inflammatory macrophage is activated by Th2 lymphokines (e.g., IL-4), apoptotic cells and corticosteroids, and secrets anti-inflammatory cytokines including IL-10 and TGF-β that mediates wound repair and fibrosis (51). Imbalance in M1/M2 polarization could lead to HSC activation and hepatocyte injury. Interestingly, macrophages from the fibrotic liver have been found to probably skew toward the M2-preponderant phenotype in both humanized mice and patients (52), which could protect hepatocytes against lethal insult-induced apoptosis and promote fibrogenesis (53). On the other hand, Ma et al. reported that cytotherapy with M1, but not M2 BM-derived macrophages significantly ameliorate liver fibrosis by regulating the activation of endogenous macrophages and NK cells in mice (54). Taken together, the anti- and pro-fibrotic effects of hepatic macrophages may be dependent on functional heterogeneity of infiltrated macrophages as well as different phase of liver injury.
Intriguingly, BM-MSC transplantation can activate M2 macrophages expressing MMP13 and inhibit M1 macrophages to further attenuate the HSC activation, which play synergistic roles in ameliorating CCL4-induced liver fibrosis (9). Moreover, a post UC-MSC transplantation decrease in the liver fibro-inflammatory reaction was found and was further promoted by hepatic macrophages depletion (55). These studies reveal the ignored role of MSC transplantation in liver fibrosis based on their immunomodulatory effect on macrophage subset-involved microenvironment. Therefore, understanding the mechanisms that regulate hepatic macrophage heterogeneity may help to develop novel MSC-based therapy combined with manipulation of the microenvironment in which MSCs are engrafted to improve the outcome for liver injury and fibrosis.
Neutrophils
Neutrophils are professional phagocytes and play a major role in innate defense against bacterial and fungal infection. Neutrophils are found to exacerbate acute liver injury by inducing inflammatory mediators (e.g., IL-1β and TNF-α) and oxidative killing of hepatocytes (56). However, an antifibrotic role of neutrophils has been reported in CCl4-induced liver fibrosis by producing MMP8 and MMP9, and depletion of neutrophils delay the regression of liver fibrosis (57). Combined administration of macrophages and BM-MSCs induce the infiltration of host neutrophils into the liver fibrotic area, which contribute to liver fibrosis resolution and promote regeneration (58).
DCs
DCs are professional antigen present cells derived from CD34+ hematopoietic progenitor cells (HPCs) initiating both innate and adaptive immunity. Hepatic DCs have a distinctly tolerogenic phenotype through induction of Tregs or active T cell depletion to mediate hepatic tolerance. After liver injury, the phenotypically transformed DCs gained a marked capacity to active HSCs, T cells, and NK cells to control the pro-inflammatory milieu via production of TNF-α (59). Depletion of DCs completely abrogated the increased production of many inflammatory mediators in the fibrotic liver, suggesting that modulation of DC function may be an attractive therapeutic method to treat fibro-inflammatory liver disease. In a P. acnes plus LPS-induced FHF model, MSC transplantation effectively ameliorated the liver injury by secretion of PGE2, which promoted the differentiation of liver regulatory DCs that induce immune tolerance (60). In particular, PGE2 can decrease pro-inflammatory cytokines (e.g., TNF-α, IFN-γ, and IL-12) and increases the anti-inflammatory cytokine IL-10 in DCs (3).
NK cells and NK T cells
NK cells and NKT cells are lymphocytes of the innate immune system that recognize and kill microbe- or virus-infected cells, injured cells, and tumorigenic cells (61). In humans, NK and NKT cells make up to 30–50% and 5–10% of total liver lymphocytes respectively (62). The cytotoxicity of NK cells contributes to liver injury in liver disease. However, the anti-fibrotic function of NK cells in liver fibrosis has also been confirmed in both animal models and patients. NK cells can produce IFN-γ, selectively kill transitional- or senescence-activated HSCs by inducing cell cycle arrest and apoptosis, and subsequently alleviates liver fibrosis (61). Similar to NK cells, NKT cells have been found to inhibit HSC activation by producing IFN-γ and direct killing. NKT cells may attenuate the liver injury in the earlier stages but not later stages of CCl4 induced liver fibrosis (63). On the other hand, NKT cells also produce profibrotic cytokines such as IL-4 and IL-13, which induce the differentiation of fibrogenic myeloid cells and contribute to the development of liver fibrosis in patients with HBV infection (64). Duman et al. reported that the number of intrahepatic NK cells significantly diminished in rats with cholestatic hepatic fibrosis. This phenomenon was markedly alleviated after BM-MSC transplantation by promoting the expansion of NK cell populations in liver and peripheral blood (10). Injection of BM-MSCs attenuate hepatitis and NKT cell induced hepatotoxicity in acute liver failure mice. BM-MSCs reduced number of IL-17-secreting NKT17 cells, and increased presence of FoxP3+IL10-secreting NKT regulatory cells (NKTregs) in a paracrine, IDO-dependent manner (65).
Innate lymphoid cells (ILCs)
ILCs derived from the common lymphoid progenitor (CLP) and share functional similarity with T helper subsets (Th1 vs. ILC1, Th2 vs. ILC2, Th17/22 vs. ILC3) (66). ILCs can response to IL-25 and IL-33 by releasing cytokines such as IL-4, IL-5, and IL-13, which contribute to the first-line immune response against infection and tissue damage. The profibrotic effects of hepatic expressed IL-33 are found to be associated with activation and expansion of liver resident ILC2. ILC2-produced IL-13, acting via regulation of the transcription factor STAT6 and HSC activation, is an important downstream cytokine of IL-33-mediated tissue remodeling and fibrosis in liver injury (67). Recently, van Hoeven et al. reported that high frequencies of IL-22-producing ILC3s during allogeneic transplantation of hematopoietic stem cells were associated with a lower risk of GvHD. By studying the interaction between MSCs and ILC3s in vitro, they found that MSCs can induce the expansion and IL-22 production of ILC3s, which contribute to epithelial homeostasis and tissue repair (68). Whether ILCs may be involved in the therapeutic effect of MSCs on liver fibrosis needs to be investigated in the future.
Effects of MSCs on adaptive immune response
T cells
Recent studies have suggested T cell lymphopenia and a disruption of T helper (Th) and cytotoxic T cells (Tc) are common in cirrhosis (69). CD4+ Th2 cells can promote fibrogenesis by secreting IL-4 and IL-13, while CD4+ Th1 cells have an anti-fibrotic effect (70). Additionally, Tregs have been found to be highly enrich in fibrotic livers, and involve in the modulation of fibrogenesis primarily through suppressing NK cells and M1 KCs, as well as via mild suppression of CD8+ T cells (71). This suppressed effect of Tregs on NK cells limits the inflammation and hepatic damage, and limits the clearance of HSCs and other pathogenic factors in the fibrotic liver as well. Therefore, regulation of T lymphocyte subsets is complex during liver fibrosis. MSCs can inhibit the T cell proliferation in vitro either by directly interacting with T cells or by secreting soluble factors (e.g., PGE2, IDO, and TGF-β1) (72). In addition, MSCs limit T cell migration into target tissues via downregulating production of chemokines, including CCL1, CCL3, CCL8, CCL17, and CCL22 (73). A recent study revealed that BM-MSCs attenuate α-galactosylceramide (α-GalCer)-induced acute liver injury in mice by promoting expansion of liver infiltrated IL-10 producing CD4+CD25+FoxP3+ Tregs in an IDO dependent manner (74). Deletion of Tregs completely abolished hepatoprotective effects of BM-MSCs and diminished their capacity to attenuate cytotoxicity of hepatic NKT cells. Thus, the differences in the regulatory effects of MSCs on different T lymphocyte subsets in liver fibrosis need to be further elucidated.
B cells
The role of B cells in pathogenesis of liver fibrosis has been also reported. In CCl4 and Mdr2−/− fibrosis mouse models, the frequency of hepatic B cells was increased and these cells exhibited state of activation with elevated IgG and TNF-α production in a MyD88-dependent manner (75). Markedly reduced collagen deposition and fibrosis was observed in CCl4-induced B cell-deficient mice (76). BM-MSC transplantation can also increase the infiltration of hepatoprotective IL10 and TGF-β-producing Bregs within the liver of mice injected with α-GalCer. However, deletion of Bregs did not alter MSC-dependent attenuation of liver injury, suggesting that Bregs were not directly involved in MSC-mediated alleviation of acute liver inflammation (74).
Conclusions
MSCs are promising therapeutic agents for the liver fibrosis and cirrhosis treatment because of their hepatic differentiation potential as well as their immunomodulatory properties and capacity to produce trophic factors. Furthermore, the anti-fibrotic and anti-oxidant activities of MSCs are also involved in their beneficial effects on liver fibrosis (3). In addition to MSC treatment alone, MSC secretome, modification of MSCs or MSCs combined with other treatment represent highly attractive therapeutic approaches in the near future. But there are still many problems to be addressed before clinical use of MSCs for liver fibrosis/cirrhosis, including sufficient cell number, optimal time, and optimal delivery route for MSC transplantation. Moreover, further studies on the improvement of the engraftment and survival rate of MSCs in the liver are needed to increase the efficacy of MSC therapy. Although in vitro and animal experiments have shown the effects of MSCs on different immune cell subsets, additional studies are needed to confirm these effects in patients with liver fibrosis/cirrhosis at different stages and different etiologies. Consequently, large randomized and controlled clinical studies with longer follow-up periods are required to increase the reliability of the clinical safety and efficacy of MSCs for fibrosis treatment.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (No. 81971495, 81571564), CAMS Innovation Fund for Medical Sciences (No. 2019-I2M-5-035), Natural Science Foundation of Jiangsu Province, China (BK20170152), the Jiangsu Provincial Medical Youth Talent (BRA2017533, QNRC2016108), Six Talent Peaks Program of Jiangsu Province (YY-081), Nanjing Technological Development Program (No. 201715054), the Foundation of Jiangsu Collaborative Innovation Center of Biomedical Functional Materials.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ling Lu) for the series “Stem Cell and Clinical Application” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/atm.2020.02.119). The series “Stem Cell and Clinical Application” was commissioned by the editorial office without any funding or sponsorship. LL serves as the unpaid Associate Editor-in-Chief of Annals of Translational Medicine from Jun 2019 to May 2024 and served as the unpaid Guest Editor of the series. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol 2019;70:151-71. [Crossref] [PubMed]
- Campana L, Iredale JP. Regression of Liver Fibrosis. Semin Liver Dis 2017;37:1-10. [Crossref] [PubMed]
- Eom YW, Shim KY, Baik SK. Mesenchymal stem cell therapy for liver fibrosis. Korean J Intern Med 2015;30:580-9. [Crossref] [PubMed]
- Volarevic V, Nurkovic J, Arsenijevic N, et al. Concise review: Therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells 2014;32:2818-23. [Crossref] [PubMed]
- de Miguel MP, Prieto I, Moratilla A, et al. Mesenchymal Stem Cells for Liver Regeneration in Liver Failure: From Experimental Models to Clinical Trials. Stem Cells Int 2019;2019:3945672.
- Mizukami A, Swiech K. Mesenchymal Stromal Cells: From Discovery to Manufacturing and Commercialization. Stem Cells Int 2018;2018:4083921.
- Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 1976;4:267-74. [PubMed]
- Choi JS, Jeong IS, Han JH, et al. IL-10-secreting human MSCs generated by TALEN gene editing ameliorate liver fibrosis through enhanced anti-fibrotic activity. Biomater Sci 2019;7:1078-87. [Crossref] [PubMed]
- Luo XY, Meng XJ, Cao DC, et al. Transplantation of bone marrow mesenchymal stromal cells attenuates liver fibrosis in mice by regulating macrophage subtypes. Stem Cell Res Ther 2019;10:16. [Crossref] [PubMed]
- Duman DG, Zibandeh N, Ugurlu MU, et al. Mesenchymal stem cells suppress hepatic fibrosis accompanied by expanded intrahepatic natural killer cells in rat fibrosis model. Mol Biol Rep 2019;46:2997-3008. [Crossref] [PubMed]
- Zhang GZ, Sun HC, Zheng LB, et al. In vivo hepatic differentiation potential of human umbilical cord-derived mesenchymal stem cells: Therapeutic effect on liver fibrosis/cirrhosis. World J Gastroenterol 2017;23:8152-68. [Crossref] [PubMed]
- Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol 2009;217:318-24. [Crossref] [PubMed]
- Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant 2016;25:829-48. [Crossref] [PubMed]
- Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294-301. [Crossref] [PubMed]
- Malgieri A, Kantzari E, Patrizi MP, et al. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med 2010;3:248-69. [PubMed]
- Prasajak P. Mesenchymal Stem Cells: Current Clinical Applications and Therapeutic Potential in Liver Diseases. J Bone Marrow Res 2014. doi: 10.4172/2329-8820.1000137. [Crossref]
- Sabapathy V, Kumar S. hiPSC-derived iMSCs: NextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine. J Cell Mol Med 2016;20:1571-88. [Crossref] [PubMed]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. [Crossref] [PubMed]
- Jin HJ, Bae YK, Kim M, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci 2013;14:17986-8001. [Crossref] [PubMed]
- Wu M, Zhang R, Zou Q, et al. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord. Sci Rep 2018;8:5014. [Crossref] [PubMed]
- Diederichs S, Tuan RS. Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor. Stem Cells Dev 2014;23:1594-610. [Crossref] [PubMed]
- Semon JA, Maness C, Zhang X, et al. Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem Cell Res Ther 2014;5:2. [Crossref] [PubMed]
- Hu C, Zhao L, Li L. Current understanding of adipose-derived mesenchymal stem cell-based therapies in liver diseases. Stem Cell Res Ther 2019;10:199. [Crossref] [PubMed]
- Yu YB, Song Y, Chen Y, et al. Differentiation of umbilical cord mesenchymal stem cells into hepatocytes in comparison with bone marrow mesenchymal stem cells. Mol Med Rep 2018;18:2009-16. [PubMed]
- Prockop D. “Stemness” Does Not Explain the Repair of Many Tissues by Mesenchymal Stem/Multipotent Stromal Cells (MSCs). Clin Pharmacol Ther 2007;82:241-3. [Crossref] [PubMed]
- van Poll D, Parekkadan B, Cho CH, et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008;47:1634-43. [Crossref] [PubMed]
- Xagorari A, Siotou E, Yiangou M, et al. Protective effect of mesenchymal stem cell-conditioned medium on hepatic cell apoptosis after acute liver injury. Int J Clin Exp Pathol 2013;6:831-40. [PubMed]
- Parekkadan B, van Poll D, Suganuma K, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One 2007;2:e941. [Crossref] [PubMed]
- Lotfinia M, Kadivar M, Piryaei A, et al. Effect of Secreted Molecules of Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells on Acute Hepatic Failure Model. Stem Cells Dev 2016;25:1898-908. [Crossref] [PubMed]
- Huang B, Cheng X, Wang H, et al. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J Transl Med 2016;14:45. [Crossref] [PubMed]
- Damania A, Jaiman D, Teotia AK, et al. Mesenchymal stromal cell-derived exosome-rich fractionated secretome confers a hepatoprotective effect in liver injury. Stem Cell Res Ther 2018;9:31. [Crossref] [PubMed]
- Chen L, Xiang B, Wang X, et al. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther 2017;8:9. [Crossref] [PubMed]
- Jiang W, Tan Y, Cai M, et al. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl4-Induced Liver Injury through Antioxidant Effect. Stem Cells Int 2018;2018:6079642.
- Liu Y, Lou G, Li A, et al. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine 2018;36:140-50. [Crossref] [PubMed]
- Zhang Y, Li Y, Zhang L, et al. Mesenchymal stem cells: potential application for the treatment of hepatic cirrhosis. Stem Cell Res Ther 2018;9:59. [Crossref] [PubMed]
- Vosough M, Moossavi S, Mardpour S, et al. Repeated Intraportal Injection of Mesenchymal Stem Cells in Combination with Pioglitazone in Patients with Compensated Cirrhosis: A Clinical Report of Two Cases. Arch Iran Med 2016;19:131-6. [PubMed]
- Sakai Y, Takamura M, Seki A, et al. Phase I clinical study of liver regenerative therapy for cirrhosis by intrahepatic arterial infusion of freshly isolated autologous adipose tissue-derived stromal/stem (regenerative) cell. Regen Ther 2017;6:52-64. [Crossref] [PubMed]
- Kharaziha P, Hellstrom PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol 2009;21:1199-205. [Crossref] [PubMed]
- Suk KT, Yoon JH, Kim MY, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology 2016;64:2185-97. [Crossref] [PubMed]
- Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol 2012;27 Suppl 2:112-20. [Crossref] [PubMed]
- Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol 2016;13:267-76. [Crossref] [PubMed]
- Albillos A, Lario M, Alvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014;61:1385-96. [Crossref] [PubMed]
- Heymann F, Tacke F. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol 2016;13:88-110. [Crossref] [PubMed]
- Gazdic M, Volarevic V, Arsenijevic N, et al. Mesenchymal stem cells: a friend or foe in immune-mediated diseases. Stem Cell Rev Rep 2015;11:280-7. [Crossref] [PubMed]
- Rivera CA, Bradford BU, Hunt KJ, et al. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol 2001;281:G200-7. [Crossref] [PubMed]
- Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 2005;115:56-65. [Crossref] [PubMed]
- Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013;14:996-1006. [Crossref] [PubMed]
- Pradere J-P, Kluwe J, De Minicis S, et al. Hepatic Macrophages But Not Dendritic Cells Contribute to Liver Fibrosis by Promoting the Survival of Activated Hepatic Stellate Cells in Mice. Hepatology 2013;58:1461-73. [Crossref] [PubMed]
- Fallowfield JA, Mizuno M, Kendall TJ, et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol 2007;178:5288-95. [Crossref] [PubMed]
- Pellicoro A, L, Aucott R, Ramachandran P, et al. Elastin Accumulation Is Regulated at the Level of Degradation by Macrophage Metalloelastase (MMP-12) during Experimental Liver Fibrosis. Hepatology (Baltimore, Md) 2012;55:1965-75. [Crossref] [PubMed]
- Xu R, Zhang Z, Wang FS. Liver fibrosis: mechanisms of immune-mediated liver injury. Cell Mol Immunol 2012;9:296-301. [Crossref] [PubMed]
- Bility M, Nio K, Li F, et al. Chronic hepatitis C infection-induced liver fibrogenesis is associated with M2 macrophage activation. Scientific Reports 2016;6:39520. [Crossref] [PubMed]
- Bai L, Liu X, Zheng Q, et al. M2-like macrophages in the fibrotic liver protect mice against lethal insults through conferring apoptosis resistance to hepatocytes. Sci Rep 2017;7:10518. [Crossref] [PubMed]
- Ma PF, Gao CC, Yi J, et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice. J Hepatol 2017;67:770-9. [Crossref] [PubMed]
- Ghanem Ly, Mansour IM, Abulata N, et al. Liver Macrophage Depletion Ameliorates The Effect of Mesenchymal Stem Cell Transplantation in a Murine Model of Injured Liver. Sci Rep 2019;9:35. [Crossref] [PubMed]
- Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol 2007;35:757-66. [Crossref] [PubMed]
- Saijou E, Enomoto Y, Matsuda M, et al. Neutrophils alleviate fibrosis in the CCl4-induced mouse chronic liver injury model. Hepatol Commun 2018;2:703-17. [Crossref] [PubMed]
- Watanabe Y, Tsuchiya A, Seino S, et al. Mesenchymal Stem Cells and Induced Bone Marrow-Derived Macrophages Synergistically Improve Liver Fibrosis in Mice. Stem Cells Transl Med 2019;8:271-84. [Crossref] [PubMed]
- Connolly MK, Bedrosian AS, Mallen-St Clair J, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-α. J Clin Invest 2009;119:3213-25. [PubMed]
- Zhang Y, Cai W, Huang Q, et al. Mesenchymal stem cells alleviate bacteria-induced liver injury in mice by inducing regulatory dendritic cells. Hepatology 2014;59:671-82. [Crossref] [PubMed]
- Gao B, Radaeva S. Natural killer and natural killer T cells in liver fibrosis. Biochim Biophys Acta 2013;1832:1061-9.
- Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol 2009;86:513-28. [Crossref] [PubMed]
- Park O, Jeong WI, Wang L, et al. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology 2009;49:1683-94. [Crossref] [PubMed]
- de Lalla C, Galli G, Aldrighetti L, et al. Production of profibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J Immunol 2004;173:1417-25. [Crossref] [PubMed]
- Milosavljevic N, Gazdic Jankovic M, Markovic B, et al. Mesenchymal stem cells attenuate acute liver injury by altering ratio between IL-17 producing and regulatory NKT cells. Liver Transpl 2017;23:1040-50. [Crossref] [PubMed]
- Jeffery HC, McDowell P, Lutz P, et al. Human intrahepatic ILC2 are IL-13positive amphiregulinpositive and their frequency correlates with model of end stage liver disease score. PLoS one 2017;12:e0188649. [Crossref] [PubMed]
- McHedlidze T, Waldner M, Zopf S, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 2013;39:357-71. [Crossref] [PubMed]
- van Hoeven V, Munneke JM, Cornelissen AS, et al. Mesenchymal Stromal Cells Stimulate the Proliferation and IL-22 Production of Group 3 Innate Lymphoid Cells. J Immunol 2018;201:1165-73. [Crossref] [PubMed]
- Morita K, Fukuda Y, Nakano I, et al. Peripheral lymphocyte subsets vary with stage of hepatitis C virus-associated liver disease. Hepatogastroenterology 2005;52:1803-8. [PubMed]
- Berardis S, Sattwika PD, Najimi M, et al. Use of mesenchymal stem cells to treat liver fibrosis: Current situation and future prospects. World J Gastroenterol 2015;21:742-58. [Crossref] [PubMed]
- Zhang X, Lou J, Bai L, et al. Immune Regulation of Intrahepatic Regulatory T Cells in Fibrotic Livers of Mice. Med Sci Monit 2017;23:1009-16. [Crossref] [PubMed]
- Singer N. I Caplan A. Mesenchymal Stem Cells: Mechanisms of Inflammation. Annu Rev Pathol 2011;6:457-78. [Crossref] [PubMed]
- Lim JY, Ryu DB, Lee SE, et al. Mesenchymal Stem Cells (MSCs) Attenuate Cutaneous Sclerodermatous Graft-Versus-Host Disease (Scl-GVHD) through Inhibition of Immune Cell Infiltration in a Mouse Model. J Invest Dermatol 2017;137:1895-904. [Crossref] [PubMed]
- Gazdic Jankovic M, Markovic B, Arsenijevic A, et al. Crosstalk between mesenchymal stem cells and T regulatory cells is crucially important for the attenuation of acute liver injury. Liver Transpl 2018;24:687-702. [Crossref] [PubMed]
- Thapa M, Tedesco D, Elrod E, et al. Chronic liver fibrosis triggers autoantibody production by B cells in a MyD88-dependent pathway (BA4P.126). J Immunol 2015;194:47.6.
- Novobrantseva TI, Majeau GR, Amatucci A, et al. Attenuated liver fibrosis in the absence of B cells. J Clin Invest 2005;115:3072-82. [Crossref] [PubMed]


