Diagnostic approach to orthostatic proteinuria: a combination of urine micro-proteinuria with ultrasonography of the left renal vein
In the current era, there has been a tremendous focus on proteinuria as a prognostic indicator of acute kidney injury and chronic kidney disease (CKD) (1-4). KDIGO in 2012 included albuminuria in staging and progression of CKD (1). Macroalbuminuria, defined as urine albumin >300 mg/day, is an independent risk factor for progression of CKD in children and adults (2-4). Proteinuria is classified as glomerular, secondary to damage to podocytes and filtration barrier (5,6). Tubular proteinuria develops when the proximal tubule fails to reabsorb the filtered low molecular proteins. Secretory proteinuria is from increased secretion of Tamm- Horsfall protein and finally Interstitial proteinuria (5,6). Proteinuria is further regrouped as persistent/fixed, transient, and orthostatic. Persistent proteinuria is seen in glomerular disorders, which can be nephrotic or non-nephrotic. Transient proteinuria is commonly associated with infections, fevers, dehydration, sepsis, and subsides with a resolution of the underlying cause (7).
Orthostatic proteinuria (OP) is a notable cause of asymptomatic proteinuria in children and young adults (8). It is frequently encountered in children with slender body habitus and low body mass index (BMI) (9). OP is defined as the presence of protein in the urine sample collected in an upright position while no protein detected in supine posture (10). It is evident that OP accounts for 15 to 20% of asymptomatic proteinuria in children (11). Although OP is a relatively benign entity, there are several reports of subtle glomerular lesions on renal biopsy specimens (12).
Pathophysiology
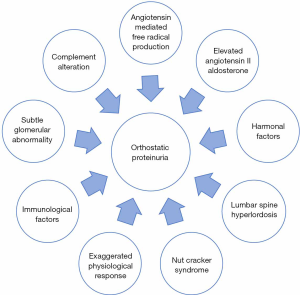
While underlying mechanism of OP is not clearly defined, multiple hypothetical concepts are proposed, Figure 1 (13). Some authors suggested that OP could be an exaggerated physiological response in an upright posture, while the total protein excretion is less than 150 mg/day (10). A probable explanation could be attributed to elevated levels of renin and angiotensin II. Hemodynamic effects of Angiotensin II results in renal efferent vasoconstriction, further leading to increased glomerular filtration pressure and elevated protein excretion (10). Angiotensin II also has non-hemodynamic local effects in releasing free oxygen radicles, cytokines, leukotrienes, and increased metalloproteins with subsequent proteinuria. Other theories include activation of the complement cascade, subtle Immunological damage to the basement membrane, and nutcracker syndrome (NCS) (13-15).
Nutcracker syndrome
NCS is originally described by El-Sadr and Mine in 1950 as compression of the left renal vein (LRV) between aorta and superior mesenteric artery (SMA) (16). Later Shintaku et al. in 1990 correlated entrapment of LRV with OP (16). The two-common developmental variants of LRV are aorto-mesenteric (anterior origin) and retrocaval (posterior origin) (17). Aorto-mesenteric or anterior nutcracker is the entrapment of LRV between Aorta and SMA. Posterior nutcracker corresponds to the compression of retro-aortic renal vein between aorta and vertebral body (18,19). It usually becomes symptomatic in second and third decades of life. Nutcracker phenomenon is distinguished from NCS where later presents with clinical features secondary to distal renal and gonadal vein dilatation. In asymptomatic individuals, the presence of engorged renal and collateral veins could be seen as an incidental finding on imaging and is termed as nutcracker phenomenon. Patients with NCS commonly present with left flank pain, pelvic congestion pain (20), autonomic dysfunction, dysmenorrhea, dyspareunia, varicose veins, microhematuria, OP (21,22).
Current issue
In the current issue, Niu et al. (8) evaluated the combination of microproteinuria along with Doppler ultrasonography in diagnosing OP. Sixty patients out of 75 are diagnosed with OP by clinical and imaging criteria. Fifty-eight subjects have entrapment of LRV, which implies nutcracker phenomenon contributes significantly to OP. Since no gold standard test is available to diagnose OP the combination of urine microprotein tests along with renal vein ultrasound significantly increased specificity, positive predictive value and positive likelihood ratio. Another interesting finding noted is that urine albumin/creatinine ratio, and IgG/creatinine ratio were subsequently high in the urine samples of patients with OP compared to patients with non-OP. Micro-molecules such as NAG and α1Mu were excreted at similar rates between both groups, which implies that glomerular proteinuria in OP mostly comprises of albumins and globulins. Doppler ultrasonography is used as an imaging modality in measuring the AP diameters and Peak velocities at different points of the LRV to yield diagnostic accuracy. Ultrasound is a safe, relatively cheap, noninvasive initial diagnostic modality used to diagnose NCS. Doppler ultrasound with flow and peak velocity measurements almost proved to be a near equivalent imaging modality to computed tomography angiography (CTA), magnetic resonance angiography (MRA). Doppler ultrasound, in combination with clinical test significantly increased the likelihood of a diagnosis of OP.
Focusing on some of the limitations, it is undoubtedly difficult for the patients to undergo all three tests, including the upright lordotic test and Robinson test, which are cumbersome. The sensitivity and specificity of ultrasound are less compared to CTA and MRA with less accurate reproducibility of the results. Also, the measurement of diameters and peak velocities of LRV can vary between operators in conveying consistent results. It could be challenging to measure the peak velocities and diameters of the narrow portion of LRV in children as the area is too small.
Approach to diagnosis
In the literature, there is no gold standard test for diagnosing OP. Thorough history taking and physical examination to delineate the extent of symptoms will be useful to determine the management approach. Clinicians can start investigations by checking urine analysis, urine culture, urine cytology, complement activity, and serological studies to rule out other causes of proteinuria (Table 1) (23). Upright and recumbent urine protein to creatinine ratio can be performed as an initial screening test. A 24-hour split urine test has been traditionally used diagnostic test. Patient is asked to collect a 16-hour urine sample during the day, followed by an 8-hour supine sample collection separately. Presence of elevated protein >150 mg/day in day sample with no protein in recumbent sample points to the diagnosis of OP. Other clinical test as narrated by Niu et al. include West test, Robinson test and upright lordotic test (8).

Full table
Renal venography is performed as a gold standard to confirm NCS (24,25). However, given the invasive nature of the test, imaging with Doppler ultrasonography, CTA or MRA are acceptable tools (26). A specific diagnostic criterion has not been standardized for diagnosis of NCS by Doppler ultrasonography. Having said that, in a study done by Kim et al. (27), the mean ratio of LRV diameter at hilum to aorto- mesenteric region >5 and ratio of peak velocities at the same points >5 has 80% sensitivity and 94% specificity. Takebayashi et al. also reported the detection of collateral veins around LRV could be a reliable criterion in diagnosing NCS (22). In a study by Takahashi et al., including 93 pediatric patients compared digital subtraction angiography, Doppler ultrasound, and venography pressures in defining imaging characteristics. They postulated several ultrasound grades in defining probable, borderline, and definite nutcracker phenomenon (28).
Management
OP is a benign entity and conservative approach is indicated especially in children <18 years old. In most children, spontaneous remissions are reported with further growth and weight gain (29,30). In an Italian study including 13 subjects, OP disappeared after 6 years in at least 9 subjects with conservative management (31). Angiotensin-converting enzyme (ACE) inhibitors can be prescribed in adults with persistent proteinuria to slow down microalbuminuria. Use of ACE Inhibitors is substantiated in patients with persistent OP given subtle glomerular changes on kidney biopsy specimens and elevated angiotensin II levels (32). However, if patients are severely symptomatic from nutcracker syndrome, surgical procedures including LRV bypass, excision of varicosities, SMA transposition, renal to inferior vena cava shunts, external and intravascular stenting and even nephrectomy have been reported (33-35).
Conclusions
OP accounts for one of the common causes of proteinuria in children and is mostly asymptomatic. However, it becomes essential to diagnose OP and rule out other conditions. NCS plays a significant role in the etiology of OP. A combination of urine micro proteinuria along with ultrasonography, has increased specificity in diagnosing this condition and can be used as a confirmatory diagnostic modality.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.02.50). WC serves as an unpaid editorial board member of Annals of Translational Medicine from Mar 2020 to Feb 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fuhrman DY, Schneider MF, Dell KM, et al. Albuminuria, Proteinuria, and Renal Disease Progression in Children with CKD. Clin J Am Soc Nephrol 2017;12:912-20. [Crossref] [PubMed]
- Fathallah-Shaykh SA, Flynn JT, Pierce CB, et al. Progression of pediatric CKD of nonglomerular origin in the CKiD cohort. Clin J Am Soc Nephrol 2015;10:571-7. [Crossref] [PubMed]
- Noone D, Licht C. Chronic kidney disease: a new look at pathogenetic mechanisms and treatment options. Pediatr Nephrol 2014;29:779-92. [Crossref] [PubMed]
- Warady BA, Abraham AG, Schwartz GJ, et al. Predictors of Rapid Progression of Glomerular and Nonglomerular Kidney Disease in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort. Am J Kidney Dis 2015;65:878-88. [Crossref] [PubMed]
- D'Amico G, Bazzi C. Pathophysiology of proteinuria. Kidney Int 2003;63:809-25. [Crossref] [PubMed]
- Waller KV, Ward KM, Mahan JD, et al. Current concepts in proteinuria. Clin Chem 1989;35:755-65. [Crossref] [PubMed]
- Hogg RJ, Portman RJ, Milliner D, et al. Evaluation and management of proteinuria and nephrotic syndrome in children: recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE). Pediatrics 2000;105:1242-9. [Crossref] [PubMed]
- Niu XL, Wu Y, Hao S, et al. Value of micro-proteinuria in combination with ultrasonography of the left renal vein in the diagnosis of orthostatic proteinuria. Ann Transl Med 2019;7:780. [Crossref] [PubMed]
- Milani G, Bianchetti MG, Bozzani S, et al. Body mass index modulates postural proteinuria. Int Urol Nephrol 2010;42:513-5. [Crossref] [PubMed]
- Mazzoni MB, Kottanatu L, Simonetti GD, et al. Renal vein obstruction and orthostatic proteinuria: a review. Nephrol Dial Transplant 2011;26:562-5. [Crossref] [PubMed]
- de Joode AA, Sluiter HE. Orthostatic proteinuria: a harmless variant of protein loss? Neth J Med 2011;69:62-5. [PubMed]
- Uehara K, Tominaga N, Shibagaki Y. Adult orthostatic proteinuria. Clin Kidney J 2014;7:327-8. [Crossref] [PubMed]
- Vehaskari VM. Orthostatic proteinuria. Arch Dis Child 1982;57:729-30. [Crossref] [PubMed]
- Devarajan P. Mechanisms of orthostatic proteinuria: lessons from a transplant donor. J Am Soc Nephrol 1993;4:36-9. [PubMed]
- Yoshioka T, Mitarai T, Kon V, et al. Role for angiotensin II in an overt functional proteinuria. Kidney Int 1986;30:538-45. [Crossref] [PubMed]
- Shintaku N, Takahashi Y, Akaishi K, et al. Entrapment of left renal vein in children with orthostatic proteinuria. Pediatr Nephrol 1990;4:324-7. [Crossref] [PubMed]
- Nalcacioglu H, Ceyhan Bilgici M, Tekcan D, et al. Nutcracker Syndrome in Children: Role of Doppler Ultrasonographic Indices in Detecting the Pattern of Symptoms. J Clin Med 2018. [Crossref] [PubMed]
- Dunphy L, Penna M, Tam E, et al. Left renal vein entrapment syndrome: nutcracker syndrome! BMJ Case Rep 2019. [Crossref] [PubMed]
- Ali-El-Dein B, Osman Y, Shehab El-Din AB, et al. Anterior and posterior nutcracker syndrome: a report on 11 cases. Transplant Proc 2003;35:851-3. [Crossref] [PubMed]
- Shin JI, Park JM, Lee JS, et al. Effect of renal Doppler ultrasound on the detection of nutcracker syndrome in children with hematuria. Eur J Pediatr 2007;166:399-404. [Crossref] [PubMed]
- Park SJ, Lim JW, Ko YT, et al. Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. AJR Am J Roentgenol 2004;182:683-8. [Crossref] [PubMed]
- Takebayashi S, Ueki T, Ikeda N, et al. Diagnosis of the nutcracker syndrome with color Doppler sonography: correlation with flow patterns on retrograde left renal venography. AJR Am J Roentgenol 1999;172:39-43. [Crossref] [PubMed]
- Takahashi Y, Akaishi K, Sano A, et al. Intra-arterial digital subtraction angiography for children with idiopathic renal bleeding: a diagnosis of nutcracker phenomenon. Clin Nephrol 1988;30:134-40. [PubMed]
- Kurklinsky AK, Rooke TW. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc 2010;85:552-9. [Crossref] [PubMed]
- Orczyk K, Wysiadecki G, Majos A, et al. What Each Clinical Anatomist Has to Know about Left Renal Vein Entrapment Syndrome (Nutcracker Syndrome): A Review of the Most Important Findings. Biomed Res Int 2017;2017:1746570. [Crossref] [PubMed]
- Cuéllar i Calàbria H, Quiroga Gómez S, et al. Nutcracker or left renal vein compression phenomenon: multidetector computed tomography findings and clinical significance. Eur Radiol 2005;15:1745-51. [Crossref] [PubMed]
- Kim SH, Cho SW, Kim HD, et al. Nutcracker syndrome: diagnosis with Doppler US. Radiology 1996;198:93-7. [Crossref] [PubMed]
- Takahashi Y, Sano A, Matsuo M. An ultrasonographic classification for diverse clinical symptoms of pediatric nutcracker phenomenon. Clin Nephrol 2005;64:47-54. [Crossref] [PubMed]
- Alaygut D, Bayram M, Soylu A, et al. Clinical course of children with nutcracker syndrome. Urology 2013;82:686-90. [Crossref] [PubMed]
- Shin JI, Park JM, Lee SM, et al. Factors affecting spontaneous resolution of hematuria in childhood nutcracker syndrome. Pediatr Nephrol 2005;20:609-13. [Crossref] [PubMed]
- Milani GP, Mazzoni MB, Burdick L, et al. Postural proteinuria associated with left renal vein entrapment: a follow-up evaluation. Am J Kidney Dis 2010;55:e29-31. [Crossref] [PubMed]
- Ha TS, Lee EJ. ACE inhibition can improve orthostatic proteinuria associated with nutcracker syndrome. Pediatr Nephrol 2006;21:1765-8. [Crossref] [PubMed]
- Wendel RG, Crawford ED, Hehman KN. The "nutcracker" phenomenon: an unusual cause for renal varicosities with hematuria. J Urol 1980;123:761-3. [Crossref] [PubMed]
- Zhang H, Li M, Jin W, et al. The left renal entrapment syndrome: diagnosis and treatment. Ann Vasc Surg 2007;21:198-203. [Crossref] [PubMed]
- Thompson PN, Darling RC 3rd, Chang BB, et al. A case of nutcracker syndrome: treatment by mesoaortic transposition. J Vasc Surg 1992;16:663-5. [Crossref] [PubMed]