
Liver regeneration and alcoholic liver disease
Introduction
Alcohol consumption is a major risk factor for morbidity and mortality worldwide. According to the Global Status Report on Alcohol and Health 2018 by the World Health Organization, approximately 3 million deaths were attributed to alcohol consumption in 2016, accounting for 5.3% of all deaths worldwide. Of note, alcohol consumption in China has been on an upward trend; per capita pure alcohol consumption was 4.1 L in 2005, 7.1 L in 2010, and 7.2 L in 2016 (1). Alcoholic liver disease (ALD) is a broad-spectrum disease caused by excessive alcohol consumption, from simple steatosis in early stages, alcoholic hepatitis, cirrhosis, and even hepatocellular carcinoma at the end stages. The development of ALD is also associated with other pathogenic factors such as gender, dietary factors, genetic polymorphisms, obesity, drinking patterns, and cigarette smoking (2). There are tremendous economic and health impacts of ALD; however, few advances have been made in the treatment of ALD patients over the past few decades. The cornerstone of treatment for ALD remains as it was 50 years ago: abstinence, nutritional support, and corticosteroids (3). Although corticosteroid treatment has been shown to improve survival, a large number of patients exhibit poor response, and there are also no effective alternative medicines for them. Recently, Sukriti et al. suggested that microvesicles in hepatic and peripheral veins could be used to identify patients who are unable to respond to corticosteroid treatment (4). For patients with decompensated cirrhosis, liver transplantation is the only definitive therapy. However, scarcity of organ donation, high operational costs, and immune rejection excludes most patients (5). Therefore, there exists an urgent problem to further understand the pathogenesis of ALD, and develop new and effective therapeutic methods.
Liver is a unique organ with the capability for complete regeneration. Under normal conditions, the recovery of liver mass is mainly by proliferation of the residual mature hepatocytes, whereas under pathologic conditions, hepatocyte proliferation is inhibited and local progenitor cells are activated and differentiate to repair the damaged liver (6). Shi et al. demonstrated that, in addition to inhibiting adult hepatocyte proliferation, ethanol can also inhibit liver progenitor cell (LPC) proliferation, and interrupt cell differentiation by Snail signaling (7). Therefore, restoration of the liver’s regenerative ability during ALD progression seems to be a possible critical mechanism in ALD patient therapy. Currently, several approaches have been tested in this field such as cytokine induction, stem cell transplantation, and establishment of 3D artificial liver, and these attempts appear to hold promise.
Pathological mechanisms and current treatment of ALD
It is estimated that more than 90% of heavy drinkers may develop steatosis, but only approximately 30% of individuals develop more severe forms of ALD, such as alcoholic hepatitis and cirrhosis (8). Early studies on the pathogenesis of ALD focused on alcohol metabolism. Ethanol can readily diffuse across cell membranes, and approximately 10% is eliminated directly by the kidneys, lungs, and in sweat (9). In the liver, ethanol is metabolised into acetaldehyde, a highly toxic substance for hepatocytes, mainly by alcohol dehydrogenase and microsomal ethanol oxidation systems, which are based on cytochrome P450 2E1 (CYP2E1). Most of the metabolite acetaldehyde is eventually metabolised to acetate by aldehyde dehydrogenase, which then enters the tricarboxylic acid cycle (10). In many cases, ethanol metabolism is associated with malnutrition, oxidative stress, gut endotoxin leakage, and glutathione and S-adenosylmethionine depletion (8). The increase in reactive oxygen species (ROS) induced by ethanol metabolism is one of the main causes of cell apoptosis and necrosis. Damage-associated molecular patterns (DAMPs) are released from dead cells, causing activation of macrophages and neutrophils, and inducing the release of inflammatory cytokines (11). In addition, these events lead to the activation of hepatic stellate cells (HSCs), leading to fibrosis and liver regeneration. Alcohol consumption increases the amount of reduced nicotinamide adenine dinucleotide (NADH) in hepatocytes, which inhibits mitochondrial β-oxidation of fatty acids. Alcohol exposure also affects the expression of lipid metabolism-associated transcription factors to enhance lipogenesis and suppresses fatty acid oxidation. Ethanol up-regulates the expression of sterol regulatory element-binding protein 1c (SREBP-1c), a transcription factor that enhances fatty acid synthesis by heightening expression of lipogenic genes. In addition, ethanol consumption suppresses the expression of the peroxisome proliferator-activated receptor (PPAR)-α, a nuclear hormone receptor that mediates transcription of a group of genes related to free fatty acid transport and oxidation, to inhibit fatty acid oxidation in hepatocytes (12). In addition, ethanol induces fatty liver and reduces fat metabolism by inhibiting AMP-activated protein kinase (AMPK), which can inactivate acetyl-CoA (ACC) and reduce malonyl-CoA levels. Studies revealed that long-term ethanol exposure also alters intestinal permeability and increases circulating pathogen-associated molecular pattern (PAMP) levels, such as lipopolysaccharide (LPS). Circulating PAMPs enter the liver via the portal vein and stimulate Kupffer cells to produce both pathogenic and beneficial cytokines, such as pro-inflammatory cytokines (TNF-a, IL-1, IL-6, and IL-10), chemokines (IL-8 and CCL2), and adhesion molecules (ICAM-1 andVCAM-1) (13). Thus, PAMPs play a crucial role in inducing liver damage in patients with alcoholic hepatitis. Other cell types such as HSCs, splenic T cells, and natural killer cells are thought to be involved in regulation of inflammation in ALD (10). Indeed, chronic ethanol consumption also results in gut microbe dysbiosis, which further increases the circulating levels of endotoxins. Previous studies have shown that the administration of probiotics can reduce hepatic inflammation via restoration of gut microbiota (14). Although understanding of the pathogenesis of ALD has made great progress in recent decades, effective medical treatments are still limited (Figure 1).
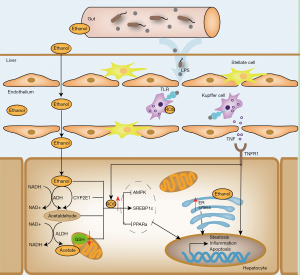
Abstinence from alcohol is extremely important for the control and possible reversal of ALD in patients. It is still the mainstay therapy for all stages of ALD. The earliest stage of ALD, simple steatosis, is asymptomatic and can be reversed by alcohol abstinence. However, when the disease progresses to hepatitis, abstinence alone is insufficient (15). Corticosteroids represent the main pharmacologic therapy for ALD patients. Although such therapy improves survival, approximately 35% of ALD patients with alcoholic hepatitis show poor response to corticosteroid-based therapy (16). Liver progenitor cells (LPCs) are liver-specific undifferentiated precursor cells, which can further differentiate towards hepatocytes or cholangiocytes, and play an important role in the repair of liver injury. Patients with poor response to corticosteroids appear to turn LPCs into cholangiocytes. Shi et al. used ethanol to treat human liver stem cells (HL-1 and HL1-ht1 cells) in vitro to reveal the effects of ethanol on LPCs. They found that ethanol inhibits cell proliferation and E-cadherin expression, stimulates collagen I, Snail and α-SMA expression, and induces cells to differentiate into a myofibroblast-like phenotype (7). Many other treatments have also been tested, such as nutritional support, drugs for anti-oxidant activity, anti-inflammatory agents, anti-apoptotic agents, and modulation of gut microbiota (17,18). Unfortunately, most methods are not effective in improving ALD-induced liver injury. For example, nutritional support may lead to obesity, which could have deleterious outcomes on long-term usage in patients with alcoholic hepatitis; anti-inflammatories may increase risk of infection and mortality, whereas anti-apoptotic agents may lead to oncogenicity (8,17). The most effective method to treat patients with advanced alcoholic cirrhosis is liver transplantation. However, its availability is limited by donor shortage, immune rejection, surgical syndrome, and high medical costs. Therefore, the development of new treatments is urgent for ALD patients. It is well known that the liver has a powerful regenerative capacity, and it could be a key target in the treatment of ALD, particularly alcoholic hepatitis; however, this process is usually prohibited by chronic consumption of alcohol (19). In recent decades, administration of liver regeneration-related cytokines or transplantation of stem cells to provoke liver regeneration has been proposed. Moreover, many studies are underway for building fully functional 3D artificial livers in vitro to resolve the donor shortage issue; these may offer new prospects for patients with advanced ALD.
Liver architecture, development, and regeneration
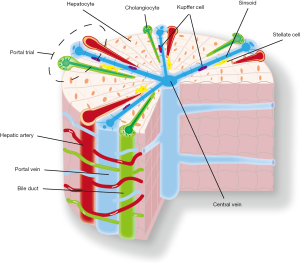
The liver is the largest metabolic and toxin-exposed organ in the body, and is composed of many types of cells, including hepatocytes, cholangiocytes, Kupffer cells, HSCs, and endothelial cells (20). It mediates digestion, detoxification, haematopoiesis, sterol synthesis, glycolysis, and urea metabolism. Hepatocytes comprise ~80% of liver mass, with the capacity to secrete bile, serum albumin, and clotting factors. Hepatic lobules are the functional units of an adult liver and are composed of polygonal hepatic cords formed by single hepatocyte sheets. The hepatic cords are radially distributed around the central vein and interlay with the peripheral portal triads, which consist of bile duct, portal vein, and hepatic artery (21). Hepatocyte cords are separated by hepatic sinusoids, through which blood travels from the portal triads to the central vein. Sinusoids are made up of endothelial cells, and Kupffer cells reside in the sinusoids to regulate liver immunity. HSCs are the main reservoir of vitamin A and are located in the space between the hepatic cords and sinusoids, and are responsible for fibrosis in liver injury. Cholangiocytes are located on the periphery of the bile duct as specialised epithelial cells, and can eliminate colloids and soluble waste macromolecules from the circulation (Figure 2) (22).
Liver tissue is derived from the endoderm, and the process of liver organogenesis is extremely conservative among vertebrate species. Endoderm is a monolayer of cells present at E7.5 in mice, or during the third week of gestation in humans. Subsequently, the endoderm forms a tube with foregut, midgut, and hindgut. By E9 in mice or 23–26 d in humans, liver diverticula appear on the ventral side of the foregut, which is adjacent to the cardiac mesoderm. Subsequently, the diverticula transition from a monolayer of endoderm cells into a multilayer of pseudostratified cells termed hepatoblasts. After that, hepatoblasts proliferate and migrate to the surrounding septum transversum to form a liver bud at E9.5 in mice or 26–32 d in humans. At this stage, haematopoietic progenitor cells begin to migrate into the bud and establish liver foetal haematopoiesis. Hepatocytes and cholangiocytes initiate differentiation from hepatoblasts at E13.5 in mouse or 56–58 d in humans, and then gradually develop into mature buds (23). Hepatic development is tightly regulated by multiple signalling pathways. Currently, signalling pathways known to play crucial roles in liver development include Wnt, Notch, transforming growth factor β, bone morphogenetic protein, and fibroblast growth factor pathways (21,24). In fact, it is important to further understand how gene transcription and signalling programs precisely coordinate, and how interactions between different cells enable liver development. However, co-culture systems, tissue printing processes, or 3D bio-scaffolds might be needed to replicate some aspects of hepatic tissue architecture, and will be a challenge to scale-up from mice to humans. These approaches could help us better conquer liver-related diseases, and enable us to produce 3D artificial livers with biological functions.
Cytokines induce liver regeneration
Many cytokines and chemokines are involved in the progression of ALD, and cytokines related to liver generation will be discussed in this review. Interleukin-22 (IL-22) is a member of the IL-10 family, and has been shown to target epithelial cells to promote tissue repair by IL-10R2 and IL-22R1. However, it has recently been found that in addition to epidermal cells, LPCs and HSCs also express high levels of IL-10R2 and IL-22R1 as IL-22 binding targets (25,26). One study documented that IL-22 treatment can relieve liver injury in both acute and chronic alcoholic mouse models (27). IL-22 not only can induce mature hepatocyte proliferation, but also provoke LPC proliferation for liver repair. It has been demonstrated that STAT3 plays an important role in IL-22-induced liver regeneration (25). Knockout of STAT3 results in poor response to IL-22-induced cell proliferation, both in vitro and in vivo. Moreover, IL-22 treatment has other beneficial effects. The expression of IL-22R1 is restricted, thus inducing fewer side effects during treatment of ALD patients compared to that with other cytokines. Meanwhile, IL-22 overexpression has anti-fibrotic consequences in liver injury. Thus, these properties of IL-22 make it a potential candidate for treatment of ALD.
Granulocyte-colony stimulating factor (G-CSF) is a glycosylated polypeptide secreted by activated monocytes and macrophages. Recombinant G-CSF is often used to treat severe neutropenia in patients who are undergoing haematopoietic cell transplantation, or receiving cancer chemotherapy (28). Studies have shown that administration of G-CSF to patients with alcoholic hepatitis can induce haematopoietic stem cell mobilisation, and improve prognosis. Subcutaneous G-CSF administration induces CD34+ stem cell mobilisation to the liver, increases circulating hepatic growth factors, and enhances LPC proliferation (29,30). Singh et al. found that combined administration of G-CSF (5 µg/kg, every 12 h for 5 days) and pentoxifylline (1,200 mg/day) significantly increases survival and decreases the (Model for end-stage liver disease) MELD score, compared to administration of pentoxifylline alone in patients with severe alcoholic hepatitis after 3 months (78.3 vs. 30.4%; P=0.001) (31,32). G-CSF treatment is well tolerated in patients, and also increases the number of leukocytes and neutrophils, improves liver function, and reduces the incidence of septic infections (33). Currently, several trials examining G-CFS for use in hepatitis and steroid-unresponsive patients are being established (34). However, the mechanisms by which G-CSF therapy improves patient survival need further elucidation in the future.
Interleukin 6 (IL-6) is a pleiotropic cytokine, with a four-helix-bundle structure. In the liver, IL-6 is not only involved in inflammatory responses, but is a potent hepatocyte mitogen that modulates hepatocyte homeostasis (35). IL-6 can prolong STAT3 phosphorylation to enhance liver regeneration via IL-6R, which is expressed on hepatocytes (36,37). Although the hepatoprotective effects of IL-6 are proven, the clinical use of IL-6 in ALD therapy is limited by a number of potential side effects that may be due to the widespread expression of the IL-6 receptor (27). In addition to the aforementioned factors, other molecules are also involved in liver regeneration, including vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1), epidermal growth factor (EGF), and basic fibroblast growth factor (bFGF) (24,38,39).
Stem cell therapy for ALD
Stem cell therapy has been proposed as a potential alternative treatment option to liver transplantation. Stem cells are a group of pluripotent cells with self-renewal ability, such as embryonic stem cells (ESCs), adult mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPSCs) (40,41). Stem cell therapy has several advantages over organ transplantation such as: (I) cells can be easy proliferated and cryopreserved; (II) they can be easily obtained from the patient for autologous transplantation; and (III) stem cell infusion does not require major invasive surgery. Adult stem cells, which include hematopoietic stem cells and MSCs, are the first candidates for stem cell therapy because of their weak teratogenic potential and low ethical controversy (42). Adult stem cells are found in all nonembryonic tissues, and are thought to play a key role in maintaining cellular homeostasis and regeneration of injured organs. MSCs are hypo-immunogenic, meaning that recipients are not required to be conditioned before transplantation (43). Nowadays, MSCs have been isolated from many tissues, including lung, liver, umbilical cord, spleen, skeletal muscle, dental pulp, and adipose tissue (19). To standardise treatment, the minimal criteria to define human MSCs have been proposed by the International Society for Cellular Therapy (ISCT) in 2006: (I) under standard culture conditions, cells must be plastic-adherent; (II) must express CD73, CD105, and CD90, and lack expression of CD14, CD45, CD34, CD19, CD11b, and class II human leukocyte antigen (HLA) surface molecules; and (III) must differentiate into osteoblasts, chondroblasts, and adipocytes in vitro (44).
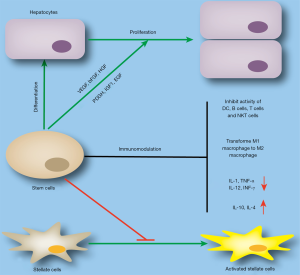
Multiple mechanisms have been revealed to be involved in amelioration of liver diseases after stem cell therapy, such as differentiation into hepatocytes, induction of endogenous hepatocyte proliferation, immunomodulation, and inhibition of fibrosis. Stem cells have the potential to differentiate into parenchymal cells, and this has been experimentally demonstrated. When MSCs were transplanted into the liver, some cells differentiated into hepatocyte-like cells (45,46). However, although the differentiated cells were found to express hepatocyte markers such as CK18, CK19, and albumin by standardised culture in vitro, they also expressed a few MSC markers such as CD90Jeny (47-49). Therefore, differentiation of MSCs to functional hepatocytes is not completely achieved, and there might be other mechanisms involved in this process. In addition to direct differentiation into hepatocytes, stem cells can also induce endogenous hepatocyte proliferation by secreting trophic factors. MSCs have been shown to secrete trophic factors both in vitro and in vivo, including HGF, VEGF, PDGF, TGF-β, IGF-1, EGF, and bFGF.
Thus, MSCs can modulate their cellular microenvironments by secreting cytokines to induce endogenous hepatocyte proliferation, prevent apoptosis, promote neovascularisation, and revert fibrosis development (50,51). Furthermore, stem cells can regulate the activity of immune cells in chronic and acute liver diseases. MSCs have been shown to inhibit the activity of dendritic cells, B cells, T cells, NK T lymphocytes and polarising M2 macrophages (19,52-54). However, MSCs inhibit the secretion of proinflammatory (e.g., IL-1β, TNF-α, IL-12, and INF-γ) cytokines, and enhance the secretion of anti-inflammatory molecules (e.g., IL-10 and IL-4) (55). In addition to the above functions, stem cell therapy can also relieve carbon tetrachloride-induced liver fibrosis by inhibiting the activity of HSCs (Figure 3) (56,57).
Although numerous studies have tried to reveal the therapeutic potential of stem cells in liver diseases, stem cell therapy for ALD is still in its infancy owing to the lack of animal models to phenocopy advanced ALD features in humans. Jang et al. treated alcoholic hepatic cirrhosis patients with autologous bone marrow-derived MSCs, and showed that MSC infusion improved 6/11 patients in terms of liver histology and decreased the expression of TGF-β1, α-SMA, and type I collagen (58). To further confirm this finding, the group recruited another 72 patients with alcoholic cirrhosis for bone marrow-derived MSC transplantation, which also suggested that autologous bone marrow-MSC (BM-MSC) transplantation was safe and could improve histologic fibrosis and liver function (40). However, Lanthier et al. suggested that the impact of bone marrow-derived cell transplantation in alcoholic hepatitis is weak, and Rajaram et al. suggested that the clinical benefits of MSC transplantation in patients with advanced cirrhosis may be temporary (59,60). Spahr et al. suggested that autologous BM-MSC transplantation was a safe method, but owing to insufficient stimulation of regeneration after transplantation in patients with decompensated alcoholic cirrhosis, no proliferation of LPCs or improvement of liver function resulted (61). One of the major obstacles to stem cell treatment is the limited number of functional stem cells available after transplantation because of the harsh microenvironment surrounding damaged tissues. Our previous work demonstrated that co-treatment with lysophosphatidic acid and sphingosine-1-phosphate could enhance stem cell transplantation efficacy in alcohol-related liver disease (62). Ge et al. found that pre-activation of TLR3 enhances the therapeutic effect of autologous BM-MSC transplantation in alcoholic liver injury (63). Thus, it is noteworthy that there is considerable evidence that stem cell therapy is relatively safe, and more than 1,000 human patients have undergone such therapy without any side effects or tumour formation (43).
Establishment of 3D artificial liver
Liver transplantation is the only clinical treatment for advanced ALD, such as decompensated alcoholic cirrhosis and alcohol-induced hepatocellular carcinoma. However, insufficient sources of qualified donor livers and immune rejection seriously restrict the application of liver transplantation. The concept of using stem cells to construct 3D artificial livers has brought new hope for patients with advanced liver diseases. Fully functional 3D artificial livers not only can induce stem cells to form hepatocytes and be deposited in three dimensions, but also require blood vessel formation, storage of vitamins, immune regulatory activity, and other hepatic functions. Here, we will summarise the research progress surrounding 3D artificial liver technology.
The lack of reliable sources of related cell types is a major obstacle to the establishment of artificial livers. Over the past decades, great progress has been made in the control of stem cell differentiation into adult somatic cells. Numerous studies have researched how to induce stem cell differentiation into hepatocytes, which represent the majority of cells in the liver. Many differentiation protocols have been proposed, and the resulting hepatocyte-like cells have most of the functions of normal hepatocytes, including secretion of α-fetoprotein, albumin, and aminotransferases (64-66). At present, hepatocyte differentiation from stem cells is relatively mature, and researchers can even easily acquire hepatocyte induction kits from commercial companies. Studies on stem cells forming HSCs are rare. Coll et al. successfully induced iPSC differentiation into stellate-like cells using BMP4, FGF1, FGF3, retinol, and palmitic acid. These cells exhibited very similar levels of function to stellate cells, and their gene expression profiles differed between quiescent and activated HSCs (67). Sinusoidal endothelial cells are the most abundant non-parenchymal cells in the liver. Du et al. used a two-step process from human iPSCs to the mesodermal lineage to endothelial cells. The final differentiated cell expressed endothelial markers, such as VE-cadherin and CD31 (68). Some studies have even reported differentiated functional endothelial cells derived from iPSC. These cells expressed endothelial cell markers, spontaneously formed tubular structures, contained endothelial nitric oxide synthase, and absorbed acetylated low-density lipoprotein (69,70). Bile duct epithelial cell cholangiocytes have also been reported to differentiate from stem cells. Cholangiocyte-like cells exhibit certain functional properties of normal cholangiocytes, including bile acid transfer, alkaline phosphatase/glutamyl-transpeptidase activity, and reaction to related secretions (71,72). To date, only Tasnim et al. have claimed to have acquired Kupffer-like cells from human iPSCs (73). Kupffer cells do not seem to be necessary in the process of establishing a 3D artificial liver, because these can be transplanted from the bone marrow and the recipient’s circulatory system into an engineered liver graft (74).
Although we have made great progress in controlling stem cell differentiation over the past few decades, building an authentic 3D structure is another obstacle to building an artificial liver. Currently, many studies are exploring suitable materials for the extracellular matrix (ECM), to construct 3D scaffolds (75). These engineered scaffolds should be fabricable, tissue-compatible, and low in toxicity to provide a qualified 3D microenvironment for cell attachment and proliferation. Scientists have made various attempts to engineer scaffolds for artificial organs, including using decellularised matrix, natural macromolecules, and synthetic biodegradable polymers (76). Although research concerning artificial scaffolds has made some progress, these methods also have a number of defects, which limits their application: (I) severe shortage of donor organs, high cost of treatment, and lifetime immune suppression for decellularised matrix (77); (II) large differences between batches and potential immune risks involving transplantation of natural macromolecules (78); (III) poor cell culture characteristics for synthetic biodegradable polymers (79). Indeed, Takebe et al. have successfully cultured hepatic buds with vascularisation and appropriate function by mixing human umbilical vein endothelial cells (HUVECs), hepatocytes, and iPSCs (80). Recently, our group also established self-assembled functional 3D human liver buds in vitro by mixing hepatocytes, HSCs, and liver sinusoidal endothelial cells (LSECs), which were derived from human MSCs. After transplantation into a murine model of acute liver failure, 3D grafts exhibited gene expression profiles similar to that of adult human livers (81). However, in vitro self-assembly of liver buds appears to be insufficient to reverse liver failure due to limited bud physical size. Thus, establishment of a sufficiently large vascularised 3D artificial liver is necessary for future clinical applications, and there is still a long way to go.
Conclusions
Long-term alcohol consumption is a major risk factor in terms of the global burden of disease and causes significant health loss. Currently, the pathogenesis of human ALD is not fully understood, and there are no modern diagnostic tools to assess an individual’s susceptibility to ALD development. Despite the advancements in basic research, the enthusiasm and energy for liver regeneration has never waned. The need for liver regeneration is urgent to address issues such as liver failure, liver transplantation, or other end-stage liver diseases. Research on molecules involved in the regulation of liver regeneration promotes our understanding of the mechanisms of liver regeneration. Stem cell transplantation represents a new therapeutic approach to treat ALD. It has been shown to trigger the regeneration of damaged liver tissue without the significant adverse effects observed in previous clinical studies. Establishing artificial livers for use in patients with advanced ALD is a potential option. Reliable source of cell types and qualified scaffold materials are essential for the acquisition of 3D artificial livers in vitro. Meanwhile, some of the potential risks associated with liver regeneration must be monitored, including immune rejection and cell proliferation leading to tumour formation. In summary, we suggest that liver regeneration is a promising therapeutic strategy to control ALD progression.
Acknowledgments
Funding: This study was supported by National Natural Science Foundation of China (81900535).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ling Lu) for the series “Stem Cell and Clinical Application” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.02.168). The series “Stem Cell and Clinical Application” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Organization WH. Global status report on alcohol and health 2018. 2018.
- Collaborators GBDA. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018;392:1015-35. [Crossref] [PubMed]
- Singh S, Osna NA, Kharbanda KK. Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World J Gastroenterol 2017;23:6549-70. [Crossref] [PubMed]
- Sukriti S, Maras JS, Bihari C, et al. Microvesicles in hepatic and peripheral vein can predict nonresponse to corticosteroid therapy in severe alcoholic hepatitis. Aliment Pharmacol Ther 2018;47:1151-61. [Crossref] [PubMed]
- Pan XN, Zheng LQ, Lai XH. Bone marrow-derived mesenchymal stem cell therapy for decompensated liver cirrhosis: a meta-analysis. World J Gastroenterol 2014;20:14051-7. [Crossref] [PubMed]
- Michalopoulos GK. Liver regeneration. J Cell Physiol 2007;213:286-300. [Crossref] [PubMed]
- Shi X, Chang CC, Basson MD, et al. Alcohol Disrupts Human Liver Stem/Progenitor Cell Proliferation and Differentiation. J Stem Cell Res Ther 2014. [Crossref] [PubMed]
- Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011;141:1572-85. [Crossref] [PubMed]
- Norberg A, Jones AW, Hahn RG, et al. Role of variability in explaining ethanol pharmacokinetics: research and forensic applications. Clin Pharmacokinet 2003;42:1-31. [Crossref] [PubMed]
- Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol 2015;12:231-42. [Crossref] [PubMed]
- Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 2014;147:765-83.e4. [Crossref] [PubMed]
- Wagner M, Zollner G, Trauner M. Nuclear receptors in liver disease. Hepatology 2011;53:1023-34. [Crossref] [PubMed]
- Kong LZ, Chandimali N, Han YH, et al. Pathogenesis, Early Diagnosis, and Therapeutic Management of Alcoholic Liver Disease. Int J Mol Sci 2019. [Crossref] [PubMed]
- Forsyth CB, Farhadi A, Jakate SM, et al. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 2009;43:163-72. [Crossref] [PubMed]
- Kim W, Kim DJ. Severe alcoholic hepatitis-current concepts, diagnosis and treatment options. World J Hepatol 2014;6:688-95. [Crossref] [PubMed]
- Singal AK, Walia I, Singal A, et al. Corticosteroids and pentoxifylline for the treatment of alcoholic hepatitis: Current status. World J Hepatol 2011;3:205-10. [Crossref] [PubMed]
- Lanthier N, Starkel P. Treatment of severe alcoholic hepatitis: past, present and future. Eur J Clin Invest 2017;47:531-9. [Crossref] [PubMed]
- Osna NA, Donohue TM Jr, Kharbanda KK. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res 2017;38:147-61. [PubMed]
- Ezquer F, Bruna F, Calligaris S, et al. Multipotent mesenchymal stromal cells: A promising strategy to manage alcoholic liver disease. World J Gastroenterol 2016;22:24-36. [Crossref] [PubMed]
- Wambaugh J, Shah I. Simulating microdosimetry in a virtual hepatic lobule. PLoS Comput Biol 2010;6:e1000756. [Crossref] [PubMed]
- Ober EA, Lemaigre FP. Development of the liver: Insights into organ and tissue morphogenesis. J Hepatol 2018;68:1049-62. [Crossref] [PubMed]
- Xu M, Wang X, Zou Y, et al. Key role of liver sinusoidal endothelial cells in liver fibrosis. Biosci Trends 2017;11:163-8. [Crossref] [PubMed]
- Gordillo M, Evans T, Gouon-Evans V. Orchestrating liver development. Development 2015;142:2094-108. [Crossref] [PubMed]
- Tao Y, Wang M, Chen E, et al. Liver Regeneration: Analysis of the Main Relevant Signaling Molecules. Mediators Inflamm 2017;2017:4256352.
- Feng D, Kong X, Weng H, et al. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology 2012;143:188-98.e7. [Crossref] [PubMed]
- Kong X, Feng D, Wang H, et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 2012;56:1150-9. [Crossref] [PubMed]
- Kong X, Feng D, Mathews S, et al. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J Gastroenterol Hepatol 2013;28 Suppl 1:56-60. [Crossref] [PubMed]
- Colony Stimulating Factors. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD) 2012.
- Spahr L, Lambert JF, Rubbia-Brandt L, et al. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology 2008;48:221-9. [Crossref] [PubMed]
- Tahan AC, Tahan V. "If a fake friend stays in your vodkabulary as a stem friend": granulocyte colony-stimulating factor promoted stem cell therapy in severe alcoholic hepatitis. Turk J Gastroenterol 2014;25:748-9. [Crossref] [PubMed]
- Moreau R, Rautou PE. G-CSF therapy for severe alcoholic hepatitis: targeting liver regeneration or neutrophil function? Am J Gastroenterol 2014;109:1424-6. [Crossref] [PubMed]
- Singh V, Sharma AK, Narasimhan RL, et al. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol 2014;109:1417-23. [Crossref] [PubMed]
- Shi X, DeLucia AL, Bao J, et al. Alcohol abuse and disorder of granulopoiesis. Pharmacol Ther 2019;198:206-19. [Crossref] [PubMed]
- Cho Y, Park YS, Kim HY, et al. Efficacy of granulocyte colony stimulating factor in patients with severe alcoholic hepatitis with partial or null response to steroid (GRACIAH trial): study protocol for a randomized controlled trial. Trials 2018;19:696. [Crossref] [PubMed]
- Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J Hepatol 2016;64:1403-15. [Crossref] [PubMed]
- Peters M, Blinn G, Jostock T, et al. Combined interleukin 6 and soluble interleukin 6 receptor accelerates murine liver regeneration. Gastroenterology 2000;119:1663-71. [Crossref] [PubMed]
- Drucker C, Gewiese J, Malchow S, et al. Impact of interleukin-6 classic- and trans-signaling on liver damage and regeneration. J Autoimmun 2010;34:29-37. [Crossref] [PubMed]
- Taniguchi E, Sakisaka S, Matsuo K, et al. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem 2001;49:121-30. [Crossref] [PubMed]
- DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest 2013;123:1861-6. [Crossref] [PubMed]
- Suk KT, Yoon JH, Kim MY, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology 2016;64:2185-97. [Crossref] [PubMed]
- Graffmann N, Ring S, Kawala MA, et al. Modeling Nonalcoholic Fatty Liver Disease with Human Pluripotent Stem Cell-Derived Immature Hepatocyte-Like Cells Reveals Activation of PLIN2 and Confirms Regulatory Functions of Peroxisome Proliferator-Activated Receptor Alpha. Stem Cells Dev 2016;25:1119-33. [Crossref] [PubMed]
- Fiore EJ, Mazzolini G, Aquino JB. Mesenchymal Stem/Stromal Cells in Liver Fibrosis: Recent Findings, Old/New Caveats and Future Perspectives. Stem Cell Rev Rep 2015;11:586-97. [Crossref] [PubMed]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726-36. [Crossref] [PubMed]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. [Crossref] [PubMed]
- Shu SN, Wei L, Wang JH, et al. Hepatic differentiation capability of rat bone marrow-derived mesenchymal stem cells and hematopoietic stem cells. World J Gastroenterol 2004;10:2818-22. [Crossref] [PubMed]
- Chamberlain J, Yamagami T, Colletti E, et al. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology 2007;46:1935-45. [Crossref] [PubMed]
- Bornstein R, Macias MI, de la Torre P, et al. Human decidua-derived mesenchymal stromal cells differentiate into hepatic-like cells and form functional three-dimensional structures. Cytotherapy 2012;14:1182-92. [Crossref] [PubMed]
- Prasajak P, Leeanansaksiri W. Developing a New Two-Step Protocol to Generate Functional Hepatocytes from Wharton's Jelly-Derived Mesenchymal Stem Cells under Hypoxic Condition. Stem Cells Int 2013;2013:762196.
- Campard D, Lysy PA, Najimi M, et al. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology 2008;134:833-48. [Crossref] [PubMed]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076-84. [Crossref] [PubMed]
- Lee T. Stem cell therapy independent of stemness. World J Stem Cells 2012;4:120-4. [Crossref] [PubMed]
- Spaggiari GM, Capobianco A, Abdelrazik H, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008;111:1327-33. [Crossref] [PubMed]
- Wang H, Zhang H, Huang B, et al. Mesenchymal stem cells reverse highfat dietinduced nonalcoholic fatty liver disease through suppression of CD4+ T lymphocytes in mice. Mol Med Rep 2018;17:3769-74. [PubMed]
- Zhao H, Shang Q, Pan Z, et al. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes 2018;67:235-47. [Crossref] [PubMed]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815-22. [Crossref] [PubMed]
- Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 2013;22:845-54. [Crossref] [PubMed]
- Alfaifi M, Eom YW, Newsome PN, et al. Mesenchymal stromal cell therapy for liver diseases. J Hepatol 2018;68:1272-85. [Crossref] [PubMed]
- Jang YO, Kim YJ, Baik SK, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int 2014;34:33-41. [Crossref] [PubMed]
- Lanthier N, Lin-Marq N, Rubbia-Brandt L, et al. Autologous bone marrow-derived cell transplantation in decompensated alcoholic liver disease: what is the impact on liver histology and gene expression patterns? Stem Cell Res Ther 2017;8:88. [Crossref] [PubMed]
- Rajaram R, Subramani B, Abdullah BJJ, et al. Mesenchymal stem cell therapy for advanced liver cirrhosis: A case report. JGH Open 2017;1:153-5. [Crossref] [PubMed]
- Spahr L, Chalandon Y, Terraz S, et al. Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLoS One 2013;8:e53719. [Crossref] [PubMed]
- Li M, Lv Y, Chen F, et al. Co-stimulation of LPAR1 and S1PR1/3 increases the transplantation efficacy of human mesenchymal stem cells in drug-induced and alcoholic liver diseases. Stem Cell Res Ther 2018;9:161. [Crossref] [PubMed]
- Ge L, Chen D, Chen W, et al. Pre-activation of TLR3 enhances the therapeutic effect of BMMSCs through regulation the intestinal HIF-2alpha signaling pathway and balance of NKB cells in experimental alcoholic liver injury. Int Immunopharmacol 2019;70:477-85. [Crossref] [PubMed]
- Pettinato G, Lehoux S, Ramanathan R, et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Sci Rep 2019;9:8920. [Crossref] [PubMed]
- Xie PY, Hu XJ, Guo RM, et al. Generation of functional hepatocyte-like cells from human bone marrow mesenchymal stem cells by overexpression of transcription factor HNF4alpha and FOXA2. Hepatobiliary Pancreat Dis Int 2019;18:546-56. [Crossref] [PubMed]
- Mallanna SK, Duncan SA. Differentiation of hepatocytes from pluripotent stem cells. Curr Protoc Stem Cell Biol 2013;26:Unit 1G 4.
- Coll M, Perea L, Boon R, et al. Generation of Hepatic Stellate Cells from Human Pluripotent Stem Cells Enables In Vitro Modeling of Liver Fibrosis. Cell Stem Cell 2018;23:101-13.e7. [Crossref] [PubMed]
- Du C, Narayanan K, Leong MF, et al. Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials 2014;35:6006-14. [Crossref] [PubMed]
- Vunjak-Novakovic G, Bhatia S, Chen C, et al. HeLiVa platform: integrated heart-liver-vascular systems for drug testing in human health and disease. Stem Cell Res Ther 2013;4 Suppl 1:S8. [Crossref] [PubMed]
- Narmada BC, Goh YT, Li H, et al. Human Stem Cell-Derived Endothelial-Hepatic Platform for Efficacy Testing of Vascular-Protective Metabolites from Nutraceuticals. Stem Cells Transl Med 2017;6:851-63. [Crossref] [PubMed]
- Sampaziotis F, de Brito MC, Madrigal P, et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol 2015;33:845-52. [Crossref] [PubMed]
- Sampaziotis F, de Brito MC, Geti I, et al. Directed differentiation of human induced pluripotent stem cells into functional cholangiocyte-like cells. Nat Protoc 2017;12:814-27. [Crossref] [PubMed]
- Tasnim F, Xing J, Huang X, et al. Generation of mature kupffer cells from human induced pluripotent stem cells. Biomaterials 2019;192:377-91. [Crossref] [PubMed]
- Uygun BE, Yarmush ML. Engineered liver for transplantation. Curr Opin Biotechnol 2013;24:893-9. [Crossref] [PubMed]
- Lee SY, Kim HJ, Choi D. Cell sources, liver support systems and liver tissue engineering: alternatives to liver transplantation. Int J Stem Cells 2015;8:36-47. [Crossref] [PubMed]
- Zhang J, Zhao X, Liang L, et al. A decade of progress in liver regenerative medicine. Biomaterials 2018;157:161-76. [Crossref] [PubMed]
- Taylor DA, Sampaio LC, Ferdous Z, et al. Decellularized matrices in regenerative medicine. Acta Biomater 2018;74:74-89. [Crossref] [PubMed]
- Abbasian M, Massoumi B, Mohammad-Rezaei R, et al. Scaffolding polymeric biomaterials: Are naturally occurring biological macromolecules more appropriate for tissue engineering? Int J Biol Macromol 2019;134:673-94. [Crossref] [PubMed]
- Zhang K, Zheng H, Liang S, et al. Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth. Acta Biomater 2016;37:131-42. [Crossref] [PubMed]
- Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013;499:481-4. [Crossref] [PubMed]
- Li J, Xing F, Chen F, et al. Functional 3D Human Liver Bud Assembled from MSC-Derived Multiple Liver Cell Lineages. Cell Transplant 2019;28:510-21. [Crossref] [PubMed]


