Effect of terlipressin on renal function in cirrhotic patients with acute upper gastrointestinal bleeding
Introduction
Renal function impairment, even acute kidney injury (AKI) (1) or hepatorenal syndrome (HRS), may develop in cirrhotic patients with acute upper gastrointestinal bleeding (AUGIB) (1-3). This is primarily associated with systemic vascular resistance reduction secondary to splanchnic arterial vasodilatation in cirrhotic portal hypertension (4) and renal perfusion reduction secondary to blood loss after gastrointestinal bleeding (5,6). On the other hand, renal dysfunction significantly decreases the survival of cirrhotic patients (7,8).
Currently, terlipressin is the first-line treatment option for the management of acute gastro-esophageal variceal bleeding and HRS (9,10). Terlipressin is a vasopressin analogue composed by a synthetic 12 amino acid peptide that acts through the V1 receptors leading to splanchnic vasoconstriction, arterial blood volume elevation, and portal pressure reduction. It also acts through the V2 receptors which can deactivate renal and systemic vasoconstriction systems, thereby ameliorating glomerular filtration rate (GFR) and improving renal perfusion and function (11). Evidence suggests that terlipressin can decrease creatinine (Cr) by increasing Cr clearance (12-22), GFR, and urine sodium concentration (23) in patients with HRS (24,25). However, until now, there is no evidence regarding effect of terlipressin on renal function in cirrhotic patients with AUGIB. Herein, we conducted a retrospective study to analyze the renal function changes during and after the treatment of AUGIB with terlipressin and to identify the factors associated with AKI development (26).
Methods
Study design
This was a retrospective study at the Department of Gastroenterology of the General Hospital of Northern Theater Command (formerly General Hospital of Shenyang Military Area) from January 2016 to June 2018. The primary objective of the study was to evaluate the renal function changes in patients treated with terlipressin. The secondary objectives were to identify the factors associated with reductions of Cr and cystatin C (CysC) values and those associated with development of AKI. The study protocol conformed to the Declaration of Helsinki and was approved by the Medical Ethical Committee of our hospital [No. k(2018)20]. All included patients were treated by an attending physician (Xingshun Qi). Inclusion criteria were: (I) patients admitted with liver cirrhosis and AUGIB; and (II) patients received terlipressin treatment. Exclusion criteria were: (I) lack of renal function results, such as Cr and CysC; and (II) renal parenchymal disease. Additionally, to further explore the role of terlipressin on renal function improvement, we established a control group of cirrhotic patients treated with somatostatin/octreotide for AUGIB during the same enrollment period.
Data collection
All data were retrospectively reviewed by electronic medical charts. The primary data were collected as follows: age, sex, liver disease etiology, ascites, hepatic encephalopathy, hepatocellular carcinoma, hemoglobin, red/white blood cell count, neutrophils, lymphocytes, platelets, total bilirubin, direct bilirubin, alanine transaminase, aspartate transaminase (AST), albumin, D-Dimer, fibrinogen, prothrombin time, activated partial thromboplastin time, international normalized ratio, Cr, CysC, serum sodium, and total dose and duration of terlipressin treatment. Severity of liver dysfunction was assessed by Child-Pugh and model of end stage liver disease (MELD) scores.
Diagnosis and definitions
Liver cirrhosis diagnosis was primarily based on the history of liver diseases, clinical presentations, laboratory tests, and imaging examinations (27,28). AUGIB was diagnosed as a development of haematemesis and/or melena within 5 days before our admission (29). Baseline Cr and CysC values were defined as the Cr and CysC values obtained before the use of terlipressin at our admission. AKI was diagnosed as an increase of Cr value ≥26.5 µmol/L within 48 hours after our admission (30,31).
Classifications
According to the patients’ conditions during the use of terlipressin or after terlipressin was stopped, the patients were divided into two groups: (I) patients at a stable condition, which was defined as the absence of further melena or hematemesis; and (II) patients at an unstable condition, which was defined as the presence of melena and/or hematemesis recurrence. According to the change of Cr value, the patients were divided into two groups: (I) patients with a Cr reduction; and (II) patients with a stable or increased Cr value. Similarly, according to the change of CysC value, the patients were also divided into two groups: (I) patients with a CysC reduction; and (II) patients with a stable or increased CysC value.
Statistical analyses
Continuous data were expressed as the mean ± standard deviation and median (range). Categorical variables were expressed as frequencies (percentages). Continuous variables between two different patient groups were compared by the independent Student’s t-test for normal distribution and non-parametric Mann-Whitney’s test for non-normal distribution. Continuous variables before and after treatment were compared by the paired Student’s t-test for normal distribution and non-parametric Wilcoxon test for non-normal distribution. Categorical variables were compared by the Chi-square test and Fisher exact test. P value less than 0.05 will be considered significant. Statistical analyses were performed using SPSS Statistics version 17.0.0. Before-after graphs were drawn to show the statistical differences by GraphPad Prism Software (La Jolla, CA, USA).
Results
Patient characteristics
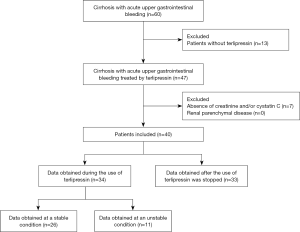
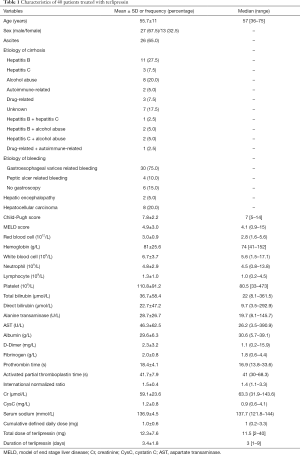
A total of 40 patients (27 males and 13 females; mean age: 55.7±11 years) were included (Figure 1). Patient characteristics were shown in Table 1. The mean Cr and CysC values were 59.1±23.6 (range: 31.9–143.6) µmol/L and 1.2±0.8 (range: 0.6–4.1) mg/L, respectively. Upper gastrointestinal endoscopy was performed in 34 patients, of whom 88.2% had gastroesophageal varices related bleeding and 11.8% had peptic ulcer related bleeding.
Full table
Terlipressin
Thirty-eight and two patients were administered by continuous intravenous infusion alone and intravenous bolus followed by continuous intravenous infusion, respectively. The mean total dose of terlipressin was 12.3±7.6 (range: 2–40) mg. The mean duration of terlipressin was 3.4±1.8 (range: 1–9) days.
Change of CysC value during the use of terlipressin
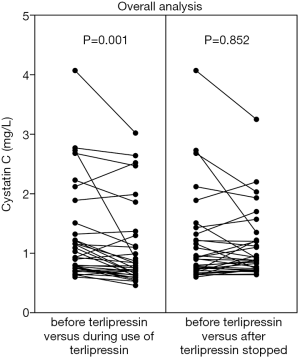
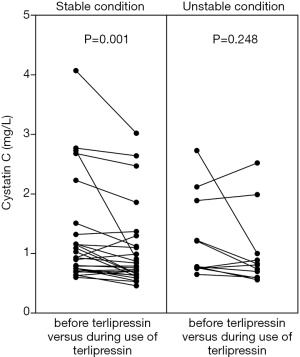
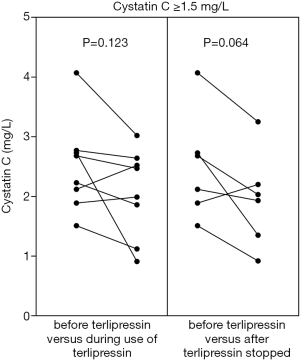
CysC value was significantly reduced during the use of terlipressin in the overall analysis (1.3±0.8 vs. 1.1±0.7, P=0.001) (Figure 2, left panel). CysC reduction during the use of terlipressin remained significant in the subgroup analysis of patients at a stable condition (1.2±0.9 vs. 1.1±0.7, P=0.001) (Figure 3, left panel), but not in the subgroup analysis of patients at an unstable condition (1.2±0.7 vs. 1.0±0.6, P=0.248) (Figure 3, right panel). CysC value was reduced during the use of terlipressin in the subgroup analysis of patients with baseline CysC value ≥1.5 mg/L, but the reduction was not statistically significant (2.5±0.8 vs. 2.1±0.7, P=0.123) (Figure 4, left panel).
As only patients at a stable condition were included, 73.1% (19/26) of patients had a CysC reduction during the use of terlipressin; by comparison, no (0/5, 0%) patient had a CysC reduction during the use of somatostatin/octreotide without terlipressin. There was a statistically significant difference between the two groups (P=0.005). As only patients at a stable condition were included, no factor at baseline was significantly associated with CysC reduction during the use of terlipressin (Table 2).
Full table
Change of Cr value during the use of terlipressin
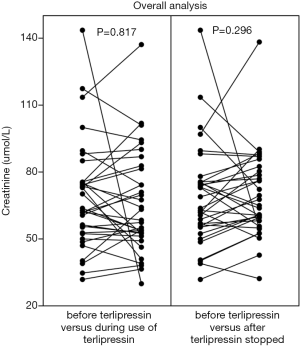
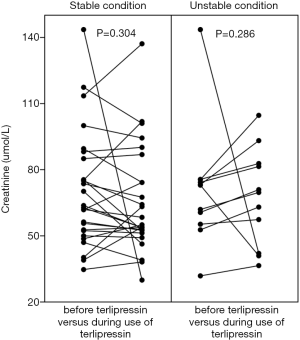
Cr value was mildly reduced during the use of terlipressin in the overall analysis (68.8±24 vs. 65.5±23, P=0.817) (Figure 5, left panel). Cr value was mildly reduced during the use of terlipressin in the subgroup analyses of patients at a stable condition (70.5±26.2 vs. 65.2±24.1, P=0.304) (Figure 6, left panel) and at an unstable condition (70.7±27.6 vs. 67.5±22.2, P=0.286) (Figure 6, right panel). Cr value was mildly reduced during the use of terlipressin in the subgroup analyses of patients with baseline Cr value ≥88.4 µmol/L (112.8±20.4 vs. 87.3±39.3, P=0.334) (Figure 7, left panel) and baseline Cr value ≥100 µmol/L (118.6±18.2 vs. 90.6±44.6, P=0.417) (Figure 8, left panel).
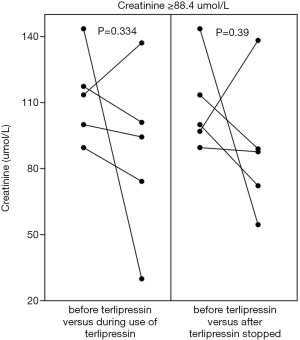

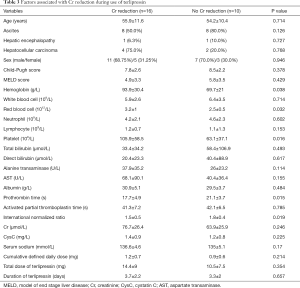
As only patients at a stable condition were included, 61.5% (16/26) of patients had a Cr reduction during the use of terlipressin; by comparison, 20% (1/5) of patients had a Cr reduction during the use of somatostatin/octreotide without terlipressin. There was no statistically significant difference between the two groups (P=0.148). As only patients at a stable condition were included, higher hemoglobin, red blood cell, and platelets and lower prothrombin time and international normalized ratio at baseline were significantly associated with Cr reduction during the use of terlipressin (Table 3).
Full table
Change of CysC value after the use of terlipressin was stopped
CysC value was mildly reduced after the use of terlipressin was stopped in the overall analysis (1.2±0.8 vs. 1.1±0.6, P=0.852) (Figure 2, right panel). CysC value was reduced after the use of terlipressin was stopped in the subgroup analysis of patients with baseline CysC value ≥1.5 mg/L, but the difference was not statistically significant (2.5±0.9 vs. 1.9±0.8, P=0.064) (Figure 4, right panel).
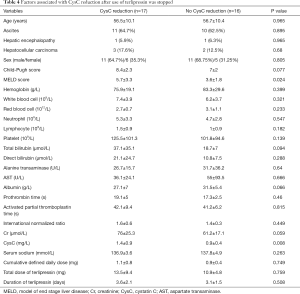
Seventeen (17/33, 51.5%) patients had a CysC reduction after the use of terlipressin was stopped; by comparison, 3 (3/9, 33.3%) patients had a CysC reduction after the use of somatostatin/octreotide was stopped. There was no statistically significant difference between the two groups (P=0.46). Higher MELD score and CysC value at baseline were significantly associated with CysC reduction after the use of terlipressin was stopped (Table 4).
Full table
Change of Cr value after the use of terlipressin was stopped
Cr value was not reduced after the use of terlipressin was stopped in the overall analysis (68.8±22.6 vs. 69.4±19.1, P=0.296) (Figure 5, right panel). Cr value was mildly reduced after the use of terlipressin was stopped in the subgroup analysis of patients with baseline Cr value ≥88.4 µmol/L (108.7±21.3 vs. 88.3±31.2, P=0.39) (Figure 7, right panel) and baseline Cr value ≥100 µmol/L (119±22.3 vs. 71.9±17.2, P=0.154) (Figure 8, right panel).
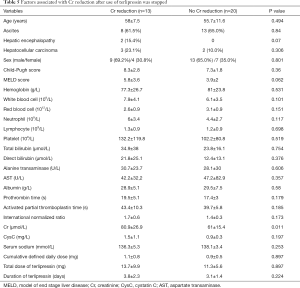
Thirteen (13/33, 39.4%) patients had a Cr reduction after the use of terlipressin was stopped; by comparison, 4 (4/9, 44.4%) patients had a Cr reduction after the use of somatostatin/octreotide was stopped. There was no statistically significant difference between the two groups (P=1.0). Higher Cr at baseline was significantly associated with Cr reduction after the use of terlipressin was stopped (Table 5).
Full table
Factors associated with the development of AKI
Three patients developed AKI during hospitalization. After excluding patients with AKI at admission or a baseline Cr value ≥133 µmol/L (n=1), no factor at baseline was significantly associated with the development of AKI (Table 6).
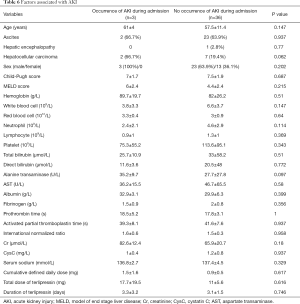
Full table
Discussion
Major findings
First, our study found that the use of terlipressin could significantly reduce the CysC value and the benefit of terlipressin was significantly superior to that of somatostatin/octreotide. However, such a benefit seemed to disappear after terlipressin was stopped. Second, there was a Cr reduction during the use of terlipressin and the absolute benefit of terlipressin seemed to be larger than that of somatostatin/octreotide. However, such a benefit did not achieve a statistical significance. Third, it seemed that higher CysC and Cr values at baseline were associated with a higher probability of developing CysC and Cr reduction after the use of terlipressin was stopped. By comparison, CysC and Cr values at baseline might not be associated with CysC and Cr reduction during the use of terlipressin.
Prior studies
Few studies explored the effect of terlipressin on renal function parameters in cirrhotic patients with acute gastrointestinal bleeding. Based on our recent systematic review regarding terlipressin for treatment of acute variceal bleeding (32), only one previous randomized controlled trial reported the relevant data. The investigators included 163 patients treated with terlipressin and 161 patients treated with octreotide. Bleeding was controlled in 92.63% of patients treated with terlipressin and 95.6% of patients treated with octreotide. Notably, Cr was decreased from 1.2±0.8 mg/dL at baseline to 1.1±0.6 mg/dL after use of terlipressin (33). However, the data were not statistically compared. Additionally, one previous retrospective study from Taiwan reported that 30-day mortality was non-significantly higher in cirrhotic patients with esophageal variceal bleeding and renal function impairment receiving somatostatin than those receiving terlipressin (52.6% vs. 42.3%). However, the change of renal function parameters was not evaluated (7). By comparison, our study focused on the change of Cr and CysC values after terlipressin in such patients.
Why should we evaluate the outcomes of patients with normal Cr at baseline?
Recently, the diagnostic criteria of AKI have been changed. Traditional diagnostic criteria for AKI are that an increase of Cr is greater than 50% and the final value is greater than 1.5 mg/dL. Current diagnostic criteria for AKI have been updated as follows: (I) an increase of Cr within 48 hours is greater than or equal to 0.3 mg/dL; or (II) an increase of Cr within 48 hours is more than 1.5 times from the baseline; or (III) the urine volume lasts less than 0.5 mL/kg/h for 6 hours (30,31). In the contemporary era, AKI should be diagnosed by a dynamic increase of Cr, but not a fixed threshold of 1.5 mg/dL. AKI may occur even if an absolute level of Cr is within normal range. The status quo suggests that the diagnostic criteria for AKI are moving forward and interventions may be initiated in patients with normal Cr level.
A recent single-center study evaluated the impact of AKI in 385 patients with liver cirrhosis awaiting liver transplantation (34). Among them, 24% and 14% of patients with a baseline Cr level of ≤0.7 mg/dL developed AKI and death, respectively; and 37% and 19% of patients with a baseline Cr level of 0.7–0.97 mg/dL developed AKI and death, respectively. It suggested the necessity of closely monitoring renal function and initiating the prophylactic measures of AKI in cirrhotic patients with normal Cr level (35). AUGIB is an important precipitating factor for AKI due to its secondary renal hypoperfusion. Thus, it might be necessary to prevent the development of AKI in cirrhotic patients with AUGIB when Cr level is normal. Our study found that terlipressin might improve renal function, especially CysC level, and potentially prevent AKI episodes in such patients.
Why has CysC, rather than Cr, been significantly improved?
Cr is a popular laboratory index for evaluating renal function and an important component of MELD score for selecting the optimal liver transplantation candidate, but has many limitations (36). First, serum bilirubin had an impact on the Cr level. An increase of bilirubin level will lead to an underestimation of Cr level. Second, the muscle wasting and low protein intake resulted in a decreased synthesis of Cr. Third, ascites and peripheral edema may result in the attenuation of Cr in the body. By comparison, current evidence suggests the superiority of CysC over Cr in assessing renal dysfunction and its associated outcomes. First, five studies evaluated the role of CysC-based equation for estimating GFR in patients with cirrhosis and suggested that CysC-based equation achieved a better performance in estimation of GFR than Cr-based equation (26,37-40). Second, two studies evaluated the role of CysC for predicting the risk of HRS. One study found that CysC was the most significant predictor for HRS in cirrhotic patients with ascites and normal Cr level (41). Another study also found that baseline CysC was predictive of renal dysfunction and HRS in patients with acute decompensated cirrhosis (42). Third, four studies evaluated the role of CysC for diagnosing AKI in patients with cirrhosis. All of them found that CysC was a useful marker and predictor for diagnosing AKI (43-46). Lastly, three studies evaluated the role of CysC for predicting the mortality of cirrhotic patients with AKI. All of them suggested that CysC was superior to Cr or MELD score for predicting the mortality after AKI (45-47). Based on the literature review mentioned above and our findings that terlipressin could significantly improve the CysC value, we suggested the potential benefit of terlipressin for improving renal function in cirrhotic patients with AUGIB.
Risk factors for AKI
As shown in previous studies (6,48-50) (Table 7), risk factors of renal failure in AUGIB patients included worse hepatic and renal function, such as higher bilirubin, AST, and Cr, lower albumin, and Child-Pugh class C. The existence of comorbidity, such as shock, bacterial infection, oliguria, hypertension, chronic heart failure, malignancy, coronary artery disease, and chronic kidney disease, was also a risk factor of renal failure in AUGIB patients. Unfortunately, our study did not identify any factor that was significantly associated with the development of AKI. This unexpected finding should be attributed to a small sample size (the number of included patients was only 40) and a few events of AKI (the number of patients with AKI was only 3).
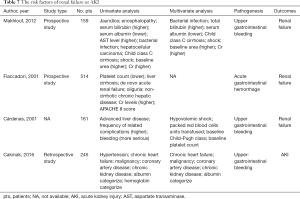
Full table
Limitations
First, the main limitation of our study is a relatively small sample size which would affect the accuracy and stability of our findings. Second, we did not evaluate Cr clearance, urine sodium concentration, and GFR. Third, there is a risk of patient selection bias due to the retrospective nature of this study.
Conclusions
Terlipressin may be effective for improving renal function and preventing the occurrence of AKI in patients with cirrhosis and AUGIB. Certainly, we need further prospective studies with larger sample size to confirm the effect of terlipressin on renal function in patients with cirrhosis and AUGIB.
Acknowledgments
This work was partially presented as a poster presentation at the Asian Pacific Association for the Study of Liver (APASL) Single Topic Conference 2018 held in Beijing on December 6, 2018.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol conformed to the Declaration of Helsinki and was approved by the Medical Ethical Committee of our hospital [No. k(2018)20].
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Egerod Israelsen M, Gluud LL, Krag A. Acute kidney injury and hepatorenal syndrome in cirrhosis. J Gastroenterol Hepatol 2015;30:236-43. [Crossref] [PubMed]
- Fede G, D'Amico G, Arvaniti V, et al. Renal failure and cirrhosis: a systematic review of mortality and prognosis. J Hepatol 2012;56:810-8. [Crossref] [PubMed]
- Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology 2008;48:2064-77. [Crossref] [PubMed]
- Arroyo V, Gines P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology 1996;23:164-76. [Crossref] [PubMed]
- Rimola A, Garcia-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol 2000;32:142-53. [Crossref] [PubMed]
- Cárdenas A, Ginès P, Uriz J, et al. Renal failure after upper gastrointestinal bleeding in cirrhosis: incidence, clinical course, predictive factors, and short-term prognosis. Hepatology 2001;34:671-6. [Crossref] [PubMed]
- Hung TH, Tsai CC, Tseng CW, et al. No difference in mortality between terlipressin and somatostatin treatments in cirrhotic patients with esophageal variceal bleeding and renal functional impairment. Eur J Gastroenterol Hepatol 2016;28:1275-9. [Crossref] [PubMed]
- Jindal A, Bhadoria AS, Maiwall R, et al. Evaluation of acute kidney injury and its response to terlipressin in patients with acute-on-chronic liver failure. Liver Int 2016;36:59-67. [Crossref] [PubMed]
- Gluud LL, Christensen K, Christensen E, et al. Terlipressin for hepatorenal syndrome. Cochrane Database Syst Rev 2012.CD005162. [PubMed]
- Ioannou G, Doust J, Rockey DC. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database Syst Rev 2003.CD002147. [PubMed]
- Papaluca T, Gow P. Terlipressin: current and emerging indications in chronic liver disease. J Gastroenterol Hepatol 2018;33:591-8. [Crossref] [PubMed]
- Sanyal AJ, Boyer T, Garcia-Tsao G, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology 2008;134:1360-8. [Crossref] [PubMed]
- Boyer TD, Sanyal AJ, Wong F, et al. Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology 2016;150:1579-89.e2. [Crossref] [PubMed]
- Neri S, Pulvirenti D, Malaguarnera M, et al. Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Dig Dis Sci 2008;53:830-5. [Crossref] [PubMed]
- Goyal O, Sidhu SS, Sehgal N, et al. Noradrenaline is as effective as terlipressin in hepatorenal syndrome type 1: a prospective, randomized trial. J Assoc Physicians India 2016;64:30-5. [PubMed]
- Alessandria C, Ottobrelli A, Debernardi-Venon W, et al. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol 2007;47:499-505. [Crossref] [PubMed]
- Ghosh S, Choudhary NS, Sharma AK, et al. Noradrenaline vs terlipressin in the treatment of type 2 hepatorenal syndrome: a randomized pilot study. Liver Int 2013;33:1187-93. [Crossref] [PubMed]
- Singh V, Ghosh S, Singh B, et al. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol 2012;56:1293-8. [Crossref] [PubMed]
- Cavallin M, Kamath PS, Merli M, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology 2015;62:567-74. [Crossref] [PubMed]
- Solanki P, Chawla A, Garg R, et al. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective, randomized placebo-controlled clinical trial. J Gastroenterol Hepatol 2003;18:152-6. [Crossref] [PubMed]
- Sharma P, Kumar A, Shrama BC, et al. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol 2008;103:1689-97. [Crossref] [PubMed]
- Saif RU, Dar HA, Sofi SM, et al. Noradrenaline versus terlipressin in the management of type 1 hepatorenal syndrome: a randomized controlled study. Indian J Gastroenterol 2018;37:424-9. [Crossref] [PubMed]
- Krag A, Moller S, Henriksen JH, et al. Terlipressin improves renal function in patients with cirrhosis and ascites without hepatorenal syndrome. Hepatology 2007;46:1863-71. [Crossref] [PubMed]
- Zhang JQ, Zhou XM, Zhao HT, et al. Adverse events of terlipressin in liver cirrhosis with acute gastrointestinal bleeding: a clinical pharmacist’s real-world observational study. Dig Med Res 2018;1:2. [Crossref]
- Zhang J, Rössle M, Zhou X, et al. Terlipressin for the treatment of hepatorenal syndrome: an overview of current evidence. Curr Med Res Opin 2019;35:859-68. [Crossref] [PubMed]
- Adachi M, Tanaka A, Aiso M, et al. Benefit of cystatin C in evaluation of renal function and prediction of survival in patients with cirrhosis. Hepatol Res 2015;45:1299-306. [Crossref] [PubMed]
- Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008;371:838-51. [Crossref] [PubMed]
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749-61. [Crossref] [PubMed]
- Zou D, Qi X, Zhu C, et al. Albumin-bilirubin score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis: a retrospective study. Turk J Gastroenterol 2016;27:180-6. [Crossref] [PubMed]
- Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol 2015;62:968-74. [Crossref] [PubMed]
- Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut 2015;64:531-7. [Crossref] [PubMed]
- Zhou X, Tripathi D, Song T, et al. Terlipressin for the treatment of acute variceal bleeding: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2018;97:e13437. [Crossref] [PubMed]
- Abid S, Jafri W, Hamid S, et al. Terlipressin vs. octreotide in bleeding esophageal varices as an adjuvant therapy with endoscopic band ligation: a randomized double-blind placebo-controlled trial. Am J Gastroenterol 2009;104:617-23. [PubMed]
- Cullaro G, Park M, Lai JC. "Normal" creatinine levels predict persistent kidney injury and waitlist mortality in outpatients with cirrhosis. Hepatology 2018;68:1953-60. [Crossref] [PubMed]
- Carrier P, Debette-Gratien M, Loustaud-Ratti V. Serum creatinine in cirrhotic patients: a cornerstone. AME Med J 2018;3:109. [Crossref]
- Carrier P, Debette-Gratien M, Essig M, et al. Beyond serum creatinine: which tools to evaluate renal function in cirrhotic patients? Hepatol Res 2018;48:771-9. [Crossref] [PubMed]
- Demirtaş S, Bozbaş A, Akbay A, et al. Diagnostic value of serum cystatin C for evaluation of hepatorenal syndrome. Clin Chim Acta 2001;311:81-9. [Crossref] [PubMed]
- Krones E, Fickert P, Zitta S, et al. The chronic kidney disease epidemiology collaboration equation combining creatinine and cystatin C accurately assesses renal function in patients with cirrhosis. BMC Nephrol 2015;16:196. [Crossref] [PubMed]
- Omar M, Abdel-Razek W, Abo-Raia G, et al. Evaluation of serum cystatin C as a marker of early renal impairment in patients with liver cirrhosis. Int J Hepatol 2015;2015:309042. [Crossref] [PubMed]
- Wang D, Feng JF, Wang AQ, et al. Role of Cystatin C and glomerular filtration rate in diagnosis of kidney impairment in hepatic cirrhosis patients. Medicine (Baltimore) 2017;96:e6949. [Crossref] [PubMed]
- Sharawey MA, Shawky EM, Ali LH, et al. Cystatin C: a predictor of hepatorenal syndrome in patients with liver cirrhosis. Hepatol Int 2011;5:927-33. [Crossref] [PubMed]
- Markwardt D, Holdt L, Steib C, et al. Plasma cystatin C is a predictor of renal dysfunction, acute-on-chronic liver failure, and mortality in patients with acutely decompensated liver cirrhosis. Hepatology 2017;66:1232-41. [Crossref] [PubMed]
- Kim DJ, Kang HS, Choi HS, et al. Serum cystatin C level is a useful marker for the evaluation of renal function in patients with cirrhotic ascites and normal serum creatinine levels. Korean J Hepatol 2011;17:130-8. [Crossref] [PubMed]
- Jaques DA, Spahr L, Berra G, et al. Biomarkers for acute kidney injury in decompensated cirrhosis: a prospective study. Nephrology (Carlton) 2019;24:170-80. [Crossref] [PubMed]
- Chung MY, Jun DW, Sung SA. Diagnostic value of cystatin C for predicting acute kidney injury in patients with liver cirrhosis. Korean J Hepatol 2010;16:301-7. [Crossref] [PubMed]
- Maiwall R, Kumar A, Bhardwaj A, et al. Cystatin C predicts acute kidney injury and mortality in cirrhotics: a prospective cohort study. Liver Int 2018;38:654-64. [Crossref] [PubMed]
- Belcher JM, Sanyal AJ, Garcia-Tsao G, et al. Early trends in cystatin C and outcomes in patients with cirrhosis and acute kidney injury. Int J Nephrol 2014;2014:708585. [Crossref] [PubMed]
- Cakmak U, Merhametsiz O, Gok Oguz E, et al. Effects of acute kidney injury on clinical outcomes in patients with upper gastrointestinal bleeding. Ren Fail 2016;38:176-84. [Crossref] [PubMed]
- Fiaccadori E, Maggiore U, Clima B, et al. Incidence, risk factors, and prognosis of gastrointestinal hemorrhage complicating acute renal failure. Kidney Int 2001;59:1510-9. [Crossref] [PubMed]
- Makhlouf NA, Morsy KH. Renal failure after upper-gastrointestinal bleeding among cirrhotic patients in Upper Egypt. Arab J Gastroenterol 2012;13:139-44. [Crossref] [PubMed]