
Changes in plasma bile acid profiles after partial internal biliary diversion in PFIC2 patients
Introduction
The accumulation of cytotoxic hydrophobic bile acids in the liver is considered to be the root cause of hepatobiliary injury in progressive intrahepatic cholestasis (PFICs) (1). The primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA) in humans, are synthesized from cholesterol in hepatocytes, conjugated, secreted into the bile, and then released into the duodenum. Ninety-five percent of biliary bile acids are reabsorbed into blood circulation through ileum enterocytes (1), and the rest enter the colon. Some of the bile acids in the colon are converted by the gut microbiome into the secondary bile acids, mainly deoxycholic acid (DCA) and lithocholic acid (LCA) (2-4), which are either reabsorbed into circulation or eliminated through feces.
Progressive familial intrahepatic cholestasis type 2 (PFIC2) is a fatal childhood disease caused by mutations in the ATP-binding cassette, sub-family B member 11 (ABCB11) gene, which encodes the Bile Salt Export Pump (BSEP) protein. PFIC2 is often associated with pruritus, jaundice, growth retardation, cirrhosis, liver failure, and death (5-9). Defects in BSEP result in the accumulation of toxic bile acids in hepatocytes with complex changes in bile acid metabolism (10-14). The recent development of a reversed-phase ultrahigh-performance liquid chromatography/multiple-reaction monitoring-mass spectrometry (UPLC/MRM-MS) method with negative ion detection allows quantitation of a wide range of modified bile acids with high sensitivity (15). Using this technique, novel polyhydroxylated bile acids have been detected in mice with a genetically impaired bile salt export pump (16-18). In a similar manner increased levels of tetrahydroxylated bile acids (THBAs) have also been detected in children with cholestatic diseases (14,19).
Biliary diversion (BD) is a first-line, non-liver transplant clinical intervention, for low gamma-glutamyl transferase (GGT) PFIC (20,21). Variants of BD (Figure 1) include partial external biliary diversion (PEBD) (20,24), partial internal biliary diversion (PIBD) (21,25), and total internal BD (22). Briefly, the PEBD procedure partially diverts the flow of bile from the gall bladder to an external stoma to reduce bile acids in the intestine for recirculation. For the PIBD procedure used in the current study, a conduit is performed between the terminolateral side of the gall bladder and distal colon using a segment of jejunum, to divert the biliary flow from the enterohepatic cycle without an external stoma (21). The procedure of total internal BD uses a jejunum conduit between the graft bile duct and the transverse colon (22).
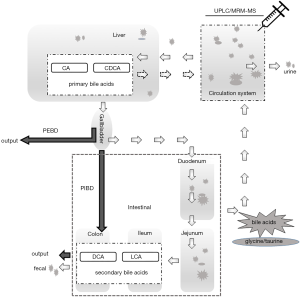
PEBD is most commonly practiced and it has been shown to lessen the clinical symptoms of PFIC including pruritus, reduce plasma bile acid levels, improve the plasma lipoprotein profile, slow disease progression, and improve liver histopathology (23,26-32). Responses do vary amongst patients and the underlying mechanism for the effectiveness of BD is not well-understood. For example, how BD affects bile acid metabolism, including the distribution and composition of bile acids, has not been well investigated yet (20). It is also not understood how the different genotypes associated with PFIC respond to BD (20,29), and detailed comparisons of bile acids pre- and post-operation have usually not been undertaken (32-35). PIBD appears an effective alternative approach to PEBD for cholestatic diseases in PFIC, although only a few cases have been reported (21,23,36-39).
In the present study, we performed detailed analyses of plasma bile acids before and after PIBD in three genetically-defined PFIC2 patients. To our knowledge, no bile acid profiling of PFIC2 patients before and after BD has been reported (20,29). We have shown previously, that the profiling of plasma bile acids has provided valuable insights into cholestasis alleviation and tracked genetic and clinical status in ABCB11 mutated patients (19). Thus, the current goal is to determine if bile acid profiling may also be useful for gaining insights into the treatment efficacy of PIBD in ABCB11 mutated PFIC2 patients.
Methods
Subjects
Study subjects were Chinese children, with informed consent, under a protocol approved by the Children’s Hospital of Fudan University, in accordance with ethical guidelines.
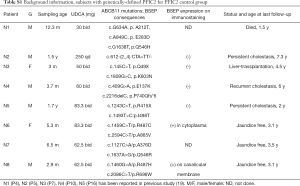
Subjects screening criteria include (I) carrying confirmed ABCB11 mutations based on the screening of a panel of 61 cholestasis-related genes (40); (II) undergone biliary diversion because of sub-optimal response to ursodeoxycholic acid (UDCA) treatment; and (III) with fasting serum samples pre- and post-operation. Immunostaining for BSEP expression was performed as previously described (13,19). The patients’ plasma bile acid profiles were determined before and after PIBD. Data for 40 age-matched healthy subjects, age from 3 months to 18 years with fasting samples and no infectious or endocrine genetic metabolic diseases, collected for another study (19), were used as healthy controls. Blood samples from eight PFIC2 patients, whom were receiving UDCA administration were collected as PFIC2 controls to differentiate the UDCA therapy effects [of whom 6 were collected for another study (19)] (Table S1).
Full table
Sample preparation and analysis of bile acids
Plasma was separated from whole blood by centrifugation (2,000 × g) for 10 min at 4 °C, with sodium heparin as an anticoagulant, and was aliquoted into 1.5-Eppemdorf tubes in 50 µL for freeze-drying before storage at −80 °C. For determination of bile acids, 50 µL of water was used to reconstitute an aliquot of each sample and 50 µL of an internal standard (IS) solution containing 14 deuterium-labeled bile acids (15) was added to each tube. After vortex-mixing and 3-s spin-down, 250 µL of methanol-acetonitrile (1:1, v/v) was added to each tube followed by 30-s vortex-mixing and 3-min sonication in an ice-water bath. Protein was pelleted by centrifugation at 21,000 ×g and 10 °C for 10 min and the clear supernatant was transferred and completely mixed with 270 µL of water inside a polymeric Strata-X reversed-phase solid-phase extraction cartridge (200 mg/3 mL, Phenomenex Inc. CA, USA), which was activated with 3 mL of methanol and reconditioned with 3 mL of water before use. Under a 3-psi positive pressure, the flow-through fraction was discarded and bile acids retained on the resin were eluted with 3 mL of methanol-acetonitrile (1:1). The collected eluant in a 5-mL glass test tube was dried down under a gentle nitrogen gas flow at 30 °C and the residue was dissolved in 200 µL fo 50% aqueous methanol. 20 µL was injected to run reversed-phase UPLC/MRM-MS with negative ion detection, on an Agilent 1290 UPLC system coupled to a Sciex QTRAP 4000 mass spectrometer, using the same procedure as described before (15). Along with preparation of the sample solutions, a mixture of 60 bile acids as reference standards as described before (13,15) was used to make serially diluted, working calibration solutions containing the same deuterium-labeled internal standards, in 50% aqueous methanol. The 60 bile acids included all of the major bile acids and some minor species, e.g., UDCA, hyocholic acid (HCA), hyodeoxycholic acid (HDCA), muricholic acids (MCAs), two THBAs [3α,6α,7α,12α-THBA, and taurine-3α,6α,7α,12α-THBA (tauro-THBA)] which were custom-synthesized (Table S2). Linear-regression calibration curves of individual bile acids were constructed with internal calibration. For those bile acids without their isotope-labeled analogues, deuteriated glyco-CDCA was used as a common internal standard. Concentrations of bile acids in plasma were calculated by interpolating the corresponding calibration curves with the analyte-to-internal standard peak area ratios measured from each sample solution.
Full table
Results
Clinical and ABCB11 mutation status of study subjects
Briefly, the patients in the current study were identified from a cohort of 77 patients with confirmed ABCB11 deficiency by genome sequencing accrued by our Center in the last 16 years. Of the ten patients from this cohort who underwent PIBD procedure, three died, one underwent liver transplantation, four suffered recurrent jaundice, and two patients were lost to follow-up. Three PFIC patients, who have undergone PIBD because of uncontrolled cholestasis (progressive jaundice and/or intractable pruritus with unsatisfied response to drug therapy) were recruited. Patient 1 carried two missense mutations (c.3457C>T, p.R1153C/c.3623 A>G, p.Y1208C). Patient 2 carried a splicing mutation and a missense mutation (c.2815-8A>G/c.3458G>A, p.R1153H), and Patient 3 carried a missense mutation and a nonsense mutation (c.1460G>A, p.R487H/c.3169C>T, p.R1057X) (Table 1). All three patients were treated with UDCA before and after PIBD. Patient P1 was a boy with no BSEP expression by immunohistochemistry, who underwent PIBD at 5 months but showed little postoperative improvement, with no alleviation in jaundice and decrease of liver enzymes (Table 1). The patient died at 1.5 y because of infection with severe cholestasis. The liver biopsy specimen acquired during PIBD indicated severe liver fibrosis (developing cirrhosis) at the time of the operation.

Full table
Patient P2 was a girl presenting with jaundice and abnormal liver indicators at admission as a 2.6-year-old. The liver biopsy specimen during PIBD procedure indicates fibrosis without obvious cirrhosis. BSEP immunostaining was not performed. No obvious improvement in jaundice and liver indicators were observed after PIBD at age of about 3.5 years old, or during later follow-ups at 4.0 and 4.3 years of age.
Patient P3 was a girl, who presented with persistent jaundice two days after birth, pruritus from 6 months of age onward, and persistent abnormal liver function (Table 1). The liver biopsy at age of 2 months suggested cholestasis, with no-detectable BSEP expression by immunohistochemistry, and no severe fibrosis/cirrhosis by histology. She underwent PIBD at age 8 because of increasing levels of abnormal liver enzymes and pruritus. Cholestasis was relieved after the PIBD, with resolved jaundice and pruritus. Liver enzymes, including TB, DB, ALT and AST levels were within the normal range except for increased ALP (Table 1). P3 suffered a relapse a year later with mild jaundice, pruritus, and elevated liver enzymes. The patient’s plasma total bilirubin rose sharply to 183 µmol/L at age 10 (up from 13.2 µmol/L at age 9.5 year, and close to the pre-PIBD level of 190.9 µmol/L) (Table 1).
Both P2 and P3 have survived with native livers as of this submission.
Plasma primary and secondary bile acids before and after PIBD
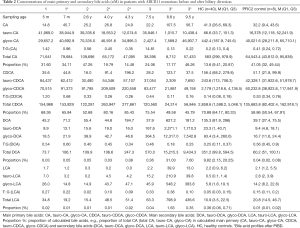
Before PIBD, all three patients exhibited bile acid profiles similar to PFIC2 controls, with much lower concentrations of unconjugated CA and CDCA and dramatically increased tauro-CA, glyco-CA, tauro-CDCA, and glyco-CDCA levels compared to healthy controls (Table 2 and Table S2). The concentrations of DCA, LCA, and their conjugates in patients before PIBD were relatively lower than the controls.
Full table
After PIBD, no significant changes in total plasma bile acid concentrations were observed in P1 and P2; however, in P3, with relief of cholestasis, as expected, the total plasma bile acid concentrations were reduced by 5.5 fold (Table 2 and Table S2) and the bile acid profile shifted toward that of the healthy controls, with increased levels of unconjugated CA, CDCA, DCA, and LCA and decreased levels of conjugated primary bile acids (tauro-CA, tauro-CDCA, and glycol-CDCA). What was not anticipated is that the level of secondary bile acids increased dramatically (25-fold) after PIBD in P3 (Figure 2). Total plasma DCA and LCA increased 26-fold and 12-fold respectively (Table 2). When cholestasis recurred in P3, the bile acid profile reverted toward that seen before PIBD and the total bile acids in the plasma increased by 3.5-fold compared to the level after PIBD, when cholestasis had been relieved (Table 2 and Table S2). Notably, unconjugated CDCA was also increased after BD in P2.
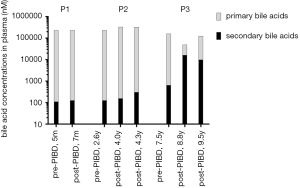
In addition, taurine conjugation was the predominant conjugated form of the primary bile acids in the plasma of these patients prior to PIBD consistent with what is observed in other cholestatic PFIC2 patients (19,41,42). The molar ratios of taurine to glycine conjugates were 1.42/0.56/14.81/0.2 for tauro-CA:glyco-CA, and 1.20/0.33/0.44/0.14 for tauro-CDCA:glyco-CDCA in P1/P2/P3/healthy controls (Table 2). In patient P3, the ratios changed towards that of healthy controls with the relief of cholestasis, with glycine conjugation predominating and molar ratios of tauro-CA:glyco-CA equal to 0.13 and tauro-CDCA:glyco-CDCA equal to 0.11.
UDCAs, MCAs, THBAs and some atypical bile acids in patients before and after PIBD
Plasma UDCA and its conjugates were present at concentrations 100s of times higher in all three patients compared to healthy controls (Table S2). This was expected since the patients were undergoing UDCA treatment. It was noted that in P3 (but not the non-responders) the tauro-UDCA-3-sulfate level was greatly reduced after PIBD (from 11,961 to 7 nM), while the glycol-UDCA-3-sulfate level remained essentially unchanged at over 100 times higher than healthy controls. It was also noted that the concentration of tauro-THBA was high in all three patients prior to PIBD (Table S2), but after PBID, tauro-THBA remained unchanged in P1, was reduced by 12-fold with no obvious relief of cholestasis in P2 and was reduced to a non-detectable level with relief of cholestasis in P3. On the recurrence of cholestasis in P3, the patient’s plasma tauro-THBA levels increased to 33 times higher than in healthy controls (Table S2).
Discussion
Biliary diversion (BD) is a first-line, non-liver transplantation, surgical intervention for low gamma-glutamyl transferase (GGT) PFIC (20,21). PEBD drains gallbladder bile to the exterior of the body to reduce enterohepatic recirculation of bile acids and thus the stress in the liver for processing and recirculation (23). PIBD involves the creation of an isolated jejunal conduit, anastomosed proximally to the gallbladder and distally to the distal colon (22) (Figure 1). The PIBD procedure redirects bile partially into the colon, which would likely change the metabolic dynamics of circulating bile acids although little is known (21,22). To our knowledge no direct comparison of these two surgical approaches as therapeutic interventions of PFIC has been performed (2,21,25,29,36-39,43). Moreover, few comparative analyses of bile acids pre- and post-operation of BD have been undertaken (32-35). Thus, the current study, albeit limited, is an attempt to determine if plasma bile acid profiling may have clinical utility in managing BD patients.
In this study, all three PFIC2 patients had uncontrolled cholestasis with 50-fold higher total plasma bile acids levels than that of healthy controls before PIBD treatment (Table S2). After PIBD, one patient (P3) showed clinically meaningful relief of jaundice and pruritus, and a decrease in liver enzymes to normal ranges. The other two patients, P1 and P2, failed to show significant symptom relief (Table 1).
Significant changes in the plasma bile acid were seen in P3 (the responder) after PIBD but not in either in P1 nor P2 (Table 2). The change in the postoperative plasma bile acid profile of P3, corresponding to relief of cholestasis, is noteworthy. P3 displayed an approximately 5-fold decrease in total plasma primary bile acids (CA and CDCA), and a 25-fold elevation in secondary bile acids (DCA and LCA). It is important to note that the level of total bile acids in the bile of a responding PEBD patient also decreased 5-fold after the operation, but that the levels of biliary LCA and DCA in that patient were reduced by 13.5-fold and 2.5-fold, respectively (29). The difference in DCA and LCA is likely due to the nature of the PIBD procedure, where much of the bile is bypassed into the colon. Thus, more bile acid becomes available to the gut microbiome (2) for conversion to secondary bile acids. These increases in the amounts of toxic secondary bile acids in circulation need to be taken into account, as they could cause additional damage post-PIBD. For example, it is known that the increased chronic exposure of secondary bile acids increases the colon cancer risk (44). In a recent study by Alrabadi et al. (45), it was found that a PIBD procedure might be associated with the development of macrovesicular steatosis post liver-transplantation, which may have been reversed by PEBD in a patient with PFIC1. This observation appears to be consistent with the clinical course of P3 reported in the current study. It is not unreasonable to assume that what Alrabadi et al. observed was associated with changes in bile acid metabolism. It is certainly worth a follow-up study. Further investigations with more cases are needed to confirm these initial observations.
Along the same vein, we note that taurine-conjugated bile acids have been shown to be more hydrophilic than their glycine counterpart and they are often increased during cholestasis likely as part of an overall compensatory mechanism to relieve cholestatic stress (19,41,42). In the current study, we found that taurine-conjugated bile acids, especially tauro-CDCA, were greatly reduced in the plasma of P3 after PIBD (Table 2), along with the relief of cholestasis by liver enzymes (Table 1). An increased molar ratio of taurine:glycine-conjugated bile acids was also found in all three PFIC2 patients. Interestingly, the fluctuation of taurine:glycine conjugate ratio in P3 coincides with the course of disease: it decreased after PIBD along with the relief of cholestasis, and increased again upon recurrence (Table 2). Thus, the taurine:glycine conjugate ratios of plasma bile acids could be potentially useful for monitoring the treatment efficacy of BD in PFIC patients, since the concentration of total serum bile acid levels could fluctuate depending on feeding status.
All three patients in this study were treated with UDCA raising the question of whether or not such treatments might affect their bile acid profiles. In a previous study (19), we have compared the bile acid profiles of PFIC2 patients with or without UDCA treatment. We observed that UDCA treatment affected levels of only a few species of bile acids, including UDCAs and ω-MCA, tau-CDCA, tau-LCA, tau-β-MCA and tau-ω-MCA within PFIC2 patients.
Conclusions
The findings from this study show that (I) the unexpectedly high levels of toxic secondary bile acids DCA and LCA in the plasma of the responding patient after PIBD, may limit the long-term effectiveness of PIBD. (II) Longer follow ups with more cases and bile acid profiling of patients’ liver, bile, stool and urine are needed to confirm these initial findings. (III) Plasma bile acid profiles should be monitored during the clinical course of PIBD patients.
Acknowledgments
Funding: This project was funded by the National Natural Science Foundation of China, Grant Numbers 81361128006 and 81873543 (to JSW, for data and sample collecting). The work was also supported by China Scholarship Council, Grant Number 201606100226 (to TL, for living expenses during the project in Canada), and the Canadian Institutes of Health Research (to VL & RW). CHB and JH are also grateful for funding for method development from The Metabolomics Innovation Centre (TMIC), from Genome Canada, Genome Alberta, and Genome British Columbia through the Genomics Technology Platform (GTP) for operations and technology development (265MET and MC3). CHB is also grateful for support from the Leading Edge Endowment Fund (University of Victoria), and for support from the Segal McGill Chair in Molecular Oncology at McGill University, and the Warren Y. Soper Charitable Trust and the Alvin Segal Family Foundation to the Jewish General Hospital.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was under a protocol approved by the Children’s Hospital of Fudan University, in accordance with ethical guidelines, with a signed consent from the parents was also achieved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med 1999;159:2647-58. [Crossref] [PubMed]
- Hylemon PB, Harder J. Biotransformation of monoterpenes, bile acids, and other isoprenoids in anaerobic ecosystems. FEMS Microbiol Rev 1998;22:475-88. [Crossref] [PubMed]
- Hofmann AF. Current concepts of biliary secretion. Dig Dis Sci 1989;34:16S-20S. [Crossref] [PubMed]
- Carey MC, Duane WC. Enterohepatic circulation. In: Arias IM, Boyer JL, Fausto N, et al. (eds), Raven Press New York 1994:719-67.
- Strautnieks SS, Bull LN, Knisely AS, et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet 1998;20:233-8. [Crossref] [PubMed]
- Hermeziu B, Sanlaville D, Girard M, et al. Heterozygous bile salt export pump deficiency: a possible genetic predisposition to transient neonatal cholestasis. J Pediatr Gastroenterol Nutr 2006;42:114-6. [Crossref] [PubMed]
- Van Mil SWC, Van Der Woerd WL, Van Der Brugge G, et al. Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology 2004;127:379-84. [Crossref] [PubMed]
- Childs S, Yeh RL, Georges E, et al. Identification of a sister gene to P-glycoprotein. Cancer Res 1995;55:2029-34. [PubMed]
- Strautnieks SS, Byrne JA, Pawlikowska L, et al. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology 2008;134:1203-14. [Crossref] [PubMed]
- Yang CH, Chen CY, Chou YY, et al. Bile acid profiles in neonatal intrahepatic cholestasis caused by citrin deficiency. Clin Chim Acta 2017;475:28-35. [Crossref] [PubMed]
- Zhou K, Wang J, Xie G, et al. Distinct Plasma Bile Acid Profiles of Biliary Atresia and Neonatal Hepatitis Syndrome. J Proteome Res 2015;14:4844-50. [Crossref] [PubMed]
- Bijleveld CM, Vonk RJ, Kuipers F, et al. Benign recurrent intrahepatic cholestasis: altered bile acid metabolism. Gastroenterology 1989;97:427-32. [Crossref] [PubMed]
- Qiu YL, Gong JY, Feng JY, et al. Defects in myosin VB are associated with a spectrum of previously undiagnosed low gamma-glutamyltransferase cholestasis. Hepatology 2017;65:1655-69. [Crossref] [PubMed]
- Lee CS, Kimura A, Wu JF, et al. Prognostic roles of tetrahydroxy bile acids in infantile intrahepatic cholestasis. J Lipid Res 2017;58:607-14. [Crossref] [PubMed]
- Han J, Liu Y, Wang R, et al. Metabolic profiling of bile acids in human and mouse blood by LC-MS/MS in combination with phospholipid-depletion solid-phase extraction. Anal Chem 2015;87:1127-36. [Crossref] [PubMed]
- Wang R, Salem M, Yousef IM, et al. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci U S A 2001;98:2011-6. [Crossref] [PubMed]
- Wang R, Liu L, Sheps JA, et al. Defective canalicular transport and toxicity of dietary ursodeoxycholic acid in the abcb11-/- mouse: transport and gene expression studies. Am J Physiol Gastrointest Liver Physiol 2013;305:G286-94. [Crossref] [PubMed]
- Marschall HU, Wagner M, Bodin K, et al. Fxr(-/-) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. J Lipid Res 2006;47:582-92. [Crossref] [PubMed]
- Liu T, Wang RX, Han J, et al. Comprehensive bile acid profiling in hereditary intrahepatic cholestasis: Genetic and clinical correlations. Liver Int 2018;38:1676-85. [Crossref] [PubMed]
- Jericho HS, Kaurs E, Boverhof R, et al. Bile acid pool dynamics in progressive familial intrahepatic cholestasis with partial external bile diversion. J Pediatr Gastroenterol Nutr 2015;60:368-74. [Crossref] [PubMed]
- Gun F, Erginel B, Durmaz O, et al. An outstanding non-transplant surgical intervention in progressive familial intrahepatic cholestasis: partial internal biliary diversion. Pediatr Surg Int 2010;26:831-4. [Crossref] [PubMed]
- Mali VP, Fukuda A, Shigeta T, et al. Total internal biliary diversion during liver transplantation for type 1 progressive familial intrahepatic cholestasis: a novel approach. Pediatr Transplant 2016;20:981-6. [Crossref] [PubMed]
- Emond JC, Whitington PF. Selective surgical management of progressive familial intrahepatic cholestasis (Byler's disease). J Pediatr Surg 1995;30:1635-41. [Crossref] [PubMed]
- Whitington PF, Whitington GL. Partial external diversion of bile for the treatment of intractable pruritus associated with intrahepatic cholestasis. Gastroenterology 1988;95:130-6. [Crossref] [PubMed]
- Erginel B, Soysal FG, Durmaz O, et al. Long-term outcomes of six patients after partial internal biliary diversion for progressive familial intrahepatic cholestasis. J Pediatr Surg 2018;53:468-71. [Crossref] [PubMed]
- Arnell H, Papadogiannakis N, Zemack H, et al. Follow-up in children with progressive familial intrahepatic cholestasis after partial external biliary diversion. J Pediatr Gastroenterol Nutr 2010;51:494-9. [Crossref] [PubMed]
- Halaweish I, Chwals WJ. Long-term outcome after partial external biliary diversion for progressive familial intrahepatic cholestasis. J Pediatr Surg 2010;45:934-7. [Crossref] [PubMed]
- Kalicinski PJ, Ismail H, Jankowska I, et al. Surgical treatment of progressive familial intrahepatic cholestasis: comparison of partial external biliary diversion and ileal bypass. Eur J Pediatr Surg 2003;13:307-11. [Crossref] [PubMed]
- Kurbegov AC, Setchell KD, Haas JE, et al. Biliary diversion for progressive familial intrahepatic cholestasis: improved liver morphology and bile acid profile. Gastroenterology 2003;125:1227-34. [Crossref] [PubMed]
- Melter M, Rodeck B, Kardorff R, et al. Progressive familial intrahepatic cholestasis: partial biliary diversion normalizes serum lipids and improves growth in noncirrhotic patients. Am J Gastroenterol 2000;95:3522-8. [Crossref] [PubMed]
- Schukfeh N, Metzelder ML, Petersen C, et al. Normalization of serum bile acids after partial external biliary diversion indicates an excellent long-term outcome in children with progressive familial intrahepatic cholestasis. J Pediatr Surg 2012;47:501-5. [Crossref] [PubMed]
- Yang H, Porte RJ, Verkade HJ, et al. Partial external biliary diversion in children with progressive familial intrahepatic cholestasis and Alagille disease. J Pediatr Gastroenterol Nutr 2009;49:216-21. [Crossref] [PubMed]
- Arnell H, Bergdahl S, Papadogiannakis N, et al. Preoperative observations and short-term outcome after partial external biliary diversion in 13 patients with progressive familial intrahepatic cholestasis. J Pediatr Surg 2008;43:1312-20. [Crossref] [PubMed]
- Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology 2010;51:1645-55. [Crossref] [PubMed]
- Lemoine C, Bhardwaj T, Bass LM, et al. Outcomes following partial external biliary diversion in patients with progressive familial intrahepatic cholestasis. J Pediatr Surg 2017;52:268-72. [Crossref] [PubMed]
- Bustorff-Silva J, Sbraggia Neto L, Olimpio H, et al. Partial internal biliary diversion through a cholecystojejunocolonic anastomosis--a novel surgical approach for patients with progressive familial intrahepatic cholestasis: a preliminary report. J Pediatr Surg 2007;42:1337-40. [Crossref] [PubMed]
- Ramachandran P, Shanmugam NP, Sinani SA, et al. Outcome of partial internal biliary diversion for intractable pruritus in children with cholestatic liver disease. Pediatr Surg Int 2014;30:1045-9. [Crossref] [PubMed]
- Mousavi SA, Karami H. Partial internal biliary diversion in progressive familial intrahepatic cholestasis: introduction of a new approach. Hepat Mon 2014;14:e13549. [Crossref] [PubMed]
- Agarwal S, Lal BB, Rawat D, et al. Progressive Familial Intrahepatic Cholestasis (PFIC) in Indian Children: Clinical Spectrum and Outcome. J Clin Exp Hepatol 2016;6:203-8. [Crossref] [PubMed]
- Wang NL, Lu YL, Zhang P, et al. A Specially Designed Multi-Gene Panel Facilitates Genetic Diagnosis in Children with Intrahepatic Cholestasis: Simultaneous Test of Known Large Insertions/Deletions. PLoS One 2016;11:e0164058. [Crossref] [PubMed]
- Murphy GM, Ross A, Billing BH. Serum bile acids in primary biliary cirrhosis. Gut 1972;13:201-6. [Crossref] [PubMed]
- Howard D, Thompson DF. Taurine: an essential amino acid to prevent cholestasis in neonates? Ann Pharmacother 1992;26:1390-2. [Crossref] [PubMed]
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: Advances and future challenges. J Hepatol 2016;65:631-42. [Crossref] [PubMed]
- Ajouz H, Mukherji D, Shamseddine A. Secondary bile acids: an underrecognized cause of colon cancer. World J Surg Oncol 2014;12:164. [Crossref] [PubMed]
- Alrabadi LS, Morotti RA, Valentino PL, et al. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: A single-center experience. Pediatr Transplant 2018.e13184. [Crossref] [PubMed]


