
Clinicopathological characteristics and prognosis of colorectal mucinous adenocarcinoma and nonmucinous adenocarcinoma: a surveillance, epidemiology, and end results (SEER) population-based study
Introduction
Colorectal cancer (CRC) is the fourth leading cause of cancer mortality with approximately 700,000 deaths estimated in 2012 (1). CRC is also the third most commonly diagnosed cancer (2-4). Among CRC subtypes, mucinous adenocarcinoma (MC) is a rare morphologic type in which more than half of tumors are composed of mucin (3,5). In addition, MC might arise from different types of carcinogenesis (6). However, the conclusion about the clinicopathological characteristics of MC is controversial. Some research found that MC patients presented with more advanced disease stage and may have a higher incidence of local extension leading to lower curative and overall resection rates (7,8). While some argued that MC histology may not be associated with greater malignant behavior (9-11) and MC may show better overall survival (OS) (12,13). Therefore, it is crucial to know the clinicopathological characteristics of MC and find out the prognosis factors of MC further.
For treatment of colorectal cancer, almost all patients choose cancer-directed surgical resection (14,15). For MC patients, apart from surgery, few studies reported other treatments for MC such as radiation therapy and chemotherapy: neoadjuvant radiochemotherapy was shown to be a standardized preoperative treatment for selected patients with rectal cancer (16,17); postoperative radiotherapy should be routinely applied to patients with stage II rectal MC (18); MC appeared to be less responsive to fluoropyrimidines, irinotecan and oxaliplatin-based chemotherapy (19). Actually, there was no credible guideline existing for CRC to treat different phenotypes such as MC, so it was quite necessary for us to perform a more intensive subgroup study on the factor of therapy.
Our population-based study systematically summarized the clinicopathological characteristics, prognosis factors of MC versus NMC patients. Meanwhile, we focus on figuring out favorable therapeutic treatments for group of MC patients.
Methods
SEER database
Our data were collected from the Surveillance, Epidemiology, and End Results (SEER) database. SEER is a large, population-based program that collects information about cancer such as incidence, prevalence and survival, covering 28% of the U.S. population (https://seer.cancer.gov/about/overview.html). The SEER program is updated every year and records patient characteristics including age, sex, race, marriage status, disease histological type, stage at diagnosis, tumor size, receipt of surgery, and radiotherapy or chemotherapy.
Patient selection
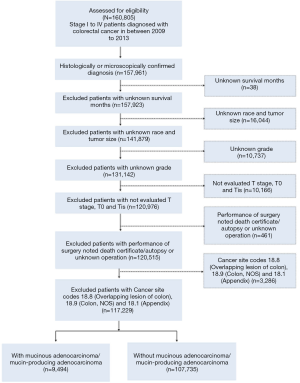
For a rigorous analysis, colorectal cancer subtypes were defined by the International Classification of Diseases for Oncology (third edition, ICD-O-3) and by TNM classification from the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 6th edition. The following inclusion criteria were applied: (I) stage I to IV patients diagnosed with colorectal cancer between 2009 and 2013, and (II) clear histological or microscopical diagnosis and had been classified into different stages according to pathological criteria in AJCC 6th. To further improve the validity and authenticity of our study, patients with unknown survival months, race, tumor size and grade were excluded. Cases without evaluated T stage and with T0 (carcinoma without evidence) or Tis (carcinoma in situ) were excluded. Additional exclusion included patients noted death certificate/autopsy or unknown operation. For colonic cancers, colon tumors were subclassified into left-side (distal to the splenic flexure) or right-side (proximal to the splenic flexure) (20). To identify the location of tumors, we did not use SEER codes for tumor location such as appendix C18.1, overlapping lesions of colon C18.8 or colon not otherwise specified (NOS) C18.9. Among 160,805 patients with a diagnosis of stage I to IV colorectal cancer from 2009 to 2013, 117,229 were eligible for potential inclusion into our study according to these selections.
After removing out patients with appendix or peritoneum patients, two other tumor histological subtypes related to MC were in ICD-O-3: mucin-producing adenocarcinoma (code: 8481) and mucinous cystadenocarcinoma (code: 8470). After comparing their prognosis, the former had a similar survival curve to MC and the latter was different so we categorized mucin-producing adenocarcinomas into MC and mucinous cystadenocarcinoma into nonmucinous adenocarcinoma (NMC), assuming that the pathological structure of mucin-producing adenocarcinoma resembled MC. Thus, 9,494 patients were diagnosed with MC (8480: mucinous adenocarcinoma; 8481: mucin-producing adenocarcinoma) and 107,735 NMC (Figure S1, Consort diagram).
Statistical analysis
Statistical analyses were by Stata Version 12 (http://www.stata.com) and conducted with R Version 3.4.2 (http://www.R-project.org/). Clinicopathological features were analyzed by chi-square test. Differences between cancer-specific survival (CSS) which means the time from the date of diagnosis to death because of the cancer and OS were analyzed by log-rank test, and survival curves were made by the Kaplan-Meier method. Cox proportional hazard regression models were performed to obtain adjusted hazard ratios (HRs) and 95% confidence interval (CIs) to estimate probable risk factors for survival outcomes. Statistical significance was defined as P less than 0.001. We used Propensity score matching (PSM) to process the analysis with a 1:1 ratio of NMC to MC patients. PSM is a statistical method reduces the impact of treatment-selection bias in estimating treatment effects using observational data (21).
Results
Clinicopathological characteristics of MC patients
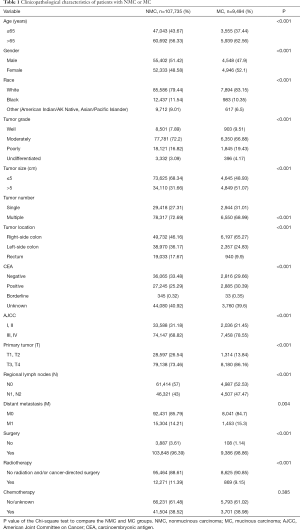
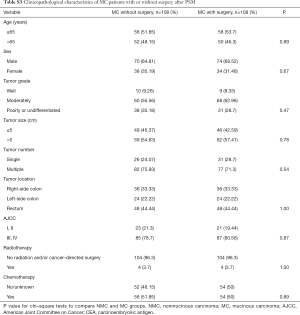
To analyze the clinicopathological characteristics of MC patients, we used other adenocarcinoma patients apart from MC (NMC) as a control group. Totally, our study included 107,735 eligible patients with NMC and 9,494 with MC in the SEER database during the 5-year study period [2009–2013]. Descriptive statistics for the study population were in Table 1. There were certain differences between NMC patients and MC patients such as patient age, tumor grade, tumor size, tumor number, tumor location, CEA status, AJCC stage and therapy patients received. Among colorectal cancer patients, the ratio of patients older than 65 years old were higher than the younger patients. Meanwhile, the MC groups showed a higher proportion than NMC groups in the patients older than 65 (NMC 56.33% and MC 62.56%, P<0.001), in accordance with the results of Wang et al. (5). The major of patients were moderately differentiated (NMC 72.2% and MC 66.88%, P<0.001), MC groups were with poorer differentiation degree compared with NMC groups, since there were more poorly (NMC 16.82% and MC 19.43%, P<0.001) or undifferentiated patients (NMC 3.09% and MC 4.17%, P<0.001). MC groups presented with later tumor stage (AJCC III and IV: NMC 68.82% and MC 78.55%, P<0.001) and larger tumor size (>5 cm: NMC 31.66% and MC 51.07%, P<0.001) in our study population. CEA positivity and right-side colon were more frequently detected in MC groups and always related to higher degree of malignancy (CEA positivity: NMC 25.29% and MC 30.39%, P<0.001; right-side of colon: NMC 46.16% and MC 65.27%, P<0.001). When it comes to the therapy, more MC patients received surgery (NMC 96.39% and MC 98.86%, P<0.001) and more NMC patients may also take radiation into consideration (NMC 11.39% and MC 9.15%, P<0.001).
Full table
Survival characteristics of MC patients and NMC patients
Then, we compared 5-year CSS and OS with NMC patients. As shown in the Kaplan-Meier plots (Figure 1A,B), MC patients showed lower 5-year CSS rate (NMC: 75.81%, 95% CI, 75.46–76.17%; MC: 70.97%, 95% CI, 69.73–72.22%, P<0.001) and lower 5-year OS rate (NMC: 58.87%, 95% CI, 58.48–59.26%; MC: 51.05%, 95% CI, 49.74–52.39%, P<0.001).
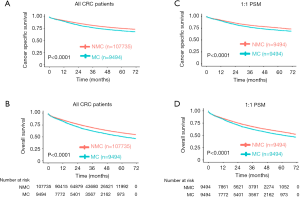
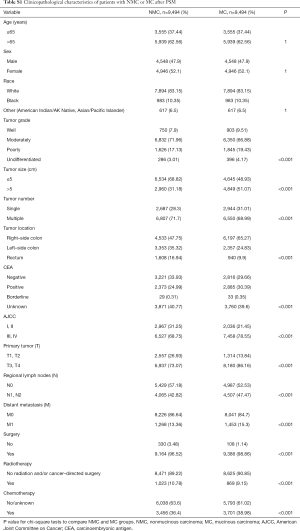
Considering that the sample size was quite different and there were some differences of population baseline characteristics between NMC and MC patients, to avoid these confounding that may made any attribution to the difference of survival between these two groups, we performed PSM with a 1:1 ratio of NMC to MC patients. During PSM, characteristics between the two groups were balanced for gender, race and age, as showed in Table S1. After PSM, these matched NMC patients still presented better 5-year CSS rate (NMC: 75.95%, 95% CI, 74.78–77.14%; MC: 70.97%, 95% CI, 69.73–72.22%, P<0.001) and better 5-year OS rate (NMC: 57.67%, 95% CI, 56.37–59.00%; MC: 51.05%, 95% CI, 49.74–52.39%, P<0.001) (Figure 1C,D).
Full table
Univariate and multivariable survival analysis for MC patients and NMC patients
The univariate Cox regression model was performed to estimate independent prognostic factors of MC patients and NMC patients. The results showed that factors associated with poor survival both in MC and NMC patients were: age at diagnosis more than 65 years, higher tumor grade, positive CEA, larger tumor size, multiple tumors and higher tumor stage (Tables 2,S2). However, there are some factors associated with poor survival only in NMC patients, included sex, race and tumor location. All the therapy were protective factors for both group patients, among them, surgery was the most important one (HR =0.23; 95% CI, 0.18–0.28, P<0.001). Through taking all the meaning variables predicted in the univariate Cox regression model into the multivariable survival analysis, we found that tumor size (HR =1.07; 95% CI, 1–1.14, P=0.056) and radio therapy (HR =0.98; 95% CI, 0.87–1.12, P=0.807) were no longer independent prognostic factors in MC group patients, but still prognostic factors among NMC patients. While other factors, such as age at diagnosis more than 65 years, positive CEA, higher tumor stage were the most poor independent prognostic factors, and surgery was the most protective factors in both groups (Tables 2,S2).
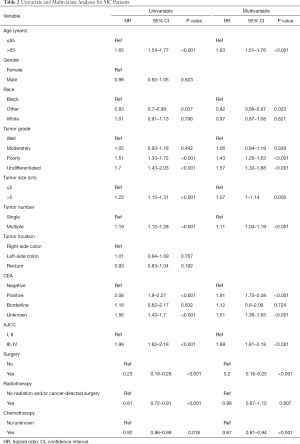
Full table
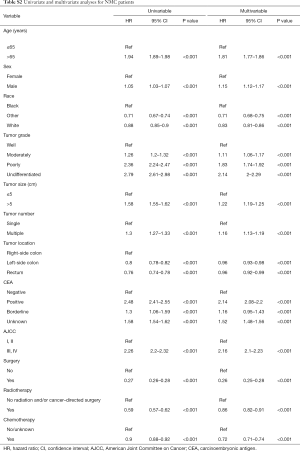
Full table
Subgroup analyses of different treatments for MC patients
Most of MC patients received surgical treatment no matter which stage they were (Table 3), and they benefited a lot from it in general, consistent with previous findings (22,23). As showed in the Kaplan-Meier plots (Figure 2A,B), MC patients receipted surgery showed much longer 5-year CSS rate (no surgery: 21.57%, 95% CI, 13.35–34.85%; surgery: 70.93%, 95% CI, 69.64–72.24%, P<0.001) and longer 5-year OS rate (no surgery: 9.28%, 95% CI, 4.55–18.92%; surgery: 50.92%, 95% CI, 49.59–52.31%, P<0.001). To avoid other covariates which may affect whether patients receipted surgery or may impact the survival of patients, we performed 1:1 PSM, which included 108 matched patients receipted surgery whose baseline characteristics were well-balanced with patients receipted no surgery. The baseline characteristics after PSM was showed in Table S3. After PSM, MC patients receipted surgery still showed better survival (P<0.0001) (Figure 2C,D).
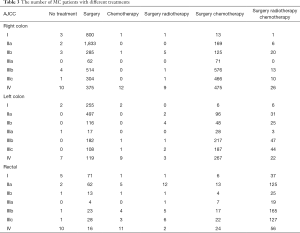
Full table
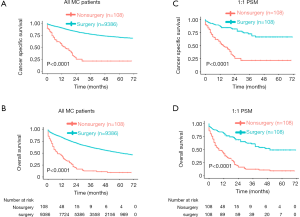
Full table
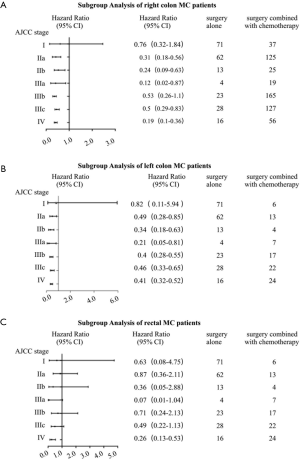
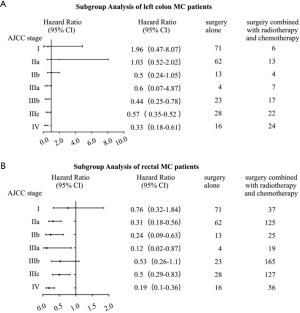
In clinical practice, we treated different stages patients with different treatments. What’s more, it was quite different between treatments of colon and rectal cancer patients, even left colon and right colon cancer were treated as different ones. As a result, we conducted a more intensive subgroup study of different stage or different location on the factor of therapy. According to Table 3, we could see that most patients with right colon cancer would receive surgery or surgery combined with chemotherapy, while patients with left colon cancer or rectal cancer would also take radiotherapy into consideration. In the subgroup analysis of right colon cancer patients where Cox’s regression models were used to estimate HR and 95% CI for each subgroup, we found that compared with surgery alone, stage II-IV patients would benefit from surgery combined with chemotherapy, while stage I patients wouldn’t (Figure 3A). The same result was found in the subgroup analysis of left colon cancer patients (Figure 3B). However, in the subgroup analysis of rectal cancer patients, surgery combined with chemotherapy was a protective factor just for stage IV patients (Figure 3C). Since a small proportion of right colon cancer patients chose radiotherapy, so we performed subgroup analysis of surgery combined with radiotherapy and chemotherapy on left colon cancer patients and rectal patients. The results showed that compared with surgery alone, surgery combined with radiotherapy and chemotherapy improved the prognosis of stage III-IV patients with left colon cancer (Figure 4A) and stage II-IV patients with rectal cancer (Figure 4B).
Discussion
Previous studies showed that MC was a rare morphologic type of CRC with advanced stage and poor survival (24). To learn more about its clinicopathological features and survival outcomes, we included a large amount of MC patients from SEER database. The result showed that MC patients had specific clinicopathological features compared with other histological type of CRC, including: a higher proportion of patients older than 65 years, poorer differentiation degree, later tumor stage, larger tumor size, a higher rate of CEA positivity and more involvement on right-side of colon. All these features indicated that MC showed higher degree of malignancy. In the survival analysis, we compared 5-year CSS and OS between MC and NMC patients, the result showed that MC presented with poorer survival. And this result still hold after adjusting for confounding factors through performing PSM.
Considering that MC patients had poor prognosis, we estimated independent prognostic factors and made a comparation with NMC groups in detail. In a univariate Cox regression model, we found that factors associated with poor survival among MC and NMC patients were: age at diagnosis more than 65 years, higher tumor grade, higher tumor stage, positive CEA, larger tumor size and multiple tumors; at the same time, all the therapy were protective factors for MC and NMC patients, among them, surgery was the most important one. Further more, in the multivariable survival analysis, the result was almost the same, the little difference was tumor size and radiotherapy were no longer prognostic factors in MC groups but they were still prognostic factors among NMC patients.
When it came to the treatment strategies for MC patients, some studies found that standard treatment strategies given to patients with NMC can also be given to patients with MC in accordance with recent guidelines (13). Ott et al. suggested the efficacy of common combination chemotherapy protocols for MC patients (25). Considering that no current, credible guidelines exist for treatment of different phenotypes (26), we performed a more intensive subgroup study on the factor of therapy. Nearly all MC patients were recommended to receive surgery which significantly improved OS. Given that multivisceral resection improves OS without increasing short-term mortality (27), appropriate surgery is critical for increasing OS of MC patients. However, there are some limitation since treatments for surgery plan for III and IV stage patients are different. There were limited sample of the latter, who tended to receive the surgery with more consideration of physical condition. Despite the limitation, the present study is significant because surgery is a favorable factor for treatment of different stages MC patients.
Many factors would be included into consideration to recommend suitable treatment strategies for patients, such as tumor characters, general condition of patients, and patients’ preference. Apart from surgery, radiotherapy and chemotherapy were both efficient tool for CRC patients as adjuvant preoperative therapies to promote possibility of surgical transformation or as postoperative systemic therapy. But there was no clear guideline on whether these were suitable for MC patients since few studies found that MC patients were not so sensitive to chemotherapy (28-30). Through subgroup analysis, we found that surgery combined with chemotherapy was a better choice compared with surgery alone for stage II–IV colon cancer patients but only for IV rectal cancer patients, which was accordance with some researches’ founding that the role of adjuvant chemotherapy was supported for stage II–III colon cancer regardless of the presence of mucinous histology (27). Radiotherapy was more often used in left colon cancer and rectal patients, and we found that the conclusion was quite different: surgery combined with chemotherapy and radiotherapy was more effective for stage II–IV rectal cancer patients but only for IV left colon cancer patients.
The SEER database had completeness and validity of data, but our study had several limitations. First and foremost, SEER did not provide information on details about medication scheme during chemotherapy and radiotherapy, and whether the patient received adjuvant therapy or neoadjuvant therapy was not clear. Second, patient comorbidity was not available so that there would be some confounding factors in the prognosis analysis. What’s more, SEER covered about 28% of the total US population and the number of MC cases we studied was relatively small compared with other population-based reports, which may result in selection bias, especially in the part of analysis of treatments. Meanwhile, the mass data may exist some small aberration, such as the data diagnosed by different pathologists and the pathological reports based on biopsy or surgery or autopsy.
Conclusions
In conclusion, based on our large, population-based, retrospective analysis, MC showed individual clinicopathological characteristics indicating higher degree of malignancy and worse survival. Age, TNM stage, tumor number and treatment were indicators of prognosis. Specific treatment should be made according to the stage and the location of cancer: (I) for stage I colorectal MC patients, surgery was not inferior to other therapy; (II) for stage II–IV colon MC patients, surgery combined with chemotherapy presented with better survival, especially, for stage IV left colon MC patients, surgery combined with chemotherapy and radiotherapy was another choice; (III) for stage II–IV rectal MC patients, surgery combined with chemotherapy and radiotherapy was more effective. We believe that our study may contribute to the treatment of MC patients and continuing efforts are needed to improve proper therapeutic options for them.
Acknowledgments
The authors of this study have no contribution to SEER data collection. We would like to thank the SEER database for its open access and International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding: This work was supported by the grants from the National Natural Science Foundation of China (NSFC Nos. 81572457 and 81702442) and the Natural Science Foundation of Jiangsu province, China (No. BK20170623).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The patients in our study were collected from the SEER database, and we received permission for using the data, for non-commercial use.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490-502. [Crossref] [PubMed]
- Hugen N, van Beek JJP, de Wilt JHW, et al. Insight into Mucinous Colorectal Carcinoma: Clues from Etiology. Ann Surg Oncol 2014;21:2963-70. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Wang MJ, Ping J, Li Y, et al. Prognostic Significance and Molecular Features of Colorectal Mucinous Adenocarcinomas: A Strobe-Compliant Study. Medicine (Baltimore) 2015;94:e2350. [Crossref] [PubMed]
- Chiang JM, Yeh CY, Changchien CR, et al. Mucinous adenocarcinoma showing different clinicopathological and molecular characteristics in relation to different colorectal cancer subgroups. Int J Colorectal Dis 2010;25:941-7. [Crossref] [PubMed]
- Bagante F, Spolverato G, Beal E, et al. Impact of histological subtype on the prognosis of patients undergoing surgery for colon cancer. J Surg Oncol 2018;117:1355-63. [Crossref] [PubMed]
- Nitsche U, Zimmermann A, Spath C, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg 2013;258:775-82; discussion 782-3. [Crossref] [PubMed]
- Hyngstrom JR, Hu CY, Xing Y, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol 2012;19:2814-21. [Crossref] [PubMed]
- Warschkow R, Tarantino I, Huttner FJ, et al. Predictive value of mucinous histology in colon cancer: a population-based, propensity score matched analysis. Br J Cancer 2016;114:1027-32. [Crossref] [PubMed]
- Hugen N, van de Velde CJ, Bosch SL, et al. Modern Treatment of Rectal Cancer Closes the Gap Between Common Adenocarcinoma and Mucinous Carcinoma. Ann Surg Oncol 2015;22:2669-76. [Crossref] [PubMed]
- Hogan J, Burke JP, Samaha G, et al. Overall survival is improved in mucinous adenocarcinoma of the colon. Int J Colorectal Dis 2014;29:563-9. [Crossref] [PubMed]
- Hosseini S, Bananzadeh AM, Salek R, et al. Prognostic Significance of Mucinous Histologic Subtype on Oncologic Outcomes in Patients With Colorectal Cancer. Ann Coloproctol 2017;33:57-63. [Crossref] [PubMed]
- Kang H, O'Connell JB, Maggard MA, et al. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum 2005;48:1161-8. [Crossref] [PubMed]
- Augestad KM, Merok MA, Ignatovic D. Tailored Treatment of Colorectal Cancer: Surgical, Molecular, and Genetic Considerations. Clin Med Insights Oncol 2017;11:1179554917690766. [Crossref] [PubMed]
- Shin US, Yu CS, Kim JH, et al. Mucinous rectal cancer: effectiveness of preoperative chemoradiotherapy and prognosis. Ann Surg Oncol 2011;18:2232-9. [Crossref] [PubMed]
- Bhangu A, Brown G, Nicholls RJ, et al. Survival outcome of local excision versus radical resection of colon or rectal carcinoma: a Surveillance, Epidemiology, and End Results (SEER) population-based study. Ann Surg 2013;258:563-9; discussion 569-71. [Crossref] [PubMed]
- Li Q, Li Y, Dai W, et al. Adjuvant radiotherapy improves cause specific survival in stage II, not stage III mucinous carcinoma of the rectum. BMC Cancer 2017;17:80. [Crossref] [PubMed]
- Mekenkamp LJ, Heesterbeek KJ, Koopman M, et al. Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. Eur J Cancer 2012;48:501-9. [Crossref] [PubMed]
- Govindarajan A, Coburn NG, Kiss A, et al. Population-based assessment of the surgical management of locally advanced colorectal cancer. J Natl Cancer Inst 2006;98:1474-81. [Crossref] [PubMed]
- Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007;26:734-53. [Crossref] [PubMed]
- Hawk NN, Long TE, Imam MH, et al. Clinicopathologic Features and Outcome of Young Adults With Stage IV Colorectal Cancer. Am J Clin Oncol 2015;38:543-9. [Crossref] [PubMed]
- O’Connell JB, Maggard MA, Liu JH, et al. Do Young Colon Cancer Patients Have Worse Outcomes? World J Surg 2004;28:558-62. [Crossref] [PubMed]
- Verhulst J, Ferdinande L, Demetter P, et al. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol 2012;65:381-8. [Crossref] [PubMed]
- Ott C, Gerken M, Hirsch D, et al. Advanced Mucinous Colorectal Cancer: Epidemiology, Prognosis and Efficacy of Chemotherapeutic Treatment. Digestion 2018;98:143-52. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii1-9. [Crossref] [PubMed]
- Catalano V, Loupakis F, Graziano F, et al. Prognosis of mucinous histology for patients with radically resected stage II and III colon cancer. Ann Oncol 2012;23:135-41. [Crossref] [PubMed]
- Catalano V, Loupakis F, Graziano F, et al. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer 2009;100:881-7. [Crossref] [PubMed]
- Maisano R, Azzarello D, Maisano M, et al. Mucinous histology of colon cancer predicts poor outcomes with FOLFOX regimen in metastatic colon cancer. J Chemother 2012;24:212-6. [Crossref] [PubMed]
- Negri FV, Wotherspoon A, Cunningham D, et al. Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol 2005;16:1305-10. [Crossref] [PubMed]


