
Is pembrolizumab monotherapy the optimal treatment for elderly patients with PD-L1 positive advanced non-small cell lung cancer?
When determining an optimal treatment strategy for a patient with advanced non-small cell lung cancer (NSCLC) over 75 years of age, clinicians are faced with the ongoing challenge of not having dedicated clinical trials on this subgroup and being forced to extrapolate findings derived from younger patients. However, we know that older adults with NSCLC may face different challenges compared to their younger counterparts, in terms of differences in the histopathology of their cancer cells (1), different body compositions, increased affliction of comorbidities, poorer performance status, higher risk for increased toxicity of systemic therapies, greater need for social support, and shorter life expectancy. The median age of NSCLC diagnosis in the United States (US) is 70 years and 68% of the patients are diagnosed after 65 years of age (2). Older patients are often under-represented in or excluded from clinical trials, with less than a third of clinical trial participants being older than 65 years. At the same time, in real-world clinical practice, almost two thirds of patients with NSCLC receiving immune checkpoint inhibitors (ICIs) were elderly patients (≥65 years) (3).
Pembrolizumab, a fully humanized IgG4 antibody against the PD-1 receptor has been studied as monotherapy in key landmark trials which led to its approval initially in second line setting for patients with advanced NSCLC with PD-L1 tumor proportion score (TPS) ≥1% [KEYNOTE-010 (4)], then earning a place as the first-line treatment for PD-L1 TPS ≥50% [KEYNOTE-024 (5)], with expansion to PD-L1 TPS ≥1% [KEYNOTE-042 (6)]. Nosaki and colleagues analyzed the data on patients ≥75 years from a pooled analysis of these large, open-label, randomized control trials (7). The pooled analysis included 264 patients ≥75 years who were randomized to receive pembrolizumab or chemotherapy. In KEYNOTE-010, patients randomized to the chemotherapy arm received docetaxel as second or subsequent line of treatment, whereas in KEYNOTE-024 and KEYNOTE-042 patients on chemotherapy arm received first line platinum-based chemotherapy. At a median follow up time of 11.7 months, the authors reported improved median overall survival (mOS) with Pembrolizumab for patients ≥75 years with PD-L1 TPS ≥1%, 15.7 versus 11.7 months (Pembrolizumab arm vs. chemotherapy), HR, 0.76 (95% CI, 0.56–1.02). As expected, this subgroup with PD-L1 TPS ≥50% yielded an even longer mOS, 23.1 months compared with 8.3 months (Pembrolizumab arm vs. chemotherapy), HR, 0.40 (95% CI, 0.25–0.64). This mOS benefit was, in fact, more impressive than that in the <75 years with PD-L1 TPS ≥50%, HR 0.67 [95% CI, 0.57–0.78]). Unfortunately, the study did not provide survival data on the subgroup of patients with a PD-L1 TPS of 1–49%.
In this analysis, patient demographics and clinical characteristics were similar in both age groups and treatment arms, though a higher percentage of older patients were noted to be treatment naive (66% vs. 60%) and with less brain metastasis (5.7% vs. 10%) compared with the <75 years patient group. As with most randomized clinical trials, the authors note that all patients were required to have an ECOG 0 or 1. In real-world practice, studies reported that 30–50% of patients with newly diagnosed NSCLC presented with an ECOG of 2 or worse (8). As expected, in this pooled analysis, a greater proportion of patients <75 years had an ECOG of 0, compared with patients ≥75 years (33% vs. 26%). The higher incidence of squamous cell carcinoma observed in older patients was reflected in this analysis. Only KEYNOTE-010 allowed EGFR-mutant or ALK-translocated tumors and accounted for 8% of all patients. The tumor mutational burden (TMB) status for patients was not provided.
With regards to toxicities, Nosaki and colleagues found that elderly patients treated with Pembrolizumab experienced lower treatment-related adverse events (TRAEs), compared with chemotherapy, 68.5% vs. 94.3%; less grade 3 AEs, 24.2% vs. 61.0% and fewer serious TRAEs, 16.1% vs. 26.7%. Around 25% of all patients treated with Pembrolizumab regardless of age, experienced immune-related AEs (iAE) and infusion reactions. Patients treated with Pembrolizumab over 75 years did experience a higher rate of grade 3 TRAEs, compared with younger patients, 24.2% vs. 16.9%, but grade 3 or higher iAEs were only slightly increased in the older patient group 9.4% vs. 7.1%. Hypothyroidism and pneumonitis were the most common iAEs (7–10%) in all patients treated with Pembrolizumab, regardless of age. Trials of other ICIs such as the PD-1 inhibitor Nivolumab, have also demonstrated similar safety profiles in older and younger patients (9). A meta-analysis of four randomized controlled trials comparing PD-1/PD-L1 blocking antibodies with docetaxel, observed significantly lower incidences of treatment emergent grade 3 or 4 AEs in patients ≥75 years compared with <65 years (47.4% vs. 23) (10). Though a large meta-analysis found that fatal toxicities associated with ICIs occurred more frequently in older patients (11). Another important consideration is that older patients typically have a lower tolerance for any toxicity events even if only low grade. Despite some conflicting data on the safety of ICIs in older patients, overall it seems that ICIs are safe in older patients and have a side effect profile more favorable than that of systemic chemotherapy.
There is concern that the efficacy of immunotherapy is diminished in older patients due to immunosenescence, the progressive decline of immune functions through complex remodeling and adaptation (12,13). However, meta-analyses comparing ICIs to systemic chemotherapy have found similar OS benefits of ICIs between older and younger patient populations (14). Another meta-analysis subgrouped the elderly patients further and demonstrated that patients ≥75 years derived less benefit from ICIs compared with patients ≥65 and <75 years (15). In addition, we reviewed several randomized clinical trials comparing ICI’s with systemic chemotherapy and found that older patients receive a comparable OS benefit from ICIs in comparison with younger patients (Table 1). Another study including 62 elderly patients with advanced NSCLC and PS of 2, found that clinical responses and toxicities with Pembrolizumab were comparable to younger patients in historical controls (20). A phase 2 study is ongoing to evaluate durvalumab as first-line treatment in patients with advanced NSCLC with a PS of 2 (NCT02879617) and will provide additional safety and efficacy data.
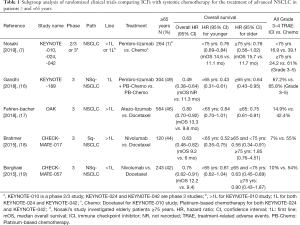
Full table
As the focus shifts towards immunotherapy with or without chemotherapy as the standard of care in patients with advanced NSCLC (with no driver mutations), we need to gain a better understanding into not only the elderly and frail patients that seek to gain benefit from mono-immunotherapy, but those that may gain benefit from combined chemo-immunotherapy. This study conveys the toxicities associated with chemotherapy and emphasizes the appropriateness of mono-immunotherapy compared with chemotherapy in elderly patients, particularly if their tumors highly express PD-L1. It would have been interesting to compare the outcomes of Pembrolizumab monotherapy with systemic chemotherapy in those with PD-L1 TPS 1–49%, as this patient group may be particularly susceptible to undertreatment. Selecting favorable elderly and frail patients for chemo-immunotherapy is challenging, as physicians seek to find the balance between preserving quality of life by minimizing toxicities, while improving symptoms and prolonging life. As the treatment paradigm expands with exploration of chemo-immunotherapy, sequencing of treatments and combinations of immunotherapy, we need to gain a better understanding into the impact of factors such as PD-L1 TPS, TMB, age, comorbidities and performance status to guide physicians in therapy selection. Elderly-specific trials will help us better define prognostication and treatment criteria for this underrepresented patient group.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.02.68). BH has received research funding from Merck, Astra-Zeneca, Boehringer-Ingelheim, Pfizer, Novartis, GSK, AbbVie, Mirati, Eli-Lilly, Takeda and BMS and consulting fees from Foundation Medicine, Guardant Health 360, Eli-Lilly, Novartis, Pfizer, BoehringerIngelheim, Astra-Zeneca, Spectrum, BMS, Merck, Genentech and Takeda. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maione P, Rossi A, Sacco PC, et al. Treating advanced non-small cell lung cancer in the elderly. Ther Adv Med Oncol 2010;2:251-60. [Crossref] [PubMed]
- Bravo-Iñiguez C, Perez Martinez M, Armstrong KW, et al. Surgical resection of lung cancer in the elderly. Thorac Surg Clin 2014;24:371-81. [Crossref] [PubMed]
- O'Connor JM, Fessele KL, Steiner J, et al. Speed of adoption of immune checkpoint inhibitors of programmed cell death 1 protein and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol 2018;4:e180798. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Nosaki K, Saka H, Hosomi Y, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with pd-l1-positive advanced non-small-cell lung cancer: pooled analysis from the keynote-010, keynote-024, and keynote-042 studies. Lung Cancer 2019;135:188-95. [Crossref] [PubMed]
- Lilenbaum RC, Cashy J, Hensing TA, et al. Prevalence of poor performance status in lung cancer patients: implications for research. J Thorac Oncol 2008;3:125-9. [Crossref] [PubMed]
- Spigel D, Schwartzberg L, Waterhouse D, et al. P3.02c-026 Is Nivolumab Safe and Effective in Elderly and PS2 Patients with Non-Small Cell Lung Cancer (NSCLC)? Results of CheckMate 153. J Thorac Oncol 2017;12:S1287-8. [Crossref]
- Marur S, Singh H, Mishra-Kalyani P, et al. FDA analyses of survival in older adults with metastatic non-small cell lung cancer in controlled trials of PD-1/PD-L1 blocking antibodies. Semin Oncol 2018;45:220-5. [Crossref] [PubMed]
- Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721-8. [Crossref] [PubMed]
- Franceschi C, Valensin S, Fagnoni F, et al. Biomarkers of immunosenescence within an evolutionary perspective: the challenge of heterogeneity and the role of antigenic load. Exp Gerontol 1999;34:911-21. [Crossref] [PubMed]
- Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol 2009;30:306-12. [Crossref] [PubMed]
- Elias R, Giobbie-Hurder A, McCleary NJ, et al. Efficacy of PD-1 and PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer 2018;6:26. [Crossref] [PubMed]
- Zhang L, Sun L, Yu J, et al. Comparison of Immune Checkpoint Inhibitors between Older and Younger Patients with Advanced or Metastatic Lung Cancer: A Systematic Review and Meta-Analysis. Biomed Res Int 2019;2019:9853701. [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Fehrenbacher L, von Pawel J, Park K, et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:1156-70. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Middleton G, Barrie C, Barone G, et al. Pembrolizumab in performance status 2 patients with non-small cell lung cancer (NSCLC): Results of the PePS2 trial. Ann Oncol 2018;29:viii493-547


