
Extranodal soft tissue metastasis as an independent prognostic factor in gastric cancer patients aged under 70 years after curative gastrectomy
Introduction
Despite a steady decrease in its incidence over the past several decades, gastric cancer (GC) remains the second most common cause of cancer-related death worldwide (1). Overall, assessment of patient characteristics to identify the risk of recurrence and poor prognosis is significantly crucial for choosing treatment strategies for cancer. To date, the extent of nodal involvement and the number of positive lymph nodes remain the focal point for determining the patient’s prognosis. The tumour-node-metastasis (TNM) classification system is widely used for tumour staging and guides the treatment and prognostic predictions of patients with GC, yet patients with the same TNM stage show a wide range of survival times and outcomes. Extranodal soft tissue metastasis (ESTM), comprising cancer cells in soft tissue discontinuous with the primary lesion, is found during routine examination of approximately 10–28% of resected gastric carcinoma specimens (2), and the prognostic significance of extranodal tumour extension among solid cancers, including GC, thyroid carcinoma, rectal cancer, has been documented (3-8). The aim of this study was to evaluate the incidence and prognostic significance of ESTM in GC patients after curative resection. In addition, we classified ESTM into several different categories based on the cut-off value of the number of ESTM and then determined whether ESTM should be combined with the American Joint Committee on Cancer (AJCC) clinical (pTNM) staging system.
Methods
Patients and operative management
Between May 1, 2003, and June 31, 2011, a total of 657 GC patients who underwent curative resection at our institution were included in a retrospective database. All procedures were followed by the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The inclusion criteria for this study were as follows: (I) pathologic diagnosis of primary adenocarcinoma of the stomach; (II) no Siewert type I or II oesophagogastric junction tumours; (III) no distant metastasis; (IV) pathologically negative resection margins (R0 resection); (V) no residual GC after surgery; (VI) no neoadjuvant chemotherapy or radiotherapy; (VII) no other synchronous malignancy or previous history of gastrectomy; (VIII) postoperative survival of at least 2 months; and (IX) under 70 years of age. In total, 580 patients met these criteria and were included. Primary tumours were resected en bloc by lymphadenectomy according to the guidelines of the Japanese Gastric Cancer Association (9), and the surgical procedures were mainly in accordance with the Japanese Gastric Cancer Treatment Guidelines (10). The TNM classification for GC (eighth edition) was adopted for the staging of all enrolled cases.
Follow-up
Patients were postoperatively followed up every 3–6 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter until the end of the study (October 2016) or death. The median follow-up duration was 41.8 [2–136] months.
Pathological assessment
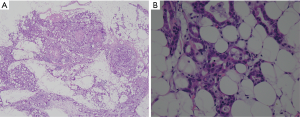
All specimens were analysed by two experienced pathologists, and different opinions were resolved through discussion to establish the ultimate diagnosis results. Carcinoma lesions along with the surrounding gastric wall were fixed in formalin and sectioned into multiple 5-mm slices in parallel with the lesser curvature. ESTM was defined as the presence of cancer cells in adipose tissue discontinuous with the primary lesion or in perinodal adipose tissue different from the lymph node (11) (Figure 1). The pathology report mainly included data regarding tumour size, ESTM, Lauren classification, depth of invasion (pT stage), number of regional LN metastases (pN stage), number of LNs examined (NELN), perineural invasion, lymphovascular invasion (LVI) and vascular invasion (VI).
Statistical analysis
The clinicopathologic features investigated for prognostic significance included gender, age, tumour size, tumour location, type of gastrectomy, Lauren classification, pT stage, pN stage, NELN, blood loss, lymphadenectomy, AJCC pathological stage (pTNM), perineural invasion, LVI, VI, signet-ring cell (SRC) variants, ESTM, blood loss and postoperative chemotherapy.
Clinicopathological characteristics significantly associated with patient survival were evaluated using the Kaplan Meier method and Cox proportional hazards analysis. Akaike information criterion (AIC) and Bayesian information criterion (BIC) values within a Cox proportional hazard regression model were calculated for each category to assess its discriminatory ability, whereby a smaller AIC or BIC value indicated a better model performance. Stratum analysis was applied to assess the influence of clinicopathological characteristics on the accuracy of the prognostic prediction of ESTM in GC patients. Differences in proportions of patients were analysed with the χ2 test. Logistic regression analysis was adopted to clarify risk factors that predict the presence of ESTM and cut point survival analysis to identify the optimal cut off values for the ESTM count. The threshold for statistical significance was P<0.05. The statistical analysis was performed using IBM SPSS Statistics, version 22.0 (IBM Corp., Armonk, NY, USA).
Results
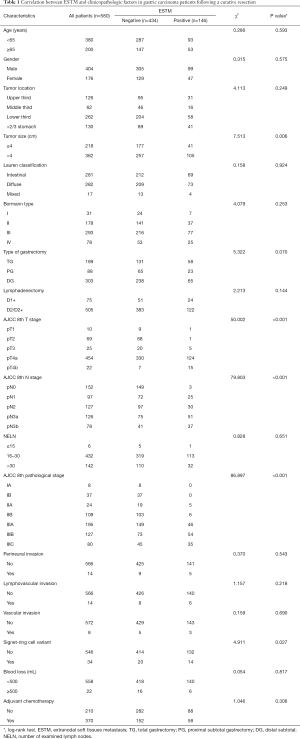
Overall, 580 patients with resected GC met the eligibility criteria. The age range of the patients included in this study was between 20 and 70 years. Among the patients examined, the absence of ESTM (ESTM−) was confirmed in 434, and the presence of ESTM (ESTM+) was confirmed in 146. The two groups (ESTM− and ESTM+) were balanced with reference to gender (P=0.575), age (P=0.593), tumour location (P=0.249), Lauren classification (P=0.924), type of gastrectomy (P=0.07), lymphadenectomy (P=0.144), NELN (P=0.651), perineural invasion (P=0.543), LVI (P=0.218), VI (P=0.690), blood loss (mL, P=0.817) and postoperative adjuvant chemotherapy (P=0.306). Simultaneously, significant differences in tumour size (P=0.006), pT category (P<0.001), pN stage (P<0.001), AJCC pathological stage (P<0.001) and signet ring cell variants (P=0.027) were observed between the ESTM negative and ESTM positive groups (Table 1).
Full table
Univariate and multivariate survival analyses of 580 GC patients
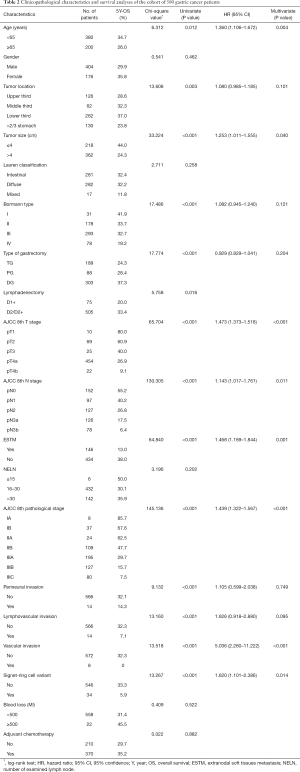
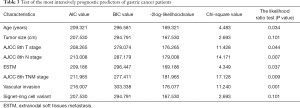
Univariate analysis revealed older age (age ≥65), advanced pT, pN, pTNM category, the presence of ESTM, LVI, VI and/or perineural invasion, the presence of SRC variants, larger tumour size (>4 cm), primary tumour invasion more than two-thirds of the stomach, Borrmann type IV GC, total gastrectomy and D1+ lymphadenectomy to be associated with a poor prognosis among GC patients. In the multivariable analysis, the variables of age, tumour size, pT stage, pN stage, pTNM stage, ESTM, VI, and SRC variants remained independent prognostic factors for the postoperative OS of all GC patients (Table 2). To measure discriminatory ability, AIC and BIC values were calculated for the independent predictors of OS. As ESTM showed the second smallest values, thus indicating that it was the better prognostic predictor of OS in this GC patients cohort (Table 3). Survival curves comparing age, tumour size, pT stage, pN stage, pTNM stage, VI and SRC variants are depicted in Figure 2.
Full table
Full table
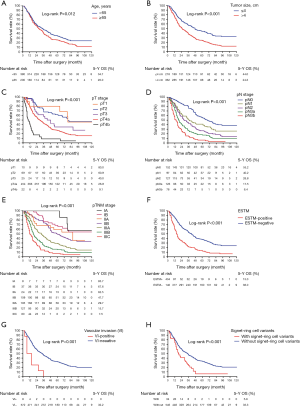
For further illustration of the potential prognostic prediction ability of ESTM for GC patients, stratum analysis within the Kaplan-Meier was adopted. The prognosis for patients in the ESTM− and ESTM+ groups stratified by tumour size, pT stage, pN stage, SRC variants and VI was compared, and we found that the 5-year survival rate of patients with ESTM-positive tumours was lower than that for patients with ESTM-negative tumours. This was also true for the stratum categories of tumour size, SRC variants and VI (Figure 3).
Logistic regression analysis to identify risk factors predictive of ESTM and LN metastasis
Logistic regression analysis was applied to identify risk factors predicting ESTM, including tumour size, tumour location, Borrmann type, Lauren classification, perineural invasion, LVI, VI, SRC variants, pT stage, and pN stage, though only pT and pN stages were correlated significantly with ESTM (OR, 2.204; 95% CI, 1.407–3.452, P=0.001; OR, 1.749; 95% CI, 1.483–2.064, P<0.001, respectively) (Table 4). Further logistic regression analysis of risk factors predictive of LN metastasis, including tumour size, tumour location, Borrmann type, Lauren classification, perineural invasion, LVI, VI, SRC variants, pT stage, pT stage and ESTM indicated that tumour size (OR, 1.643; 95% CI, 1.077–2.507, P=0.021), ESTM (OR, 19.285; 95% CI, 6.002–61.973, P<0.001) and pT stage (OR, 1.748; 95% CI, 1.377–2.219, P<0.001) were significantly associated with LN metastasis (Table 5).
Full table
Full table
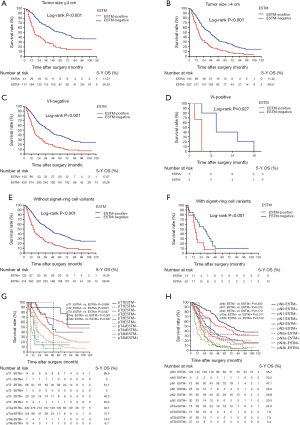
Incorporation of ESTM into the AJCC pTNM staging system (eighth edition) and stage migration analysis of ESTM
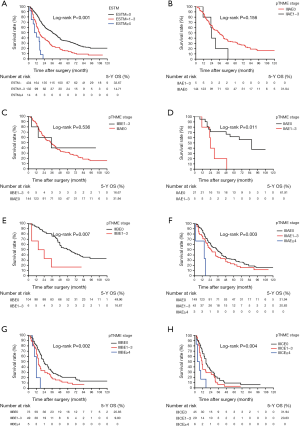
Cut point analysis was performed to determine the optimal ESTM count cut off values for discriminating survival differences among various subgroups, and the results are presented in Table S1. Appropriate ESTM count cut-off values to verify statistically significant survival differences among subgroups were identified as 0, 1–3, and ≥4. In addition, Kaplan Meier analysis indicated significant survival differences among these three ESTM count subgroups (P<0.001, Figure 4A). Additionally, the ESTM was incorporated into the eighth edition of the pTNM classification and the prognostic prediction of pTNME classification were calculated directly. No statistically significant survival differences were observed between pIIAE1-3 patients and pIIIAE0 patients (P=0.156; Figure 4B), or between pIIBE1-3 patients and pIIIAE0 patients (P=0.536; Figure 4C). Simultaneously, there were significant survival differences between pIIAE0 and pIIAE1-3 patients (P=0.011, Figure 4D), pIIBE0 and pIIBE1-3 patients (P=0.007, Figure 4E). Also, significant survival differences can be detected among the pIIIAE0, pIIIAE1-3 and pIIIAE ≥4 patients (P=0.003, Figure 4F); pIIIBE0, pIIIBE1-3 and pIIIBE ≥4 (P=0.002, Figure 4G), especially among pIIICE0, pIIICE1-3 and pIIICE ≥4 patients (P=0.004, Figure 4H). These results manifested that pTNME is a promising prognostic classification and might be an alteration of the eighth edition of pTNM classification; however, it requires further validations.

Full table
Discussion
The histologically complete resection (R0) of tumours is the only potentially curative treatment for patients with gastric carcinoma. The AJCC recommends curative gastrectomy with the systematic lymph node dissection up to second-tier nodes (D2) when tumours are confined to the primary lesion and regional lymph nodes (12). However, the significance of extranodal soft tissue in lymph node dissection has not been mentioned in all guidelines for GC, even though pathological examination of surgical specimens has revealed a rate of extranodal metastasis reaching 10% to 20% (2). Furthermore, previous studies have reported that ESTM is more likely to occur in large tumours, tumours with invasive growth characteristics, undifferentiated carcinoma, and lymph node, peritoneal, hepatic metastasis or recurrent lymphatic vessel metastasis (2,13).
To date, patient prognosis has primarily been predicted by the extent of nodal involvement and the number of metastasized lymph nodes (LNs). Indeed, the TNM classification system is widely used for tumour staging and guides treatment decisions and prognostic predictions of patients with cancer (1). However, patients with the same pTNM stage have a wide range of survival times and treatment outcomes. Localized disease often recurs after curative resection, even for pT1 tumours. Anticipating the prognosis of patients who undergo curative surgery, especially for early disease, is difficult, which implies that the current staging system is inaccurate for prognostic predictions and does not provide a good basis for adjuvant treatment decisions. A prognostic factor that can ascertain patients with a high risk of recurrence and death would be conducive to more accurately predict patient prognoses as well as elect GC patients who have a high risk of death and who might profit from adjuvant chemotherapy. To date, many histological and biological markers in addition to T and N have been reported and discussed as prognostic factors (14,15).
Recently, it has been suggested that extra-nodal involvement is related to an advanced stage and appears to be a reliable prognostic factor for GC (2-4,16). In addition, previous studies have confirmed that ESTM, which is an intermediate between LN metastasis and peritoneal metastasis, is an independent factor influencing the prognosis of GC patients (17). What’s more, a previous study also showed that extra-nodal extension was the significant prognostic factor in patients with early GC and nodal metastases (18). The researchers demonstrated that pT1 or pN1 GC patients with ESTM+ had a worse prognosis than those pT2 or pN2 patients without ESTM. Therefore, they suggested adjuvant therapy to be taken into consideration for GC patients in early stages with ESTM (18). However, most of previous studies have enrolled patients aged older than 80 years. As the average lifetime of men and women in China are 74 and 77 years old, respectively, results would not be as dependable if elderly patients older than these are evaluated (19,20). Moreover, death among patients older than 70 years within 5 years after the surgery may be wing to their own lifespans rather than due to recurrence of metastasis of GC; the 5-YSR of patients is also an important indicatrix for patients with cancer. Therefore, the patients with resected GC included in present study were aged under 70 years, which may have resulted in age selection bias.
In this study, we investigated the clinical parameters and prognostic value of ESTM in the GC patients who underwent radical resection, and we found that the incidence of ESTM was 146 (25.2%) among 580 patients. Our univariate and multivariate survival analyses indicated that tumour size, pT stage, pN stage, pTNM stage, ESTM, VI, and SRC variants are independent poor prognostic factors. Tumour size t4 cm, presence of ESTM, VI and SRC variants, higher pT stage, pN stage and pTNM stage were associated with a poorer 5Y-OS. It is generally known that different histotypes sometimes means different biological behaviour. In the literature, GC with SRC variants tends to metastasize to the peritoneum and has favorable prognosis in early stages but poor prognosis in advanced tumor stages in comparison to non-SRC adenocarcinoma (21,22). Therefore, the prognosis for patients in the ESTM− and ESTM+ groups stratified by SRC variants as well as other prognostic factors including tumour size, pT stage, pN stage, and VI was compared. And the results indicated that 5-YSR of patients in the ESTM group was significantly lower than that of patients without ESTM stratified by SRC variants as well as the other prognostic variables of tumour size and VI. These results demonstrated the ESTM might be considered as a common prognostic factor independent by histotypes, tumour size and VI.
Considering that pN stage is the most valuable prognostic indicator of GC patients, logistic regression analysis on risk factors that predict LN metastasis were performed (23,24), revealing a close relationship between LN metastasis with pT stage, tumour size and ESTM. Importantly, cancer patients with ESTM were at higher risk of LN metastasis. Our results emphasize the importance of ESTM in patient prognosis and its association with LN metastasis. ESTM can also be an efficient predictor of LN metastasis, and pT may be conducted as an indicator of ESTM. Thus, GC patients in an advanced pT stage may be at a higher risk of LN metastasis. Therefore, patients with advanced clinical T stage (cT stage) should be monitored more closely, and as many LNs as possible should be retrieved for accurate staging (25,26).
Likewise, the logistic regression analysis was also conducted to identify risk factors of the existence of ESTM and pT stage and pN stage were found to be important risk predictor of ESTM in this regard. Accordingly, ESTM patients with higher pT and pN stages may have a shorter survival time, which is in agreement with previous studies demonstrating that ESTM is closely associated with tumour aggressiveness (27,28). Our analysis results also foreground the important value of ESTM for prognosis and its relationship with LN metastasis and tumour invasion. ESTM may also be a valid predictor of LN metastasis. In view of these results, we propose that ESTM be included in the current pTNM staging system as an important prognostic factor.
Etoh et al. (3) have demonstrated that ESTM is an independent prognostic factor and should therefore be incorporated in the pTNM staging system. ESTM has been associated with a high recurrence risk, and was found to be a better prognostic factor than lymph node status (29). It is also reported an increasing number of positive lymph nodes with ESTM to be associated with poorer survival outcomes (3). In this study, outcomes were poorer with an increasing number of ESTMs. To identify whether ESTM should be included in the pTNM category, we stratified patients into three subgroups according to the cut-off analysis of ESTM number and incorporated ESTM into the eighth edition pTNM stage system. And this analysis showed the pTNME classification might reduce stage migration and might be a more appropriate prognostic classification for predicting the OS of GC patients after curative surgery than the eighth edition of pTNM classification, especially for advanced-stage GC. However, it requires further validations.
As there may be a tendency towards bias in a retrospective study, further multicentre, randomized controlled trials, especially utilizing postoperative pathology reports, are required.
Overall, our results demonstrated that incorporating ESTM into a new edition of the pTNM classification for GC might help offer better prognostic prediction. Accurate classification of lymphatic spread in the resected gastric specimens is crucial not only for estimating prognosis but also for stratifying patients in future clinical trials and for providing personalized adjuvant strategies, providing additional information for the AJCC pN category and pTNM stage. This approach will also be conducive to identifying GC patients with inferior prognoses. In future staging systems, the number of positive lymph nodes should be considered as well as also the presence of ESTM in GC. Therefore, lymph nodes, soft tissues, fascia and adipose tissue should be removed en bloc at the same time of lymph node dissection; only in this way can the purpose of R0 surgery be achieved.
Acknowledgments
Funding: This work was supported in part by grants from the Programs of National Natural Science Foundation of China (No. 81572372), National Key Research and Development Program “major chronic non-infectious disease research” (2016YFC1303202), National Key Research and Development Program “precision medicine research” (2017YFC0908304).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures were followed by the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol 2013;107:230-6. [Crossref] [PubMed]
- Tanaka T, Kumagai K, Shimizu K, et al. Peritoneal metastasis in gastric cancer with particular reference to lymphatic advancement; extranodal invasion is a significant risk factor for peritoneal metastasis. J Surg Oncol 2000;75:165-71. [Crossref] [PubMed]
- Etoh T, Sasako M, Ishikawa K, et al. Extranodal metastasis is an indicator of poor prognosis in patients with gastric carcinoma. Br J Surg 2006;93:369-73. [Crossref] [PubMed]
- Koike H, Ichikawa D, Kitamura K, et al. Perinodal involvement of cancer cells in gastric cancer patients. Surgery 2004;135:266-72. [Crossref] [PubMed]
- Yamashita H, Noguchi S, Murakami N, et al. Extracapsular invasion of lymph node metastasis is an indicator of distant metastasis and poor prognosis in patients with thyroid papillary carcinoma. Cancer 1997;80:2268-72. [Crossref] [PubMed]
- van der Velden J, Lindert ACMV, Lammes FB, et al. Extracapsular growth of lymph node metastases in squamous cell carcinoma of the vulva. The impact on recurrence and survival. Cancer 1995;75:2885-90. [Crossref] [PubMed]
- Ueno H, Mochizuki H, Tamakuma S. Prognostic significance of extranodal microscopic foci discontinuous with primary lesion in rectal cancer. Dis Colon Rectum 1998;41:55-61. [Crossref] [PubMed]
- Brasilino de Carvalho M.. Quantitative analysis of the extent of extracapsular invasion and its prognostic significance: A prospective study of 170 cases of carcinoma of the larynx and hypopharynx. Head Neck 1998;20:16-21. [Crossref] [PubMed]
- Jaehne J, Meyer HJ, Maschek H, et al. Lymphadenectomy in gastric adenocarcinoma. A prospective and prognostic study. Arch Surg 1992;127:290-94. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Veronese N, Fassan M, Wood LD, et al. Extranodal Extension of Nodal Metastases Is a Poor Prognostic Indicator in Gastric Cancer: a Systematic Review and Meta-analysis. J Gastrointest Surg 2016;20:1692-8. [Crossref] [PubMed]
- Okamoto T, Tsuburaya A, Kameda Y, et al. Prognostic value of extracapsular invasion and fibrotic focus in single lymph node metastasis of gastric cancer. Gastric Cancer 2008;11:160-67. [Crossref] [PubMed]
- Kumagai K, Tanaka T, Yamagata K, et al. Liver metastasis in gastric cancer with particular reference to lymphatic advancement. Gastric Cancer 2001;4:150-5. [Crossref] [PubMed]
- Yoshikawa T, Tsuburaya A, Kobayashi O, et al. Protein levels of tissue inhibitor of metalloproteinase-1 in tumor extracts as a marker for prognosis and recurrence in patients with gastric cancer. Gastric Cancer 2006;9:106-13. [Crossref] [PubMed]
- Cai J, Ikeguchi M, Maeta M, et al. Clinicopathological value of immunohistochemical detection of occult involvement in pT3N0 gastric cancer. Gastric Cancer 1999;2:95-100. [Crossref] [PubMed]
- Nakamura K, Ogoshi K, Makuuchi H. Subclassification of extranodal involvement in gastric cancer patients. Hepatogastroenterology 2010;57:968-74. [PubMed]
- Wang XN, Ding XW, Zhang L, et al. Correlation analysis of gastric cancer with extranodal metastasis. Zhonghua Wei Chang Wai Ke Za Zhi 2007;10:436-9. [PubMed]
- Lee IS, Park YS, Ryu MH, et al. Impact of extranodal extension on prognosis in lymph node-positive gastric cancer. Br J Surg 2014;101:1576-84. [Crossref] [PubMed]
- Zong L, Abe M, Seto Y, et al. Randomized Controlled Trial of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: How Should We Define the Age of Included Patents? J Clin Oncol 2016;34:3706. [Crossref] [PubMed]
- Zhang CD, Ning FL, Zeng XT, et al. Lymphovascular invasion as a predictor for lymph node metastasis and a prognostic factor in gastric cancer patients under 70 years of age: A retrospective analysis. Int J Surg 2018;53:214-20. [Crossref] [PubMed]
- Mariette C, Carneiro F, Grabsch HI, et al. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer 2019;22:1-9. [Crossref] [PubMed]
- Chon HJ, Hyung WJ, Kim C, et al. Differential Prognostic Implications of Gastric Signet Ring Cell Carcinoma: Stage Adjusted Analysis From a Single High-volume Center in Asia. Ann Surg 2017;265:946-53. [Crossref] [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Survival results of a multicentre phase II study to evaluate D2 gastrectomy for gastric cancer. Br J Cancer 2004;90:1727-32. [Crossref] [PubMed]
- de Manzoni G, Verlato G, Roviello F, et al. The new TNM classification of lymph node metastasis minimises stage migration problems in gastric cancer patients. Br J Cancer 2002;87:171-4. [Crossref] [PubMed]
- Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol 2007;14:317-28. [Crossref] [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Choi WH, Kim S, Shen J, et al. Prognostic significance of perinodal extension in gastric cancer. J Surg Oncol 2007;95:540-5. [Crossref] [PubMed]
- Nakamura K, Ozaki N, Yamada T, et al. Evaluation of prognostic significance in extracapsular spread of lymph node metastasis in patients with gastric cancer. Surgery 2005;137:511-7. [Crossref] [PubMed]
- Wang W, Li Y, Zhang Y, et al. Incorporation of extranodal metastasis of gastric carcinoma into the 7th edition UICC TNM staging system. PLoS One 2011;6:19557.


