Robotic assisted nephrectomy for living kidney donation (RANLD) with use of multiple locking clips or ligatures for renal vascular closure
Introduction
Nephrectomy for living kidney donation has been performed laparoscopically for more than two decades (1). Minimally invasive surgical techniques have substantially contributed to the future development of transplant programs and among others significantly increased the number of potential living donors (2,3). This evolution was fueled by the fact that minimally invasive operative techniques generate significant patients’ benefits such as reduction in postoperative pain, decreased length of hospital stay, better cosmetic results and lower morbidity rates (4-6).
In this context, robotic assisted nephrectomy for living kidney donation has become a well-established procedure, competing for supremacy with laparoscopic and hand assisted minimally invasive approaches (7,8). Unlike in other surgical interventions, living kidney donation involves healthy altruistic individuals, who are willing to undergo major surgery for the wellbeing of another human being. Minimization of any potential risk factors thus maximizing donor safety is even more highest priority (9).
Hemorrhagic complications resulting from inadequate renal artery closure in living kidney donation surgery have been shown to jeopardize donor life and health. Opinions about the safety of specific techniques of renal vascular closure are heterogeneous and predominantly investigated in open and laparoscopic procedures of living kidney donation. In general, transfixing techniques using various vascular stapling devices and surgical sutures are considered superior when compared with non-transfixing techniques like clips and ligatures. A survey among surgeon-members of the American Society of Transplant Surgeons (ASTS) however, also revealed that multiple locking clips are still commonly used in laparoscopic cases of living kidney donation (10,11).
From a technical perspective robotic assisted surgery provides the surgeon with enhanced 3D visualization of the operative field and the use of wristed instrumentation allows tremor free articulation with seven degrees of freedom. Surgeons intraoperative comfort while using the robotic unit has been described to be extremely high, resulting in continuous steep reproducibility rates of various major surgical interventions (12,13).
The first series of robotic assisted laparoscopic donor nephrectomies were reported in 2001 (14,15). Recent data suggest that robot assisted nephrectomies account for a fraction of 3,8% of all minimally invasive donor nephrectomies, numbers increasing (16). Published data suggest that renal vascular closure in robotic assisted nephrectomy for living donation (RANLD) has been prevalently performed safely with vascular staplers (13,17).
The da Vinci Surgical System® (Intuitive Surgical, Sunnyvale, CA) is currently the most used device with more than 3,500 operating platforms worldwide (18). As the company’s name suggests, the design and handling of the robot is meant to be intuitive for a first-time user, which allows for a steep learning curve for novices.
Here we describe the experience of our first 40 consecutive robotic assisted donor nephrectomies with regard to pre- and intraoperative strategies and postoperative outcome. In contrast to previous studies we used multiple locking clips or a combination of clips and suture ligatures for renal vascular closure.
Methods
Patient evaluation
According to the German law, living donors can be either directly related to the recipient or altruistic. In any case, a close relationship without a direct degree of kindship must be evident and will be reviewed by an ethics committee of the German medical association (Bundesärztekammer).
Donor selection was based on a medical and psychological evaluation as recommended by the KDIGO Clinical Practice Guidelines of the Transplantation Society (19). Table 1 gives an overview of the standard parameters investigated during the assessment process. Older age and comorbidities such as moderate hypertension and a BMI >30 kg/m2 are no contraindications at our center, since previous studies have proven safe results for these types of donors (20-22). Preoperative CT or MRI angiography of the kidneys was performed with special emphasis on vascular supply. Split renal function was determined by MAG3 kidney scintigraphy. Presuming that isotope clearance of the organ selected for donation did not differ more than 5% of the total isotope clearance, the kidney with the fewer artery supply was chosen for donation. The institutional review board composed of a multidisciplinary team of transplant surgeons, nephrologists and anesthesiologists approved every case before schedule. Informed consent from all parties involved into the transplant process was obtained before the procedure.

Full table
Surgical technique
All robotic assisted donor nephrectomies were performed by two board certified urologic and transplant surgeon specialists using the da Vinci® Si Surgical System. As described earlier (17), the patient was placed in a left or right 45° lateral decubitus position, depending on the nephrectomy side. Following the team-timeout, under general anesthesia and sterile conditions, the 12mm camera trocar was placed on the hemiclavear line, next to the umbilicus. Capnoperitoneum was maintained at 12 mmHg. The 3 trocars (8 mm) for the robotic arms were subsequently placed in an imaginary semicircular line equidistant to the camera. Another 12 mm trocar used by the assistant for suction-irrigation, introduction and removal of suture materials and clip application was placed in the lower lateral abdomen. The “docking maneuver”, which virtually means connecting the robotic arms with all trocars, was performed after the robot was positioned lateral at the backside of the donor. Standard instruments used for all operations comprised hot shears (MSC), fenestrated bipolar forceps, Cadiere forceps and a large SutureCut needle driver (Endowrist®, Intuitive Surgical).
In short, for left donor nephrectomy (LDN) the descending colon was mobilized and displaced medially to allow opening of the Gerota’s fascia. In a first step the ureter was identified at the level of the iliac axis and dissected free up to the lower pole of the kidney preserving the periureteral tissue commonly known as “golden triangle”. After dissection of the lateral and posterior region of the kidney the renal vein was dissected free until its outlet into the inferior vena cava, and its branches (lumbar, gonadal and adrenal) are clipped and transected free. In a subsequent step the renal artery was identified and dissected free up to the level of its aortic origin. When the kidney was completely mobilized the ureter got clipped with one hemlock clip and was divided leaving the clip in situ. Subsequently the kidney was placed into an endobag for extraction. Before removal of the wrapped kidney through a Pfannenstiel or Rutherford-Morrison incision, the renal artery and renal vein was either double clipped with locking hemlock clips (Video 1) or addressed by one locking hemlock clip and one non-transfixing ligature (Vicryl 3-0).
For right donor nephrectomy (RDN), in a similar fashion the ascending colon was mobilized and displaced medially to allow access to the Gerota’s fascia and perirenal fat. In contrast to the left side the renal vascular stalk, especially the right renal vein is shorter. Ligation and clipping close to the inferior vena cava is even more crucial for a simple and successful subsequent transplant procedure. Again, the kidney was extracted manually in an endo-bag and afterwards flushed with 200 mL of heparinized ice-cold perfusion solution (Custodiol®). Before wound closure the renal bed and vascular stumps were inspected for bleeding. All trocars were removed under direct vision during evacuation of the pneumoperitoneum and occasionally a 12 Chr. Robinson drain was inserted into the wound bed depending on the surgeon’s decision.
Data analysis
Data collection was performed retrospectively in context with quality inspection for all 40 consecutive RANLD. Evaluated donor parameters included, relationship to the recipient, gender, age, BMI, preoperative creatinine and GFR CDK EPI as well as vascular supply of the organ selected for donation and transplantation. Data related to the surgical procedure included operation time (operation time was defined as time between skin incision and placement of the last skin suture), warm ischemia time during the donation operation(warm ischemia time was defined as the time from renal artery clipping to the beginning of back-table perfusion), intra- and postoperative complications in terms of Clavien-Dindo classification as well as the pre- and postoperative laboratory parameters including serum creatinine (µmol/L) and glomerulus filtration rate (mL/min/1.73 m2) which were estimated using the CDK-EPI-formula. Laboratory parameters were evaluated preoperatively and at date of discharge. Furthermore, days of intermediate care unit (ICU) and length of hospital stay (LOS) were assessed.
Data analysis was performed using descriptive statistics. Continuous variables were reported as mean values ± standard deviation (SD). SD was calculated using Microsoft® Excel (Redmond, WA, USA). Categorical variables were presented as the absolute values and/or percentage of the group. Statistical analysis was performed using Graph Pad Software (La Jolla, CA, USA) and statistical significance was calculated using Student’s t test, with significance defined as P<0.05.
Results
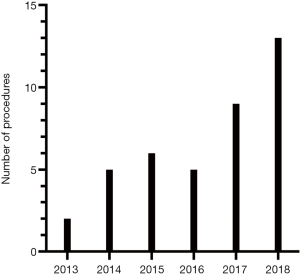
From November 2013 till August 2018, forty consecutive RANLD were performed at our center. Within this time period an exponential increase of donation surgeries could be detected (Figure 1). A number of 13 cases alone was performed within the last 8 months, which represents 32.5% of the total patient volume. Sex distribution among donors was n=21 (52.5%) women and n=19 (47.5%) men, with a donor’s average age of 53.075±11.68 years (range, 28–70) and an average body mass index of 25.99±3.58 kg/m2 (range, 19.9–35.3 kg/m2). All donors were Caucasian. Relation to the recipient was distributed in parent (n=18, 45%), sibling (n=5, 12.5%), spouse (n=9, 22.5%) and children (n=1, 2.5%) for direct relation as well as other (n=7, 17.5%) for a very close relationship. Preoperative laboratory parameters showed average creatinine values of 72.7±11.95 µmol/L and a mean glomerular filtration rate of 92.48±12.46 mL/min/1.73 m2 (see all Table 2).
Full table
A total of n=21 (52.5%) left and n=19 (47.5%) right kidneys were used for donation. Hereof n=31 (77.5%) kidneys had a single arterial vascular supply and n=9 (22.5%) a multiple (seven donors with 2 arteries and two with 3 arteries). Venous vascular supply was as follows: n=37 (92.5%) single and n=3 (7.5%) multiple (maximum of two veins) (see all Table 3). Eighteen cases, (including all cases with multiple vessel supply) were operated with double clipping of renal vessels using locking hemlock clips and in 22 cases vessels were addressed with one locking hemlock clip and one Vicryl ligature (Vicryl 3-0). No significant difference in warm ischemia time (WIT; defined as time from renal artery closure to cold perfusion) was evident in both groups (WIT double clipping: 3.9±1.2 min vs. clip + ligature: 3.8±1.5 min, P=n.s.).

Full table
Overall mean operative time was 150.75±27.30 min with no individual difference between the two surgeon specialists (surgeon A with n=18 RANLD and a mean operation time of 153.11±30.92 min, surgeon B with n=22 RANLD with a mean operative time of 148.8±24.53 min, P=0.636). Also, the mean operative time for patients with single 150.9±26.99 min or multiple 150.27±28.99 min vascular supply (arterial and or venous) showed no significant difference (P=0.949) (no figure provided).
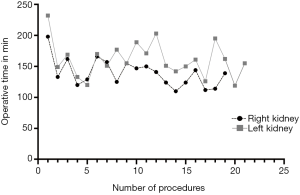
Interestingly a significant difference in operation time could be detected when looking at the donation side. Right donor nephrectomy (139±22 min) was performed significantly faster than left (160.95±27.93 min) (P=0.01) (Figure 2).
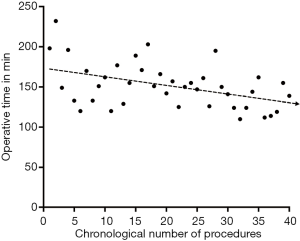
Total operation time gradually decrease with the numbers of robotic donor nephrectomies performed (Figure 3). There was also a significant decrease in operation time when comparing the first 20 RANLD (161±29.16 min) with the last 20 cases (140.5±21.38 min) (P=0.016).
Average warm ischemia time (WIT) was 2.25±0.63 min. 30 patients received an intraoperative drainage (Redon, 16 Charr), placed in the nephrectomy loge. First eight Patients were transferred to the ICU postoperatively for safety reasons. The mean length of stay was 1.25±0.54 days (range, 0–2). Average length of hospital stay was 7.25±3.59 days (range, 4–21). Mean value of postoperative serum creatinine at day of discharge was 114.05±18.42 µmol/L and average GFR was 55.89±11.17 mL/min/1.73 m2.
No conversion to hand assisted, laparoscopic or total open surgery was necessary. There were no intraoperative complications. A number of five (12.5%) postoperative complications were identified. Clavien-Dindo grade I complications occurred in n=2 (5%), lymphatic fistula, urinary tract infection), grade IIIa complications occurred in n=1 (2.5%) (abscess of the trocar insertion site) and grad IIIb complications occurred in n=2 (5%) n=1 fascial dehiscence and n=1incisional hernia). Grad IV and V complications did not occur (see all Table 4).

Full table
Similar to results of operative time, there is a small difference for complication rate between the first 20 (n=3, 7.5%) and the second 20 (n=2, 5%) cases. Donor mortality was 0%. Thirteen kidneys (32.5%) were transplanted across the AB0 barrier, requiring therapeutic apheresis as well as T and B cell directed immunosuppressive therapy for the recipient.
Discussion
Kidney transplantation is the treatment of choice for patients with end-stage renal disease (23). In particular, live kidney donation has been shown to be superior to deceased donor kidney transplantation mainly in terms of long-term benefits and better graft survival (24,25). In Germany, living donor kidney transplantation is currently available in 37 transplant centers, and 39% of all kidney transplants in 2018 were living donor related (www.DSO.org).
As surgical robots begin to diversify into all different fields of surgery worldwide, innovations in robotic surgical procedures continuously strive to improve patients’ outcome, including the field of transplant surgery.
Our data support the evidence of a limited number of articles highlighting the feasibility and safety of robotic assisted living kidney donation (13,26). The majority of European, American and Asian transplant centers performing living donor kidney transplantation offer their patients a minimally invasive donor organ procurement strategy (27,28). The elective type surgery allows transplantation of kidney grafts with excellent function to recipients under optimal conditions. Living kidney donation furthermore allows to reduce wait time to transplantation and time on dialysis and should therefore additionally be propagated in the field of transplant surgery. As described earlier (20-22), older age and comorbidities such as moderate hypertension and a BMI >30 kg/m2 are no contraindications at our center and have again proven safe outcomes for the donor.
Interestingly the introduction of robotic assisted donor surgery has furthermore propelled the living kidney donation program in our center. A similar effect has already been described by Oberholzer et al. for the kidney transplantation program at the University of Illinois at Chicago (3). In 2001 for the first time the number of living kidney donors has exceeded the number of transplantations with organs from deceased donors in several specialized transplant centers (5,29). In 2018 almost 39% (n=638) of all kidneys transplanted (n=1,653) in Germany were living donor related (www.DSO.org). This mirrors our numbers of living donations which have almost quintupled within the last 5 years since our debut of robotic assisted donor surgery.
Renal scintigraphy and volumetry for split renal function assessment are key decision parameters for donor side selection, which generally falls for the side with inferior function. Accurate preoperative evaluation of renal vascular anatomy using CT angiography (30) or MRI imaging (31) is vital for successful and safe postoperative outcomes. As a principle we selected the kidney with inferior function and smaller number of vessels. If split renal function was well balanced, we generally selected the left kidney for anatomical reasons which are mainly the longer renal vein. We share the idea that in contrast to early publications, kidney grafts with multiple vessels are no longer considered as a relative contraindication for living donation (32). In this context our cohort included an amount of 27.5% donor organs with multiple (n=2 or n=3) renal arteries, all originating from the aorta. In case of a significant difference of split renal function (more than 5% difference) we did not hesitate to select the qualitatively inferior kidney for donation, even if the number of vessels was increased.
Although uncommon, major vascular complications during minimally invasive donor nephrectomy can be potentially fatal (10). Based on data from laparoscopic donor nephrectomies, it has been postulated that vascular clips alone are inadequate for safe closure of the main renal artery and vascular staplers would be saver due to transfixion of the vessel wall (27). In 2006 the US Food and Drug administration (FDA) and the manufacturer of the hemlock clip, Teleflex, as a reaction to two death of living kidney donors, announced a recall stating that the use of hemlock clips in laparoscopic donor nephrectomy is contraindicated. In addition, three live kidney donors, two form the United States and one from India reportedly died after the release of the statement and several other donors obviously suffered severe hemorrhagic events from clipping failures during laparoscopic kidney donation (11). This is a substantial dilemma, and we strongly believe that the incidence of fatal vascular complications in living kidney donation is even under-reported. By now, from all vessel sealing devices and techniques that were used for laparoscopic kidney donation [intracorporal knot tying (33), bipolar vascular sealing devices (34) ultrasonic shears (35)] staplers and clips still remain the prevalent techniques used for vascular control (36). The reason why staplers may not have entirely replaced clips in this surgical scenario may be the fact that stapler use is itself is associated with a considerable amount of malfunction (37). In addition to malfunction (38) stapler use in laparoscopic kidney donation is furthermore associated with donor vessel length shortening (39) and increased operative costs (40).
In our patients, we either used two locking hemlock clips, or a combination of one locking hemlock clip and one Vicryl 3-0 ligature for renal artery closure. Informed consent on this technique was obtained by all patients. Our strategy is supported by a recent metanalysis on vessel controlling devices for renal pedicle ligation in laparoscopic live donor nephrectomy, which revealed no significant differences between hemlock clips and staplers with regard to device failure rate, death rate and severe hemorrhage rate (41). Our strategy utilizes the full capacity of the robot, namely enhanced 3-D vision and when indicated, the ability of delicate intracorporal tying. This method does not waste significant millimeters of vessel length when compared to vascular stapling and seems to be cost effective. When compared to the robotic clip applicator, the conventional re-usable laparoscopic applicator proved sufficient, allowing for even more cost savings in our setup.
Furthermore, the simultaneous use of two laparoscopic clip applicators, one used for clipping and another one ready to be applied might furthermore reduce warm ischemia time, since the repeat loading of one robotic clip applicator might be more time consuming.
In case of multiple vascular supply, the individual vessel diameter trends to be smaller. Under these circumstances our strategy of double clipping proved to be save. Our indigenous technique furthermore does not significantly delay the operation time or increase warm ischemia time. We believe that vessel length might be of crucial importance in case of multiple artery supply, requiring microvascular reconstruction before transplantation. Reduction of material costs in terms of saving expenses for stapling devices, might in our situation furthermore compensate for the current global higher costs associated with robotic surgery (42).
In general, robotic operations are predominantly performed by two surgeons, one handling the robotic console and the second one scrubbed in the operating field for assistance. In contrast, fully laparoscopic, laparoscopic hand assisted, or open donor nephrectomies may require up to three surgeons depending on the strategy and technique.
In comparison to previous studies (13,17) our mean operative time was relatively low. This is probably due to the fact, that both surgeons have a work experience with the robotic platform of more than 10 years, having completed more than 400 complex robotic assisted kidney surgeries each. Still a learning curve was evident after the first 10 operations for each surgeon, resulting in a decrease of operation time and patient morbidity. In parallel, steep learning curves have also been reported for both minimally invasive retroperitoneoscopic and laparoscopic donor nephrectomies (43). 3-D vision which is not only exclusively restricted to robotic assisted operations, but also broadly available for laparoscopic procedures in general seem to reduce operation time and warm ischemia times when compared to 2-D techniques (44).
Interestingly multiple vessel supply did not have a significant impact on operation time or warm ischemia time, nor did it have an impact on graft survival or morbidity after transplantation (data not shown). This supports our strategy of vessel occlusion, using a combination of both ligatures and clips.
A new surgical technique must always be compared to the gold standard. RALDN has been reported to be feasible and save, although operation time and warm ischemia time in some studies may be slightly longer when compared to laparoscopic kidney donation (17,45). A recent study however, revealed that skin to skin time of RALDN approached that of laparoscopic which each subsequent procedure, and the standard operation time of the laparoscopic kidney donation procedure could be reached at the 22nd case (46). Longer operation times might hypothetically be explained with an initial cautious and slower approach of the surgeon to an unfamiliarly procedure (47).
Our study presented here is not devoid of limitations, which are mainly a small sample size and the retrospective character, which has to be taken into consideration. We used a total of six trocars for the robotic procedure which is two more than in the average laparoscopic procedure. In addition to the 4 robotic trocars (three working trocars and one camera trocar) our additional two trocars are necessary for simultaneous suction irrigation and clip application. Admittedly we also have a fairly high complication rate, especially when it comes to fascia dehiscence and early hernia development. This might be directly related to the Rutherford Morrison incision, since no complications developed when a Pfannenstiel incision was used for kidney extraction.
However, to date no data using explicitly hemlock clips or ligatures for renal vascular control in robotic assisted living kidney donation have been published. Further prospectively randomized studies involving robotic surgery in living kidney donation are in demand.
We consider the robotic assisted nephrectomy for living kidney donation as the method of choice for the experienced robotic surgeon which incorporates lowest donor risks and highest reproducibility rates. Vascular closure with two locking clips or a combination of one locking clip and one ligature have been proven save and feasible using the robotic platform. Laparoscopic or hand-assisted laparoscopic techniques may always be taken into consideration for selected cases or at institutions where a robotic device is currently not available.
Acknowledgments
We acknowledge support from the German Research Foundation (DFG) and Leipzig University within the program of Open Access Publishing.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the local ethics committee of the University of Leipzig (AZ: Nr: 111–16-14,032,016). Informed consent from all parties involved into the transplant process was obtained before the procedure.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ratner LE, Ciseck LJ, Moore RG, et al. Laparoscopic live donor nephrectomy. Transplantation 1995;60:1047-9. [PubMed]
- Segev DL. Innovative strategies in living donor kidney transplantation. Nat Rev Nephrol 2012;8:332-8. [Crossref] [PubMed]
- Oberholzer J, Testa G, Sankary H, et al. Kidney transplantation at the University of Illinois at Chicago from 1988-2004. Clin Transpl 2004.143-9. [PubMed]
- Black CK, Termanini KM, Aguirre O, et al. Solid organ transplantation in the 21(st) century. Ann Transl Med 2018;6:409. [Crossref] [PubMed]
- Schweitzer EJ, Wilson J, Jacobs S, et al. Increased rates of donation with laparoscopic donor nephrectomy. Ann Surg 2000;232:392-400. [Crossref] [PubMed]
- Pradel FG, Limcangco MR, Mullins CD, et al. Patients' attitudes about living donor transplantation and living donor nephrectomy. Am J Kidney Dis 2003;41:849-58. [Crossref] [PubMed]
- Shockcor NM, Sultan S, Alvarez-Casas J, et al. Minimally invasive donor nephrectomy: current state of the art. Langenbecks Arch Surg 2018;403:681-91. [Crossref] [PubMed]
- Hubert J, Renoult E, Mourey E, et al. Complete robotic-assistance during laparoscopic living donor nephrectomies: an evaluation of 38 procedures at a single site. Int J Urol 2007;14:986-9. [Crossref] [PubMed]
- Janki S, Dor FJ, IJzermans JN. Surgical aspects of live kidney donation: an updated review. Front Biosci (Elite Ed) 2015;7:346-65. [PubMed]
- Friedman AL, Peters TG, Jones KW, et al. Fatal and nonfatal hemorrhagic complications of living kidney donation. Ann Surg 2006;243:126-30. [Crossref] [PubMed]
- Friedman AL, Peters TG, Ratner LE. Regulatory failure contributing to deaths of live kidney donors. Am J Transplant 2012;12:829-34. [Crossref] [PubMed]
- Unterberg SH, Patel SH, Fuller TW, et al. Robotic-assisted Proximal Perineal Urethroplasty: Improving Visualization and Ergonomics. Urology 2019;125:230-3. [Crossref] [PubMed]
- Giacomoni A, Di Sandro S, Lauterio A, et al. Evolution of robotic nephrectomy for living donation: from hand-assisted to totally robotic technique. Int J Med Robot 2014;10:286-93. [Crossref] [PubMed]
- Horgan S, Vanuno D. Robots in laparoscopic surgery. J Laparoendosc Adv Surg Tech A 2001;11:415-9. [Crossref] [PubMed]
- Horgan S, Vanuno D, Sileri P, et al. Robotic-assisted laparoscopic donor nephrectomy for kidney transplantation. Transplantation 2002;73:1474-9. [Crossref] [PubMed]
- Kortram K, Ijzermans JN, Dor FJ. Perioperative Events and Complications in Minimally Invasive Live Donor Nephrectomy: A Systematic Review and Meta-Analysis. Transplantation 2016;100:2264-75. [Crossref] [PubMed]
- Janki S, Klop KWJ, Hagen SM, et al. Robotic surgery rapidly and successfully implemented in a high volume laparoscopic center on living kidney donation. Int J Med Robot 2017. [Crossref] [PubMed]
- Simorov A, Otte RS, Kopietz CM, et al. Review of surgical robotics user interface: what is the best way to control robotic surgery? Surg Endosc 2012;26:2117-25. [Crossref] [PubMed]
- Lentine KL, Kasiske BL, Levey AS, et al. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation 2017;101:S1-109. [Crossref] [PubMed]
- Klop KW, Dols LF, Weimar W, et al. Quality of life of elderly live kidney donors. Transplantation 2013;96:644-8. [Crossref] [PubMed]
- Ahmadi AR, Lafranca JA, Claessens LA, et al. Shifting paradigms in eligibility criteria for live kidney donation: a systematic review. Kidney Int 2015;87:31-45. [Crossref] [PubMed]
- Dols LF, Kok NF, Roodnat JI, et al. Living kidney donors: impact of age on long-term safety. Am J Transplant 2011;11:737-42. [Crossref] [PubMed]
- Shapiro R. End-stage renal disease in 2010: Innovative approaches to improve outcomes in transplantation. Nat Rev Nephrol 2011;7:68-70. [Crossref] [PubMed]
- Innocenti GR, Wadei HM, Prieto M, et al. Preemptive living donor kidney transplantation: do the benefits extend to all recipients? Transplantation 2007;83:144-9. [Crossref] [PubMed]
- Davis CL, Delmonico FL. Living-donor kidney transplantation: a review of the current practices for the live donor. J Am Soc Nephrol 2005;16:2098-110. [Crossref] [PubMed]
- Giacomoni A, Di Sandro S, Lauterio A, et al. Robotic nephrectomy for living donation: surgical technique and literature systematic review. Am J Surg 2016;211:1135-42. [Crossref] [PubMed]
- Tzvetanov I, Bejarano-Pineda L, Giulianotti PC, et al. State of the art of robotic surgery in organ transplantation. World J Surg 2013;37:2791-9. [Crossref] [PubMed]
- Merlin TL, Scott DF, Rao MM, et al. The safety and efficacy of laparoscopic live donor nephrectomy: a systematic review. Transplantation 2000;70:1659-66. [Crossref] [PubMed]
- Schnitzler MA, Whiting JF, Brennan DC, et al. The expanded criteria donor dilemma in cadaveric renal transplantation. Transplantation 2003;75:1940-5. [Crossref] [PubMed]
- Raman SS, Pojchamarnwiputh S, Muangsomboon K, et al. Utility of 16-MDCT angiography for comprehensive preoperative vascular evaluation of laparoscopic renal donors. AJR Am J Roentgenol 2006;186:1630-8. [Crossref] [PubMed]
- Blankholm AD, Pedersen BG, Ostrat EO, et al. Noncontrast-Enhanced Magnetic Resonance Versus Computed Tomography Angiography in Preoperative Evaluation of Potential Living Renal Donors. Acad Radiol 2015;22:1368-75. [Crossref] [PubMed]
- Hung CJ, Lin YJ, Chang SS, et al. Kidney grafts with multiple renal arteries is no longer a relative contraindication with advance in surgical techniques of laparoscopic donor nephrectomy. Transplant Proc 2012;44:36-8. [Crossref] [PubMed]
- Chon CH, Chung SY, Ng CS, et al. Looped silk tie: surgical technique for management of the renal vein during laparoscopic live-donor nephrectomy. J Endourol 2005;19:401-5. [Crossref] [PubMed]
- Constant DL, Florman SS, Mendez F, et al. Use of the LigaSure vessel sealing device in laparoscopic living-donor nephrectomy. Transplantation 2004;78:1661-4. [Crossref] [PubMed]
- Landman J, Kerbl K, Rehman J, et al. Evaluation of a vessel sealing system, bipolar electrosurgery, harmonic scalpel, titanium clips, endoscopic gastrointestinal anastomosis vascular staples and sutures for arterial and venous ligation in a porcine model. J Urol 2003;169:697-700. [Crossref] [PubMed]
- McGregor TB, Patel P, Chan G, et al. Hilar control during laparoscopic donor nephrectomy: Practice patterns in Canada. Can Urol Assoc J 2017;11:321-4. [Crossref] [PubMed]
- Chan D, Bishoff JT, Ratner L, et al. Endovascular gastrointestinal stapler device malfunction during laparoscopic nephrectomy: early recognition and management. J Urol 2000;164:319-21. [Crossref] [PubMed]
- Breda A, Veale J, Liao J, et al. Complications of laparoscopic living donor nephrectomy and their management: the UCLA experience. Urology 2007;69:49-52. [Crossref] [PubMed]
- Chueh SC, Wang SM, Lai MK. Use of Hem-o-lok clips effectively lengthens renal vein during laparoscopic live donor nephrectomy. Transplant Proc 2004;36:2623-4. [Crossref] [PubMed]
- Siqueira TM Jr, Mitre AI, Simoes FA, et al. A cost-effective technique for pure laparoscopic live donor nephrectomy. Int Braz J Urol 2006;32:23-8; discussion 28-30. [Crossref] [PubMed]
- Liu Y, Huang Z, Chen Y, et al. Staplers or clips?: A systematic review and meta-analysis of vessel controlling devices for renal pedicle ligation in laparoscopic live donor nephrectomy. Medicine (Baltimore) 2018;97:e13116. [Crossref] [PubMed]
- Herron DM, Marohn M. SAGES-MIRA Robotic Surgery Consensus Group. A consensus document on robotic surgery. Surg Endosc 2008;22:313-25; discussion 311-2. [Crossref] [PubMed]
- Serrano OK, Bangdiwala AS, Vock DM, et al. Defining the Tipping Point in Surgical Performance for Laparoscopic Donor Nephrectomy Among Transplant Surgery Fellows: A Risk-Adjusted Cumulative Summation Learning Curve Analysis. Am J Transplant 2017;17:1868-78. [Crossref] [PubMed]
- Wahba R, Kleinert R, Hellmich M, et al. Optimizing a living kidney donation program: transition to hand-assisted retroperitoneoscopic living donor nephrectomy and introduction of a passive polarizing three-dimensional display system. Surg Endosc 2017;31:2577-85. [Crossref] [PubMed]
- Serrano OK, Kirchner V, Bangdiwala A, et al. Evolution of Living Donor Nephrectomy at a Single Center: Long-term Outcomes With 4 Different Techniques in Greater Than 4000 Donors Over 50 Years. Transplantation 2016;100:1299-305. [Crossref] [PubMed]
- Yang A, Barman N, Chin E, et al. Robotic-assisted vs. laparoscopic donor nephrectomy: a retrospective comparison of perioperative course and postoperative outcome after 1 year. J Robot Surg 2018;12:343-50. [Crossref] [PubMed]
- Creta M, Calogero A, Sagnelli C, et al. Donor and Recipient Outcomes following Robotic-Assisted Laparoscopic Living Donor Nephrectomy: A Systematic Review. Biomed Res Int 2019;2019:1729138.