Association between lipoprotein(a) concentration and the risk of stroke in the Chinese Han population: a retrospective case-control study
Introduction
Stroke has become the second leading cause of disease burden worldwide and the first leading cause of disability among Chinese populations (1). Chinese populations have a higher overall incidence of stroke and greater proportion of hemorrhagic stroke than Caucasian populations. Despite a greater incidence of ischemic stroke, hemorrhagic stroke contributes to a similar number of deaths in China (2). Conventional risk factors include hypertension, diabetes, and smoking; however, approximately 25% of all ischemic strokes lack a clear cause (3). Therefore, it is important to identify other potential stroke risk factors.
Lipoprotein (a) [Lp(a)] is formed from a low-density lipoprotein (LDL)-like particle and the glycoprotein, apolipoprotein(a) [apo(a)], linked to apolipoprotein B in the LDL by a single disulphide bond (4). Lp(a) plays a role in atherogenic and pro-thrombotic/anti-fibrinolytic processes (5,6). In addition, studies have reported roles in inflammatory responses, binding of oxidized phospholipids, and vascular remodeling (7).
A causal association between elevated Lp(a) and increased risk of myocardial infarction has been proved (8); however, there are conflicting reports regarding the relationship between stroke risk and Lp(a) concentration. Several meta-analyses with large samples have shown that Lp(a) is a risk factor for ischemic stroke (9-11). However, a systematic literature review with 16 prospective studies found no significant effect of Lp(a) on stroke risk (12). Additionally, the concentration of serum Lp(a) is determined by genetic factors primarily and differs substantially across ethnicities. Few studies have assessed the effects of Lp(a) on stroke in Asian populations. Moreover, previous studies have used unclassified stroke or ischemic stroke patients only; fewer studies have assessed the effect of Lp(a) on hemorrhagic stroke. Therefore, we sought to detect the association between Lp(a) and ischemic or hemorrhagic stroke risk in this case-control study in the Chinese Han population.
Methods
Study participates
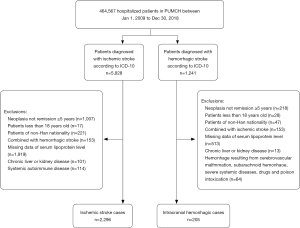
This was a retrospective case-control study assessing the relationship between serum Lp(a) and ischemic and hemorrhagic stroke risk in the Chinese Han population. Cases were extracted from administrative data from the Peking Union Medical College Hospital (PUMCH), a national comprehensive hospital. Patients >18 years old were eligible for inclusion if they had been diagnosed with ischemic (International Classification of Diseases, 10th edition (ICD-10, code I63) or hemorrhagic stroke (ICD-10; codes I61, I62) between January 1, 2009 and December 31, 2018. Patients with transient ischemic attack were excluded.
All diagnostic information and laboratory data were obtained from discharge files and the hospital’s electronic laboratory database. Exclusion criteria were non-Han nationality, malignant tumor with ≤5 years remission, chronic liver disease (>3-fold transaminase elevation), chronic kidney disease (serum creatinine, ≥152.5 µmol/L), and systemic autoimmune disease. For patients with multiple hospitalizations, we included records during hospitalization for the first stroke diagnosis only. Eligible healthy individuals without stroke history were selected from the health examination center of PUMCH during the same period with the same exclusion criteria. Control participants were selected and matched by age (±5 years) and gender for the ischemic stroke (1:1 ratio) and hemorrhagic (1:2 ratio) group, respectively. The study protocol was approved by the Ethics Committee of the PUMCH who waived the requirement for informed consent because this study used anonymized data in a retrospective analysis.
Hypertension was defined as the use of antihypertensive drugs or with a diagnosis in the medical records. Diabetes (DM) was diagnosed when the percentage of hemoglobin A1c (HbA1c%) was >6.5%, use of glucose-lowering drugs, or with a diagnosis in the medical records. Coronary atherosclerotic heart disease (CAD) refers to those with a record of coronary atherosclerotic stenosis or occlusion, or coronary stent implantation. Hyperlipidemia was defined as LDL >4.1 mmol/L, HDL <1.0 mmol/L, or the use of lipid-lowering medication (13).
Lp(a) measurement
Lp(a) and other plasma biochemical parameters were analyzed in the Department of Laboratory Medicine at PUMCH and archived data were identified using computer retrieval. Blood samples were obtained after a 12-hour overnight fast and centrifuged at 3,500 r/min for 15 min after standing for 30 min. The concentration of serum Lp(a) was measured using the latex agglutination turbidimetric method with mouse monoclonal anti-Lp(a) antibody (Beckman AU5800 automatic biochemistry, Sekisui Medical Company, Tokyo, Japan). The detection range was 0–100 mg/dL with a normal value of below 30 mg/dL. The intra- and inter-assay coefficients of variation (CV) were below 5.0% and 10%, respectively. Triglyceride (TG), total cholesterol (TC), LDL cholesterol (LDL-c), and homocysteine (HCY) were measured using an enzymatic method. High density lipoprotein cholesterol (HDL-c) was determined by direct methods. Hypersensitive C-reactive protein (hsCRP) was detected using immune turbidimetric analysis. All plasma biochemical parameters were assayed by an automatic biochemistry analyzer (AU5800, Beckman Coulter, Brea, CA).
Statistical analysis
Data are presented as mean ± standard deviation (SD) for continuous normally distributed variables and median with interquartile ranges for skewed distributed variables. Student’s t-test was applied to compare normally distributed variables or skewed distributed variables after logarithm transformation, and Pearson’s χ2 test was applied for categorical comparisons. A common logarithm (Lg) transformation was applied for the skewed distribution of Lp(a). Further, analysis was undertaken to assess the relationship between quartiles of Lg-transformed Lp(a) levels and stroke risk. Univariate and multivariate conditional logistic regression models for matched case-control data were used to calculate the odds ratios (ORs) and 95% confidence intervals (95% CI) of Lg-transformed Lp(a) as a categorical variable. We adjusted for diabetes, hyperlipidemia, and hypersensitive CRP. In addition, we also adjusted for variables that changed the matched odds ratio by at least 10% when added to this model. Interaction and stratified analyses were conducted by age (<55 and ≥55 years) and sex. A two-tailed P value of <0.05 was considered significant. All statistical analyses were performed with R software, EmpowerStats (X&Y Solutions, Boston, MA) and SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Clinical characteristics
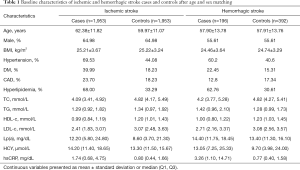
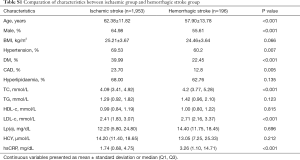
Among the 464,567 hospitalizations in the study cohort, we enrolled 2,296 and 205 ischemic and hemorrhagic stroke patients, respectively, based on the inclusion and exclusion criteria (Figure S1). We randomly selected 1,953 and 392 age- and sex-matched healthy controls for the ischemic and hemorrhagic groups, respectively. The characteristics of the controls are presented in Table 1. The ischemic group had a higher mean age and higher proportion of males compared with the hemorrhagic group. The proportions of DM and CAD were higher in the ischemic group. The median Lp(a) concentration in the two groups was similar (Table S1). Cases and controls were similar in age, sex and BMI in the ischemic group. There was a greater burden of hypertension, diabetes, cardiovascular disease, and hyperlipidemia, in ischemic stroke cases compared with controls. In addition, data showed lower median HDL, LDL, total cholesterol, and triglyceride levels and higher homocysteine levels in ischemic stroke cases when compared with controls. A similar distribution was found in the hemorrhagic group, except that the hemorrhagic cases showed lower CAD ratios and higher triglyceride levels when compared with controls.
Full table
Full table
Lp(a) and ischemic stroke risk
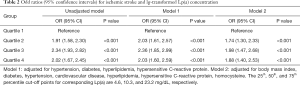
The median concentration of Lp(a) was 12.2 and 8.60 mg/dL in the ischemic stroke and control groups, respectively (Table 1). We used conditional logistic regression models to assess whether Lp(a) was independently associated with ischemic stroke (Table 2). After adjustment for hypertension, diabetes, hyperlipidemia, and hsCRP, we found that lg-transformed Lp(a) was significantly associated with ischemic stroke with odd radios (ORs) and 95% confidence intervals (CI) for lg-transformed Lp(a) quartiles 4, 3, and 2 (vs. quartile 1 as a reference) of 2.03 (1.61, 2.57), 2.36 (1.85, 2.99) and 2.03 (1.60, 2.59), respectively. After further adjustment for BMI, cardiovascular disease, and homocysteine on Model 2, the relationship persisted (all P values for the log-transformed Lp(a) quartiles 2, 3, and 4 vs. quartile 1 were <0.001). In the stratified analysis, we found a significant association between elevated Lg-transformed Lp(a) and ischemic stroke in men. No statistically significant association was found in women in the Model adjusted for hypertension, hyperlipidemia, and hsCRP; and there was a significant interaction (P=0.006). Interestingly, younger patients (<55 years) showed greater increased risk than older patients for ischemic stroke in the multivariable analysis, and no significant differences for interactions were detected (Figure 1).
Full table
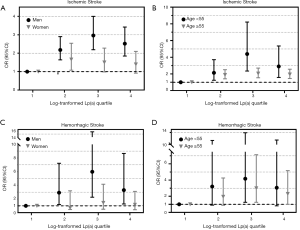
Lp(a) and hemorrhagic stroke risk
There was a higher median Lp(a) concentration in the hemorrhagic group (14.40 mg/dL) compared with the control group (13.40 mg/dL; Table 1). The association between log-transformed Lp(a) and hemorrhagic stroke was assessed using conditional logistic regression models (Table 3). Compared with patients in the lowest quartile, the ORs and 95%CIs for log-transformed Lp(a) quartiles 2–4 were 1.88 (1.08, 3.12), 2.46 (1.43, 4.25) and 2.18 (1.28, 3.72), respectively, in the unadjusted model. Additional adjustment for hypertension, hyperlipidemia, and hsCRP yielded similar results [OR =1.93 (1.02, 3.67), 3.24 (1.65, 6.39), and 2.19 (1.12, 4.29) for quartiles 2–4, respectively, vs. the reference quartile 1, P=0.043, P<0.001 and P=0.022]. The correlation between Lp(a) level and the risk of hemorrhagic stroke also survived full adjustment for diabetes, hypertension, cardiovascular disease, hyperlipidemia, and hsCPR in Model 2. The stratified analysis that adjusted for hypertension, hyperlipidemia, and hsCPR was conducted according to sex and age. Similar to the ischemic stroke, elevated Lg-transformed Lp(a) concentration was associated with a higher risk of hemorrhagic stroke in men alone, after adjusting for potential confounding factors. Increased hemorrhagic stroke risk was associated with elevated Lg-transformed Lp(a) in both younger (<55 years) and older patients (≥55 years), but the effects were smaller in the older group (Figure 1).

Full table
Discussion
In this retrospective case-control study, we identified the associations between elevated Lp(a) and ischemic or hemorrhagic stroke risk compared with the age- and gender-matched healthy controls. Furthermore, we found an increased stroke risk associated with Lp(a) among younger patients. Interestingly, the effect of elevated Lp(a) on both stroke types was significant among men alone. Surprisingly, LDL-c, TC, and TG levels were lower in the stroke groups when compared with the controls, which may be due to the higher proportion of dyslipidemia in the stroke group with a lower proportion and target value for lipid-lowering treatment.
The effect of serum Lp(a) level on the risk of coronary heart disease is well-established; however, the effects on stroke risk remain controversial. Our results correspond with several previous studies on ischemic stroke. A meta-analysis of 126,634 individuals by the Emerging Risk Factors Collaboration demonstrated a significantly higher risk for ischemic stroke with a risk ratio (RR) of 1.10 (95% CI: 1.02–1.18) per 3.5-fold higher Lp(a) concentrations (9). However, >90% of the participants in this study were of European continental ancestry that attenuated the generalization to patients of all ethnicities. Nave et al. have summarized 20 articles comprising 90,904 cases and concluded that Lp(a) might be an independent risk factor for ischemic stroke with a pooled OR of 1.41 (95% CI: 1.26–1.57) for case-control studies and 1.29 (95% CI: 1.06–1.58) for prospective studies (10). In contrast, data from the China Kadoorie Biobank (CKB) nested case-control study showed no significant association between Lp(a) and ischemic stroke in a sample of 32,869 incident ischemic stroke cases (14). It should be noted that the female rate in the ischemic group was higher in the CKB study, which might reduce the intergroup differences because of gender differences of the association between Lp(a) and ischemic stroke. Overall, the different conclusions of studies assessing Lp(a) and ischemic stroke risk might be due to differences in study design, definition of vascular end points, and participant sampling. Another factor related to the discrepancy between these results is the long-term storage of samples, since the Lp(a) measurement could be less reliable if samples are stored for longer than 6 weeks (4).
Mechanisms underlying the effect of Lp(a) on vascular diseases remain unclear and require further research. Nevertheless, the number of studies that have explored the correlation between ischemic subtypes and Lp(a) is limited. It has been reported that serum Lp(a) is significantly higher in large-artery atherosclerosis compared with other stroke subtypes (15,16). Additionally, the association between large-artery atherosclerosis subtypes of ischemic stroke and Lp(a) has been supported by genetic variant analysis of the Lp(a) related gene, LPA (4). However, we were unable to stratify our patient group by ischemic stroke subtypes due to limitations in our data collection.
Most previous studies have focused on ischemic stroke or unclassified stroke. Our study also investigated the effect of Lp(a) on hemorrhagic stroke; however, the literature offers mixed results, ranging from negative to positive associations between Lp(a) and hemorrhagic stroke. Consistent with our results, a multicenter case-control study in Chinese patients with 499 events found increased Lp(a) levels led to a 1.64-fold increase (95% CI: 1.21–2.21) in the risk of hemorrhagic stroke (17). In contrast, results from a population-based prospective cohort study with 84 hemorrhagic stroke events suggest that decreased Lp(a) may be a predictive marker for future cerebral hemorrhage (18). A different study has reported no significant association between Lp(a) levels and hemorrhagic stroke (14). Furthermore, the Emerging Risk Factors Collaboration concluded that there was no significant association between Lp(a) and hemorrhagic stroke (adjusted RR: 1.06, 95% CI: 0.90–1.26) (9). The limited number of hemorrhagic events included in these studies might be a contributing factor for the inconsistent results. In addition, the causes of cerebral hemorrhage are varied and no study has detected a relationship with the stratification of hemorrhagic types, which might be another contributing factor.
In this study, we observed that the concentration of Lp(a) had a greater effect on ischemic and hemorrhagic stroke in men. The same trend was also observed in previous studies assessing ischemic stroke (10,19). This might be due to genetically predetermined differences in Lp(a) levels between sexes. Moreover, most studies did not adjust for differences in living habits, including smoking and dietary habits between the sexes. Studies that take into account sex differences in the association between Lp(a) and hemorrhagic stroke are lacking. Nonetheless, consistent with previous findings, we found a more pronounced association between Lp(a) and stroke risk in younger patients (10). A meta-analysis of four case-control studies also showed a positive association between elevated Lp(a) and stroke in children (OR: 4.24, 95% CI: 2.94–6.11) (20). This finding might be due to a lower absolute risk in younger patients was lower. Additionally, Lp(a) levels are closely linked to genetic factors (4), while environmental risk factors play an increasingly important role in stroke with age. Thus, Lp(a) might be a potential risk factor for stroke in young adults.
Compared with observational studies, studies assessing whether the therapeutic lowering of Lp(a) reduces stroke risk could provide powerful evidence. However, this is not easy because effective, targeted Lp(a)-lowering therapies are limited. The currently available therapies for lowering Lp(a) include lipid apheresis, niacin, inhibitors of proprotein convertase subtilisin/kexin type 9, and cholesteryl ester transfer protein inhibitors (21). Antisense oligonucleotides targeting Apo(a), including IONIS-APO(a)Rx and IONIS-APO(a)LRx, have shown reductions in Lp(a) by ~65% and 92–99%, respectively (22). A further study selected patients with high Lp(a) levels, and reported that isolated lowering of Lp(a) could lower the incidence rate of cardiovascular events (23). Further prospective studies are required to evaluate this therapeutic effect on stroke risk.
There are several limitations in our study. Firstly, as a case-control study, the results can only show associations between Lp(a) levels and stroke; however, and they do not allow any assessment of causality. Secondly, although the concentration of Lp(a) is relatively stable throughout life (24), its concentration collected after a stroke may not accurately reflect pre-stroke exposure. Thirdly, the time intervals between Lp(a) measurement and stroke were differed between individuals. We were also limited by the selection of controls, who were recruited from the health examination center rather than a population-based sample. Fourthly, studies have reported that statins increase Lp(a) levels (25), which could be a bias of this study. Lastly, this study did not analyze the effect of Lp(a) concentration on the risk of different ischemic stroke types according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST), which might diminish the actual absolute risk and limit the detection of a pathogenic mechanism for Lp(a).
In conclusion, our study found that the concentration of serum Lp(a) was independently associated with both ischemic and hemorrhagic stroke risk, especially among younger individuals in a Chinese Han population. Further, this effect was only significant among men. A powerful and specific Lp(a) lowering method is required to demonstrate causality and guide methods of stroke prevention.
Acknowledgments
Funding: This study was supported by the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS), Grant Number: 2016-I2M-1-002.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Ethics Committee of the PUMCH who waived the requirement for informed consent because this study used anonymized data in a retrospective analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1260-344. [Crossref] [PubMed]
- Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology 2013;81:264-72. [Crossref] [PubMed]
- Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429-38. [Crossref] [PubMed]
- Kronenberg F. Human Genetics and the Causal Role of Lipoprotein(a) for Various Diseases. Cardiovasc Drugs Ther 2016;30:87-100. [Crossref] [PubMed]
- Vogt A. Lipoprotein(a)-apheresis in the light of new drug developments. Atheroscler Suppl 2017;30:38-43. [Crossref] [PubMed]
- Cho T, Romagnuolo R, Scipione C, et al. Apolipoprotein(a) stimulates nuclear translocation of β-catenin: a novel pathogenic mechanism for lipoprotein(a). Mol Biol Cell 2013;24:210-21. [Crossref] [PubMed]
- Riches K, Porter KE. Lipoprotein(a): Cellular Effects and Molecular Mechanisms. Cholesterol. 2012;2012:923289. [Crossref] [PubMed]
- Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, et al. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 2009;301:2331-9. [Crossref] [PubMed]
- Emerging Risk Factors Collaboration, Erqou S, Kaptoge S, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009;302:412-23. [Crossref] [PubMed]
- Nave AH, Lange KS, Leonards CO, et al. Lipoprotein (a) as a risk factor for ischemic stroke: a meta-analysis. Atherosclerosis 2015;242:496-503. [Crossref] [PubMed]
- Zhang J, Du R, Peng K, et al. Serum lipoprotein (a) is associated with increased risk of stroke in Chinese adults: A prospective study. Atherosclerosis. 2019;289:8-13. [Crossref] [PubMed]
- Genser B, Dias KC, Siekmeier R, et al. Lipoprotein (a) and risk of cardiovascular disease--a systematic review and meta analysis of prospective studies. Clin Lab 2011;57:143-56. [PubMed]
- Beheshtian A, Shitole SG, Segal AZ, et al. Lipoprotein (a) level, apolipoprotein (a) size, and risk of unexplained ischemic stroke in young and middle-aged adults. Atherosclerosis 2016;253:47-53. [Crossref] [PubMed]
- Sun L, Clarke R, Bennett D, et al. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat Med 2019;25:569-74. [Crossref] [PubMed]
- Hong XW, Wu DM, Lu J, et al. Lipoprotein (a) as a Predictor of Early Stroke Recurrence in Acute Ischemic Stroke. Mol Neurobiol 2018;55:718-26. [Crossref] [PubMed]
- Aronis KN, Zhao D, Hoogeveen RC, et al. Associations of Lipoprotein(a) Levels With Incident Atrial Fibrillation and Ischemic Stroke: The ARIC (Atherosclerosis Risk in Communities) Study. J Am Heart Assoc 2017;6. [Crossref] [PubMed]
- Sun L, Li Z, Zhang H, et al. Pentanucleotide TTTTA repeat polymorphism of apolipoprotein(a) gene and plasma lipoprotein(a) are associated with ischemic and hemorrhagic stroke in Chinese: a multicenter case-control study in China. Stroke 2003;34:1617-22. [Crossref] [PubMed]
- Ishikawa S, Kotani K, Kario K, et al. Inverse association between serum lipoprotein(a) and cerebral hemorrhage in the Japanese population. Thromb Res 2013;131:e54-8. [Crossref] [PubMed]
- Boden-Albala B, Kargman DE, Lin IF, et al. Increased stroke risk and lipoprotein(a) in a multiethnic community: the Northern Manhattan Stroke Study. Cerebrovasc Dis 2010;30:237-43. [Crossref] [PubMed]
- Sultan SM, Schupf N, Dowling MM, et al. Review of lipid and lipoprotein(a) abnormalities in childhood arterial ischemic stroke. Int J Stroke 2014;9:79-87. [Crossref] [PubMed]
- Ferretti G, Bacchetti T, Johnston TP, et al. Lipoprotein(a): A missing culprit in the management of athero-thrombosis?. J Cell Physiol 2018;233:2966-81. [Crossref] [PubMed]
- Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016;388:2239-53. [Crossref] [PubMed]
- Leebmann J, Roeseler E, Julius U, et al. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation 2013;128:2567-76. [Crossref] [PubMed]
- Langsted A, Kamstrup PR, Nordestgaard BG. Lipoprotein(a): fasting and nonfasting levels, inflammation, and cardiovascular risk. Atherosclerosis 2014;234:95-101. [Crossref] [PubMed]
- Enkhmaa B, Berglund L. Statins and Lp(a): The plot thickens. Atherosclerosis 2019;289:173-5. [Crossref] [PubMed]